Research Progress on the Anti-tumor Activity and Mechanism of Narciclasine
-
摘要: 水仙环素(narciclasine,NCS)是从石蒜科水仙属植物水仙的鳞茎中提取出的水鬼蕉碱型生物碱,被证实对多种肿瘤细胞具有显著的抑制活性。NCS的抗肿瘤作用机制多样,通过不同途径产生抗肿瘤作用,适应了当前研发多靶点抗肿瘤药物的趋向。本文结合NCS抑制乳腺癌细胞、结肠癌细胞、胃癌细胞、黑色素瘤细胞、多形性胶质母细胞瘤细胞、口腔癌细胞和原发性渗出性淋巴瘤细胞的作用及分子机制,对NCS近年来的抗肿瘤活性及作用机制研究进行综述,旨在为将来NCS类抗肿瘤药物的研发与设计提供思路和借鉴。Abstract: Narciclasine(NCS), a hymenocallis littoralis alkaloid extracted from the bulbs of the genus Narcissus in the Lycoriaceae family, has been proven to have significant anti-tumor activity against a variety of tumor cells. The antitumor mechanisms of NCS are diverse and NCS exhibits antitumor effects through different pathways, which adapts to the current trend of developing multi-target anti-tumor drugs. This review introduces the research progress of the anti-tumor activity and mechanism of NCS in recent years based on the inhibitory effect of NCS on gastric cancer cells, oral cancer cells, polymorphous glioblastoma cells, colon cancer cells, breast cancer cells, melanoma cells and primary exudative lymphoma cells, aiming to provide ideas and references for the research and development, and design of NCS type anti-tumor drugs in the future.
-
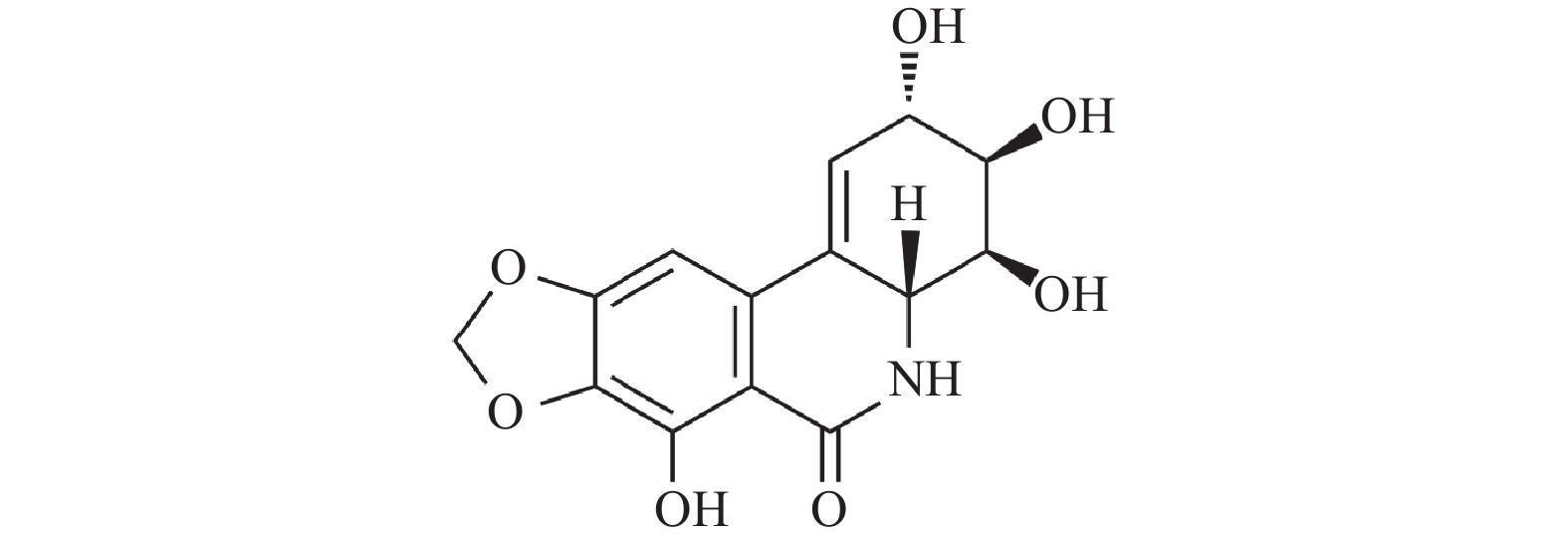
图 1 水仙环素的化学结构[4]
Figure 1. Chemical structure of NCS
-
[1] 毕凯健. 水仙环素衍生物的设计、合成及抗肿瘤活性研究[D]. 上海: 第二军医大学, 2016. [2] Antoszczak M. A medicinal chemistry perspective on sali-nomycin as a potent anticancer and anti-CSCs agent[J]. Eur J Med Chem,2019,164:366-377. doi: 10.1016/j.ejmech.2018.12.057 [3] Dagogo-Jack I,Shaw A T. Tumour heterogeneity and re-sistance to cancer therapies[J]. Nat Rev Clin Oncol,2018,15(2):81-94. doi: 10.1038/nrclinonc.2017.166 [4] Ingrassia L,Lefranc F,Dewelle J,et al. Structure-activity relationship analysis of novel derivatives of narciclasine (an Amaryllidaceae isocarbostyril derivative) as potential anticancer agents[J]. J Med Chem,2009,52(4):1100-1114. doi: 10.1021/jm8013585 [5] Ceriotti G. Narciclasine: An antimitotic substance from Narcissus bulbs[J]. Nature,1967,21(5076):595-596. [6] Fürst R. Narciclasine - an amaryllidaceae alkaloid with potent antitumor and anti-inflammatory properties[J]. Planta Med,2016,82(16):1389-1394. doi: 10.1055/s-0042-115034 [7] Rárová L,Ncube B,Van Staden J,et al. Identification of Narciclasine as an in vitro anti-inflammatory component of Cyrtanthus contractus by correlation-based metabolomics[J]. Nat Prod,2019,82(5):1372-1376. doi: 10.1021/acs.jnatprod.8b00973 [8] Bräutigam J,Bischoff I,Schürmann C,et al. Narciclasine inhibits angiogenic processes by activation of rho kinase and by downregulation of the VEGF receptor 2[J]. Mol Cell Cardiol,2019,135:97-108. doi: 10.1016/j.yjmcc.2019.08.001 [9] De Castro Barbosa E,Alves T M A,Kohlhoff M,et al. Searching for plant-derived antivirals against dengue virus and Zika virus[J]. Virol J,2022,19(1):31. [10] Dumont P,Ingrassia L,Rouzeau S,et al. The Amaryllidaceae isocarbostyril narciclasine induces apoptosis by activation of the death receptor and/or mitochondrial pathways in cancer cells but not in normal fibroblasts[J]. Neoplasia,2007,9(9):766-776. doi: 10.1593/neo.07535 [11] Min B S,Cao J J,Nakamura N,et al. Cytotoxic alkaloids and a Fla Villi from the bulbs of Crinum asiaticum var japonicum[J]. Chem Plmrm Bull,2001,49(9):1217-1219. doi: 10.1248/cpb.49.1217 [12] Van Goietsenoven G,Mathieu V,Lefranc F,et al. Narciclasine as well as other Amaryllidaceae isocarbostyrils are promising GTP-ase targeting agents against brain cancers[J]. Med Res Rev,2013,33(2):439-455. [13] Qiu Y,Fang B,Thuy N T T,et al. Narciclasine suppresses esophageal cancer cell proliferation and migration by inhibiting the FAK signaling pathway[J]. Eur J Pharmacol,2022,921:174669. [14] 马楠楠,陈宁,季宇彬,等. 水鬼蕉生物碱抗肿瘤研究进展[J]. 黑龙江医药,2013,26(2):205-208. doi: 10.3969/j.issn.1006-2882.2013.02.017 [15] Kornienko A,Evidente A. Chemistry,biology,and medicinal potential of NCS and its congeners[J]. Chem Rev,2008,108(6):1982-2014. doi: 10.1021/cr078198u [16] Cao C,Huang W,Zhang N,et al. NCS induces autophagy-dependent apoptosis in triple-negative breast cancer cells by regulating the AMPK-ULK1 axis[J]. Cell Prolif,2018,51(6):12518. doi: 10.1111/cpr.12518 [17] Beebe J D,Li J Y,Zhang J T. Two decades of research in discovery of anticancer drugs targeting STAT3,how close are we?[J]. Pharmacol Ther,2018,191:74-91. doi: 10.1016/j.pharmthera.2018.06.006 [18] Mohan C D,Rangappa S,Preetham H D,et al. Targeting STAT3 signaling pathway in cancer by agents derived from Mother Nature[J]. Cancer Biol,2022,80:157-182. doi: 10.1016/j.semcancer.2020.03.016 [19] Lv C,Huang Y,Huang R,et al. Narciclasine targets STAT3 via distinct mechanisms in tamoxifen-resistant breast cancer cells[J]. Mol Ther Oncolytics,2022,24:340-354. doi: 10.1016/j.omto.2021.12.025 [20] Wang M,Liang L,Wang R,et al. Narciclasine,a novel topoisomerase I inhibitor,exhibited potent anti-cancer activity against cancer cells[J]. Nat Prod Bioprospect,2023,13(1):27. doi: 10.1007/s13659-023-00392-1 [21] Liu L F,Wang J C. Supercoiling of the DNA template during transcription[J]. Proc Natl AcadSci USA,1987,84(20):7024-7027. doi: 10.1073/pnas.84.20.7024 [22] Lichota A,Gwozdzinski K. Anticancer Activity of Natural Compounds from Plant and Marine Environment[J]. Int J Mol Sci,2018,19(11):3533. doi: 10.3390/ijms19113533 [23] Pommiier Y,Pourquier P,Urasaki Y,et al. Topoisomerase 1 inhibitors: selectivity andcellular resistance[J]. Drug Resist Updat,1999,2(5):307-318. doi: 10.1054/drup.1999.0102 [24] Said A H,Raufman J P,Xie G. The role of matrix metalloproteinases in colorectal cancer[J]. Cancers,2014,6:366-375. doi: 10.3390/cancers6010366 [25] Pezeshkian Z,Nobili S,Peyravian N,et al. Insights into the Role of Matrix Metalloproteinases in Precancerous Conditions and in Colorectal Cancer[J]. Cancers,2021,13(24):6226. doi: 10.3390/cancers13246226 [26] Ara ú jo R F,Jr,Lira G A,et al. Prognostic and diagnostic implications of MMP-2,MMP-9,and VEGF-α expressions in colorectal cancer[J]. Pathol Res Pract,2015,211(1):71-77. doi: 10.1016/j.prp.2014.09.007 [27] Wang K,Zheng J,Yu J,et al. Knockdown of MMP-1 inhibits the progression of colorectal cancer by suppressing the PI3K/Akt/c-myc signaling pathway and EMT[J]. Oncol Rep,2020,43(4):1103-1112. [28] Mathieu V,Laguera B,Masi M,et al. Amaryllidaceae alkaloids decrease the proliferation,invasion,and secretion of clinically relevant cytokines by cultured human colon cancer cells[J]. Biomolecules,2022,12(9):1267. doi: 10.3390/biom12091267 [29] Bie Y, Ge W, Yang Z, et al. The crucial role of CXCL8 and its receptors in colorectal liver metastasis[J]. Dis Markers, 2019, 2019: 8023460. [30] Wang Y,Wang K,Han GC,et al. Neutrophil infiltration favors colitis-associated tumorigenesis by activating the interleukin-1 (IL-1)/IL-6 axis[J]. Mucosal Immunol,2014,7(5):1106-1115. doi: 10.1038/mi.2013.126 [31] Lin Y,He Z,Ye J,et al. Progress in Understanding the IL-6/STAT3 Pathway in Colorectal Cancer[J]. Onco Targets Ther,2020,13:13023-13032. doi: 10.2147/OTT.S278013 [32] Yuan Y,He X,Li X,et al. NCS induces autophagy-mediated apoptosis in gastric cancer cells through the Akt/mTOR signaling pathway[J]. BMC Pharmacol Toxicol,2021,22(1):70. doi: 10.1186/s40360-021-00537-3 [33] White E,Mehnert J M,Chan C S. Autophagy,metabolism,and Cancer[J]. Clin Cancer Res,2015,21:5037-5046. doi: 10.1158/1078-0432.CCR-15-0490 [34] Deng S,Shanmugam M K,Kumar A P,et al. Targeting autophagy using natural compounds for cancer prevention and therapy[J]. Cancer,2019,125(8):1228-1246. doi: 10.1002/cncr.31978 [35] Marino G, Niso-Santano M, Baehrecke E H, et al Self-consumption: the interplay of autophagy and apoptosis[J]. Nat Rev Mol Cell Biol, 2014, 15(2): 81–94. [36] Moosavi M A,Haghi A,Rahmati M,et al. Phytochemicals as potent modulators of autophagy for cancer therapy[J]. Cancer Lett,2018,424:46-69. doi: 10.1016/j.canlet.2018.02.030 [37] Liu R,Li J,Zhang T,et al. Itraconazole suppresses the growth of glioblastoma through induction of autophagy: involvement of abnormal cholesterol trafficking[J]. Autophagy,2014,10(7):1241-1255. doi: 10.4161/auto.28912 [38] Elgendy M,Sheridan C,Brumatti G,et al. Oncogenic ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival[J]. Mol Cell,2011,42(1):23-35. doi: 10.1016/j.molcel.2011.02.009 [39] He C,Klionsky D J. Regulation mechanisms and signaling pathways of autophagy[J]. Annu Rev Genet,2009,43:67-93. doi: 10.1146/annurev-genet-102808-114910 [40] Jung C H,Ro S H,Cao J,et al. mTOR regulation of autophagy[J]. FEBS Lett,2010,584(7):1287-1295. doi: 10.1016/j.febslet.2010.01.017 [41] Van Goietsenoven G,Hutton J,Becker J P,et al. Targeting of eEF1A with Amaryllidaceae isocarbostyrils as a strategy to combat melanomas[J]. FASEB J,2010,24(11):4575-4584. doi: 10.1096/fj.10-162263 [42] Lefranc F,Sauvage S,Van Goietsenoven G,et al. NCS,a plant growth modulator,activates Rho and stress fibers in glioblastoma cells[J]. Mol Cancer Ther,2009,8(7):1739-1750. doi: 10.1158/1535-7163.MCT-08-0932 [43] Scott R W,Olson M. LIM kinases: function,regulation and association with human disease[J]. J Mol Med,2007,85(6):555-568. doi: 10.1007/s00109-007-0165-6 [44] Arber S,Barbayannis F A,Hanser H,et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase[J]. Nature,1998,393(6687):805-809. doi: 10.1038/31729 [45] Le Mercier M,Mathieu V,Haibe-Kains B,et al. Knocking down galectin-1 in human Hs683 glioblastoma cells impairs both angiogenesis through ORP150 depletion and endoplasmic reticulum stress responses[J]. J Neuropathol Exp Neurol,2008,67(5):456-469. doi: 10.1097/NEN.0b013e318170f892 [46] Raftopoulou M,Hall A. Cell migration: Rho GTPases lead the way[J]. Dev Biol,2004,265(1):23-32. doi: 10.1016/j.ydbio.2003.06.003 [47] Tabu K,Ohba Y,Suzuki T,et al. Oligodendrocyte lineage transcription factor 2 inhibits the motility of a human glial tumor cell line by activating RhoA[J]. Mol Cancer Res,2007,5(10):1099-1109. doi: 10.1158/1541-7786.MCR-07-0096 [48] Shieu M K,Ho H Y,Lin C C,et al. NCS suppresses oral cancer metastasis by modulating cathepsin B and extracellular signal-related kinase pathways[J]. Biomed Pharmacother,2023,158:114159. doi: 10.1016/j.biopha.2022.114159 [49] Q Peng,Deng Z,Pan H,et al. Mitogen-activated protein kinase signaling pathway in oral cancer[J]. Oncol Lett,2018,15(2):1379-1388. [50] Gopalakrishnan R,Matta H,Choi S,et al. NCS,an isocarbostyril alkaloid,has preferential activity against primary effusion lymphoma[J]. Sci Rep,2020,10(1):5712. doi: 10.1038/s41598-020-62690-9 [51] Meyer N,Penn L Z. Refecting on 25 years with MYC[J]. Nat Rev Cance,2008,8(12):976-990. doi: 10.1038/nrc2231 [52] Tolani B,Gopalakrishnan R,Punj V,et al. Targeting Myc in KSHV-associated primary effusion lymphoma with BET bromodomain inhibitors[J]. Oncogene,2014,33(22):2928-2937. doi: 10.1038/onc.2013.242 -





 下载:
下载: