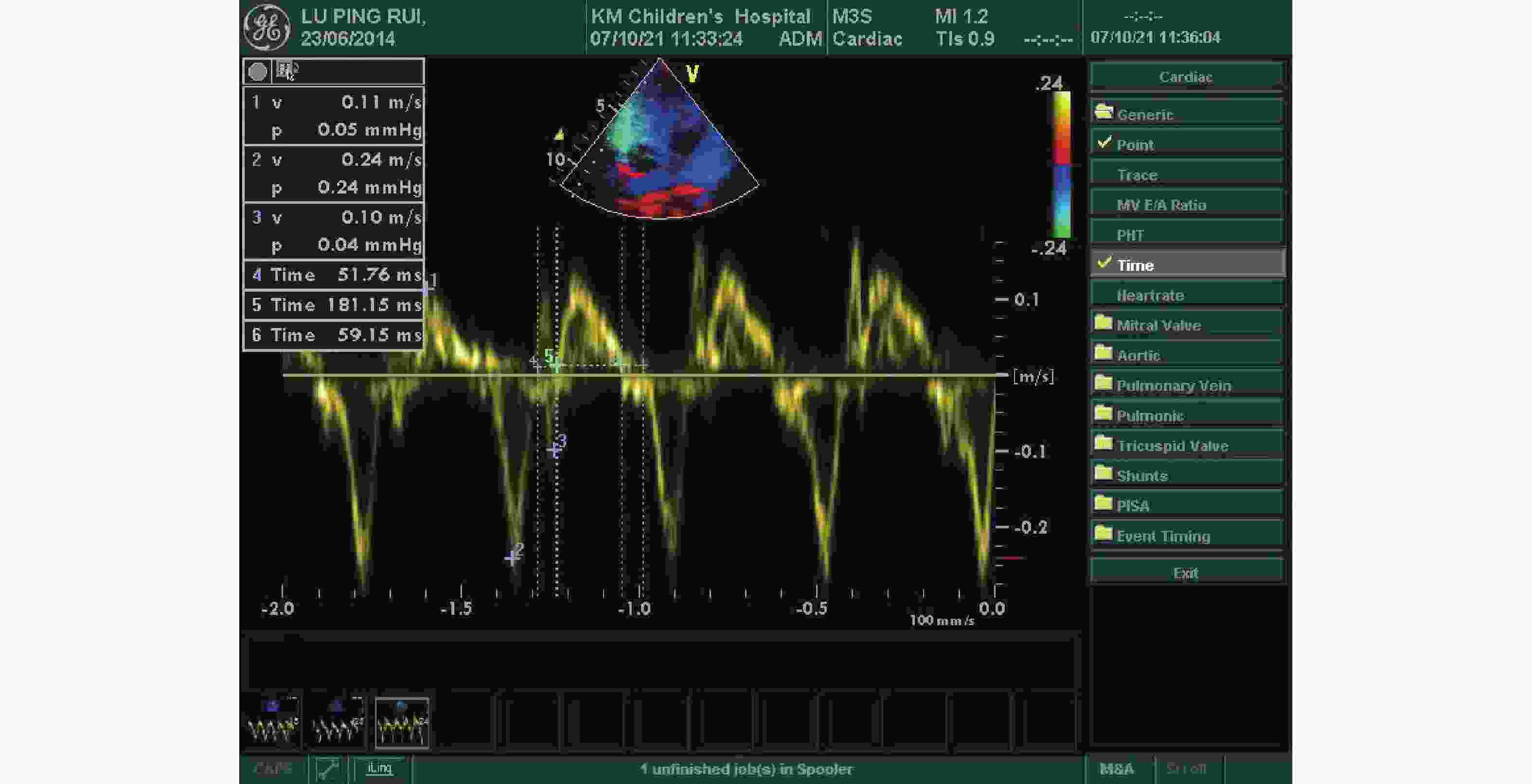
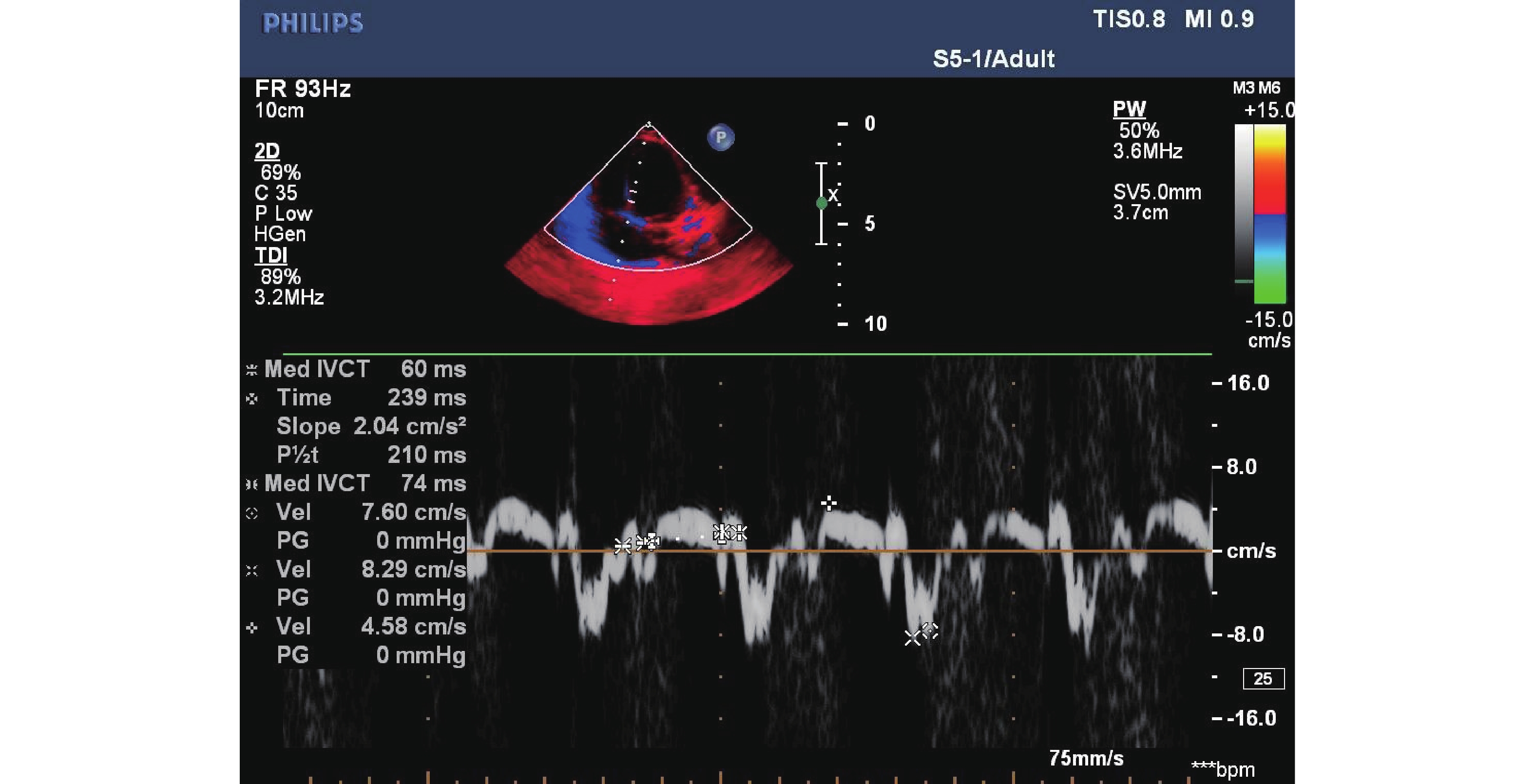
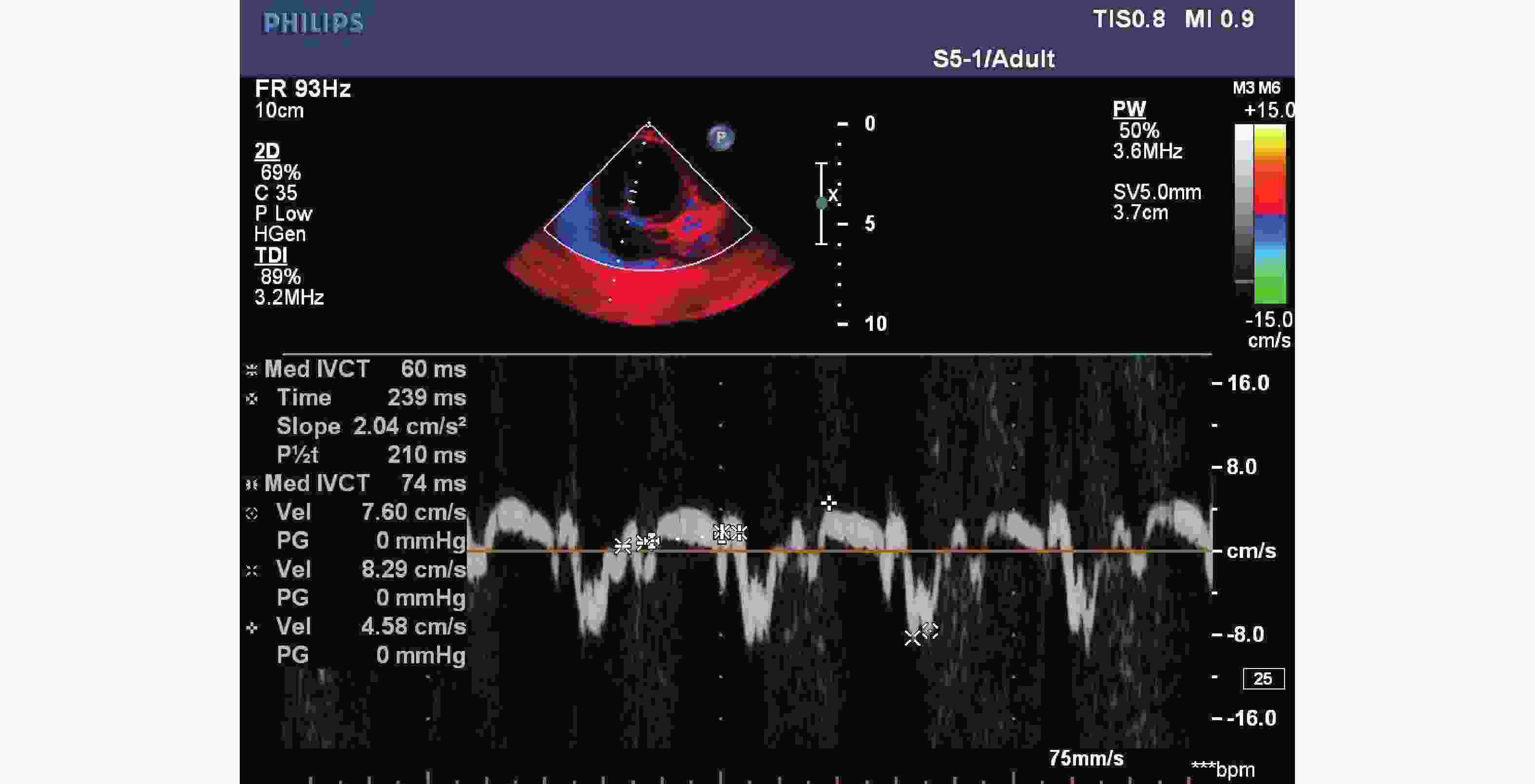
Evaluation of Cardiac Toxicity of Anthracyclines in Children with Acute Leukemia Based on Tei Index
-
摘要:
目的 应用超声技术监测急性白血病患儿使用蒽环类药物后的心功能,以期得到心功能早期变化的指标。 方法 按照入组与排除标准选取2018年3月至2020年12月昆明医科大学附属儿童医院白血病患儿,记录其常规心脏超声指标和组织多普勒,并应用TeiS、TeiRL、TeiM和TeiT评估心脏收缩功能的变化。 结果 正常组和用药前实验组常规心脏超声指标中LVEF 的均值都在 60%以上,FS、SV及EDV均在正常范围,常规指标、TDI和 Tei 指数无统计学意义(P>0.05);实验组累积剂量为200和250 mg/m2组的TeiM与用药前存在显著差异(P<0.05);200和250 mg/m2组与用药前的TeiRL存在显著差异(P<0.05);200和250 mg/m2组与用药前的TeiT存在显著差异(P<0.05)。 结论 Tei指数可作为白血病患儿应用蒽环类药物后左右心功能的早期改变较敏感监测指标。 -
关键词:
- 蒽环类药物 /
- 心脏毒性 /
- 超声心动图 /
- 室间隔基底段心肌做功指数 /
- 右室侧壁基底心肌做功指数 /
- 左心心肌做功指数 /
- 左心心肌做功指数
Abstract:Objective To apply ultrasound to monitor cardiac function changes after anthracycline exposure in children with acute leukemia, in order to obtain the indicators of early changes in their cardiac function. Methods Children with acute leukemia from 2018 March to December 2020 in the Children’ s Hospital of Kunming Medical University were enrolled according to the inclusion and exclusion criteria, their routine cardiac ultrasound and tissue Doppler condition were recorded, and the changes in systolic function were evaluated by Tei index including TeiS, TeiRL, TeiM and TeiT. Results The mean values of LVEF in the normal and the experimental group were both above 60%. FS, SV, and EDV were all in the normal range. While common indicant, the index of TDI or Tei was not statistically significant(P>0.05). The levels of TeiM, TeiRL and TieT in the groups that received a total dose of 200 mg/m2 anthracyclines and 250 mg/m2 were significantly different from that before treatment(P<0.05). Conclusion Tei index can be utilized as a sensitive indicator for early changes in left and right heart function after children with acute leukemia are exposed to anthracyclines. -
Key words:
- Anthracyclines /
- Cardiac toxicity /
- Cardiac ultrasound /
- TeiS /
- TeiRL /
- TeiM /
- TeiT
-
表 1 基线资料比较统计表 ($\bar x \pm s $)
Table 1. Baseline data comparison statistical table ($\bar x \pm s $)
项目 组别 χ2/t P 对照组(n=21) 病例组(n=15) 性别[n(%)] 男 14(66.7) 10(66.7) 0 1 女 7(33.3) 5(33.3) 年龄(岁) 8.49±2.631 6.887±3.4303 1.589 0.121 BSA 1.112±0.219 1.136±0.2234 −0.318 0.752 表 2 对照组与实验照组用药前常规超声指标统计表 [($\bar x \pm s $)/M (Q1,Q3)]
Table 2. Statistical table of routine ultrasound indexes between control group and before medication of experimental group [($\bar x \pm s $)/M (Q1,Q3)]
项目 组别 t/t’/z P 对照组(n=21) 实验组(n=15) LVEF(%) 69.119±6.09431 67.8133±5.45957 0.661 0.513 FS(%) 38.0571±4.82779 36.82±4.44574 0.783 0.439 SV(mL)# 50(25~75) 30.7(23.5~39.4) −0.546 0.585 EDV(mL) 48.1952±14.13147 48.9933±15.13621 −0.162 0.872 s’M(m/s)# 30.8(25.75~40.45) 0.05(0.045~0.06) −1.816 0.069 e’M(m/s) 0.1133±0.02266 0.116±0.02063 −0.361 0.72 a’M(m/s)# 0.06(0.05~0.0725) 0.05(0.05~0.07) −0.05 0.96 e’T(m/s)# 0.06(0.05~0.06) 0.13(0.115~0.145) −0.147 0.883 TeiS# 0.07(0.06~0.0725) 0.38(0.37~0.41) −0.766 0.444 TeiRL# 0.4(0.375~0.4) 0.42(0.395~0.42) −0.492 0.622 TeiM 0.3881±0.01806 0.384±0.02473 0.575 0.569 TeiT 0.4±0.02408 0.3967±0.0216 0.427 0.672 #表示经过SK正态性检验得出非正态分布。 表 3 实验组用药前与各累计剂量的常规超声指标统计表 [($\bar x \pm s $)/M (Q1,Q3)]
Table 3. Statistical table of routine ultrasound indexes of the experimental group before medication and each cumulative dose [($\bar x \pm s $)/M (Q1,Q3)]
项目 组别 χ2/F P 用药前 100 200 250 LVEF(%)# 68.4(64.3~72.4) 65.5(61.3~69.4) 65.4(63.5~70.6) 67.3(64.9~70.2) 1.221 0.748 FS(%)# 37.2(33.2~40.6) 35.1(31.1~39.4) 35.4(33.7~40.9) 37.3(35.9~40.2) 2.398 0.494 SV(mL)# 33.3(26~40.9) 31.3(26.3~40.2) 35.1(27.5~40.2) 35.2(28.1~41) 0.922 0.82 EDV(mL)# 50.3(37.8~58.2) 49.6(36.2~55.4) 50.6(38.9~53.2) 50.2(39.9~57.3) 0.497 0.92 s’M(m/s)# 0.06(0.05~0.07) 0.06(0.06~0.07) 0.06(0.06~0.07) 0.06(0.05~0.07) 2.024 0.567 e’M(m/s)# 0.12(0.1~0.13) 0.11(0.11~0.13) 0.11(0.11~0.13) 0.11(0.1~0.12) 1.926 0.588 a’M(m/s)# 0.06(0.05~0.06) 0.06(0.05~0.07) 0.06(0.05~0.06) 0.06(0.05~0.06) 2.047 0.563 e’T(m/s)# 0.13(0.12~0.14) 0.13(0.12~0.14) 0.13(0.13~0.14) 0.12(0.12~0.13) 8.077 0.044* TeiS# 0.4(0.38~0.4) 0.4(0.38~0.42) 0.4(0.4~0.42) 0.4(0.4~0.42) 6.746 0.08 TeiRL# 0.4(0.4~0.42) 0.42(0.41~0.44) 0.44(0.43~0.44)a 0.44(0.44~0.44)a 18.371 <0.001* TeiT# 0.4(0.38~0.41) 0.42(0.39~0.43) 0.42(0.42~0.43)a 0.42(0.42~0.44)a 15.687 0.001* TeiM 0.384±0.025 0.394±0.01957 0.4027±0.0166a 0.4053±0.0203a 3.307 0.027* #表示经过SK正态性检验得出非正态分布;P<0.05;a表示与用药前存在显著差异。 -
[1] 吴剑秋,沈波,曹军宁,等. 中国蒽环类药物治疗淋巴瘤专家共识[J]. 癌症,2021,40(11):465-474. [2] Banke A,Fosbol E L,Moller J E,et al. Long-term effect of epirubicin on incidence of heart failure in women with breast cancer: insight from a randomized clinical trial[J]. Eur J Heart Fail,2018,20(10):1447-1453. doi: 10.1002/ejhf.1168 [3] Lipshultz E R,Chow E J,Doody D R,et al. Cardiometabolic risk in childhood cancer survivors: a report from the children’s oncology group[J]. Cancer Epidemiol Biomarkers Prev,2022,31(3):536-542. doi: 10.1158/1055-9965.EPI-21-0360 [4] Samosir S M,Utamayasa I,Andarsini M R,et al. Risk factors of daunorubicine induced early cardiotoxicity in childhood acute lymphoblastic leukemia: A retrospective study[J]. Asian Pac J Cancer Prev,2021,22(5):1407-1412. doi: 10.31557/APJCP.2021.22.5.1407 [5] Bayram C,Cetin I,Tavil B,et al. Evaluation of cardiotoxicity by tissue doppler imaging in childhood leukemia survivors treated with low-dose anthracycline[J]. Pediatr Cardiol,2015,36(4):862-866. doi: 10.1007/s00246-015-1096-6 [6] Leger K,Slone T,Lemler M,et al. Subclinical cardiotoxicity in childhood cancer survivors exposed to very low dose anthracycline therapy[J]. Pediatr Blood Cancer,2015,62(1):123-127. doi: 10.1002/pbc.25206 [7] Amedro P,Vincenti M,Abassi H,et al. Use of speckle tracking echocardiography to detect late anthracycline-induced cardiotoxicity in childhood cancer: A prospective controlled cross-sectional study[J]. Int J Cardiol,2022,354(5):75-83. [8] Seara F,Kasai-Brunswick T H,Nascimento J,et al. Anthracycline-induced cardiotoxicity and cell senescence: New therapeutic option?[J]. Cell Mol Life Sci,2022,79(11):568. doi: 10.1007/s00018-022-04605-7 [9] 中国蒽环类药物治疗骨肉瘤专家共识[J]. 癌症, 2022, 41(8): 368-384. [10] 国家卫生健康委办公厅. 儿童急性早幼粒细胞白血病诊疗规范(2018年版)[S], 国卫办医函〔2018〕868号. [11] 国家卫生健康委办公厅. 儿童急性淋巴细胞白血病诊疗规范(2018年版)[S], 国卫办医函〔2018〕868号. [12] Chang H M,Moudgil R,Scarabelli T,et al. Cardiovascular complications of cancer therapy: best practices in diagnosis,prevention,and management: Part 1[J]. J Am Coll Cardiol,2017,70(20):2536-2551. doi: 10.1016/j.jacc.2017.09.1096 [13] Ganatra S,Nohria A,Shah S,et al. Upfront dexrazoxane for the reduction of anthracycline-induced cardiotoxicity in adults with preexisting cardiomyopathy and cancer: A consecutive case series[J]. Cardiooncology,2019,5(1):1-12. [14] 邰宵辉,张玲芳,党春艳,等. 抗肿瘤药物致心脏损伤的药物防治进展[J]. 临床肿瘤学杂志,2023,28(1):70-76. [15] 欧晓霞. 超声心动图E/Em、TDI-Tei指数及STI评价乳腺癌患者蒽环类化疗后早期心脏损伤[D]. 长沙: 湖南中医药大学, 2022. [16] Klaeboe L G,Edvardsen T. Echocardiographic assessment of left ventricular systolic function[J]. J Echocardiogr,2019,17(1):10-16. doi: 10.1007/s12574-018-0405-5 [17] van den Bogaard V,van Luijk P,Hummel Y M,et al. Cardiac function after radiation therapy for breast cancer[J]. Int J Radiat Oncol Biol Phys,2019,104(2):392-400. doi: 10.1016/j.ijrobp.2019.02.003 [18] 罗银丽,陶方依,陈小维,等. 组织多普勒Tei指数评价蒽环类药物对乳腺癌患者左室功能的影响[J]. 温州医科大学学报,2022,52(1):47-50. [19] 徐海燕,茅玲,刘海浪,等. 三维斑点追踪成像对乳腺癌病人蒽环类化疗早期心室收缩功能障碍的评价研究[J]. 中西医结合心脑血管病杂志,2023,21(3):515-519. [20] Tei C,Ling L H,Hodge D O,et al. New index of combined systolic and diastolic myocardial performance: A simple and reproducible measure of cardiac function-a study in normals and dilated cardiomyopathy[J]. J Cardiol,1995,26(6):357-366. [21] Kang Y,Scherrer-Crosbie M. Echocardiography imaging of cardiotoxicity[J]. Cardiol Clin,2019,37(4):419-427. doi: 10.1016/j.ccl.2019.07.006 [22] 邓曼,彭格红. 超声心动图用于蒽环类药物乳腺癌化疗后右心功能评估的研究进展[J]. 海南医学,2021,32(4):522-525. [23] 韩若凌,尹笑笑,赵娜. Tei指数评价蒽环类药物对恶性肿瘤患儿的右心损害[J]. 中华医学超声杂志(电子版),2018,15(6):433-439. -





 下载:
下载: