Efficacy and Safety of Bevacizumab Combined with Temozolomide for Recurrent High-Grade Glioma
-
摘要:
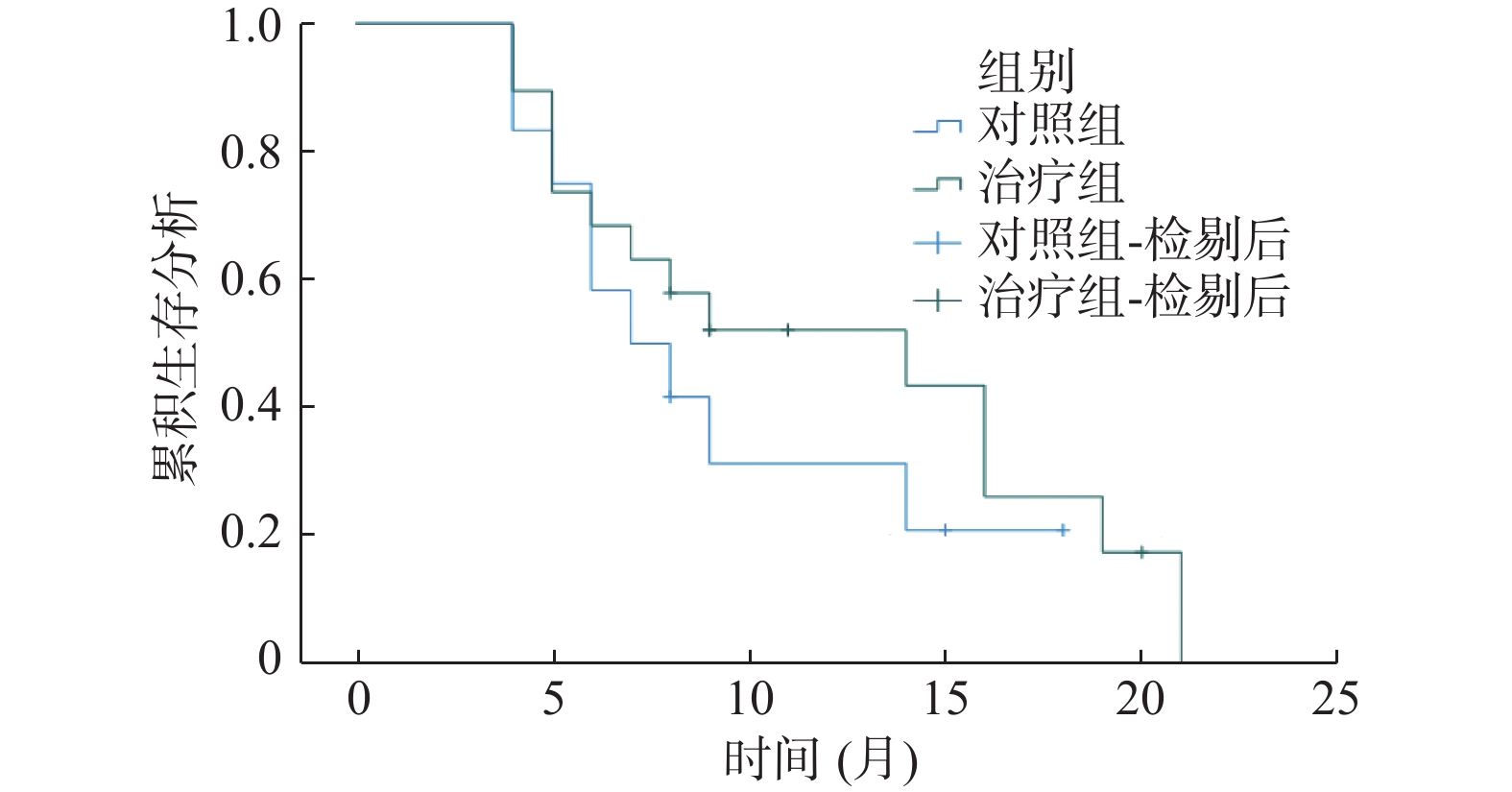
目的 探讨贝伐珠单抗(bevacizumab,BEV)单用及贝伐珠单抗联合替莫唑胺(temozolomide,TMZ)治疗复发性高级别脑胶质瘤(high-grade glioma,HGG)的近期疗效、安全性及影响复发性高级别脑胶质瘤患者总生存期(overall survival,OS)的预后因素。 方法 按照脑胶质瘤治疗效果评估(response assessment in neuro oncology,RANO)标准,以客观有效率(objective response rate,ORR)、疾病控制率(disease control rate,DCR)为指标,回顾性分析云南省肿瘤医院2020年8月至2023年7月31例复发性HGG患者的临床病历资料,分为对照组(BEV)和治疗组(BEV+TMZ),评价2组近期疗效。采用Cox单因素和多因素分析方法分析组别(对照组与治疗组)、性别、年龄、疾病分级、入院评分、组织病理型分类等对31例复发性HGG患者OS的影响;通过χ2检验比较2组不良反应(adverse reactions,ADR),评价安全性。 结果 治疗组ORR = 63.16%,对照组ORR = 16.67%,差异有统计学意义(χ2 = 6.419,P = 0.011);治疗组DCR = 89.47%,对照组DCR = 75.00%,DCR比较差异无统计学意义(χ2 = 0.320,P = 0.571)。对照组中位生存期(mOS)为7个月(95%CI:3.605~10.395),治疗组中位生存期(mOS)为14个月(95%CI:3.853~24.147),2组mOS差异无统计学意义(χ2 = 0.829,P = 0.363)。31例复发性HGG患者Cox单因素分析入院评分和组织病理型分类差异有统计学意义(P < 0.05),多因素分析组织病理型分类差异有统计学意义(P < 0.05)。2组治疗后ADR比较,恶心呕吐、乏力及腹泻,差异无统计学意义(P > 0.05);血小板及白细胞降低率比较,差异有统计学意义(P < 0.05),2组出现的ADR对症处理后均得到了缓解。 结论 治疗组治疗复发性HGG近期疗效优于对照组,2组治疗复发性HGG后的ADR可耐受,安全性良好。组织病理型分类可能是影响31例复发性HGG患者OS的预后因素。 Abstract:Objective To explore the short-term efficacy, safety, and prognostic factors affecting the overall survival (OS) of patients with recurrent high-grade glioma (HGG) treated with bevacizumab (BEV) alone and bevacizumab in combination with temozolomide (TMZ). Methods According to the response assessment in neurooncology (RANO) criteria for the treatment of gliomas, objective response rate (ORR) and disease control rate (DCR) were used as indicators to retrospectively analyze the clinical medical records of 31 patients with recurrent HGG at Yunnan Cancer Hospital from August 2020 to July 2023. The patients were divided into a control group (BEV) and a treatment group (BEV+TMZ), and the recent efficacy of the two groups was evaluated. Cox univariate and multivariate analysis methods were used to analyze the impact of group (control group vs. treatment group), gender, age, disease grading, admission score, histopathological classification, etc., on the overall survival (OS) of 31 patients with recurrent HGG. Adverse reactions (ADR) between the two groups were compared using a chi-square test to evaluate the safety. Results The ORR of the treatment group was 63.16%, and the ORR of the control group was 16.67%. The difference was statistically significant (χ2 = 6.419, P = 0.011). The DCR of the treatment group was 89.47%, and the DCR of the control group was 75.00%. There was no significant difference in DCR (χ2 = 0.320, P = 0.571). The median survival time (mOS) in the control group was 7 months (95%CI: 3.605~10.395), and the median survival time (mOS) in the treatment group was 14 months (95%CI: 3.853~24.147). There was no significant difference in mOS between the two groups (χ2 = 0.829, P = 0.363). There were significant differences in Cox univariate analysis admission score and histopathological classification among 31 patients with recurrent HGG (P < 0.05), and statistically significant differences in histopathological classification by multivariate analysis (P < 0.05). There was no significant difference in nausea, vomiting, fatigue and diarrhea between the two groups after treatment (P > 0.05). There were statistically significant differences in the reduction rates of platelets and white blood cells (P < 0.05), after symptomatic treatment, both groups of ADRs were alleviated. Conclusion The short-term curative effect of the treatment group was better than that of the control group in the treatment of recurrent HGG, the ADRs after recurrent HGG treatment in 2 groups were tolerable and safe, and the histopathological classification may be a prognostic factor affecting the OS of 31 patients with recurrent HGG. -
Key words:
- High-grade glioma /
- Bevacizumab /
- Temozolomide
-
表 1 2组患者入院时临床资料分析[n(%)]
Table 1. Clinical data analysis of patients between the two groups at admission [n(%)]
临床资料 对照组 治疗组 T/Z/χ2 P 年龄(岁) 46.25±13.13 43.37±15.123 0.543 0.591 性别 男 7(58.3) 12(63.2) 0.072 0.788 女 5(41.7) 7(36.8) 组织病理型分类 胶质母细胞瘤 2(16.7) 7(36.8) 4.033 0.203 少突胶质瘤 4(33.3) 9(47.4) 星形细胞瘤 6(50.0) 3(15.8) 疾病分级 3 9(75.0) 12(63.2) 0.086 0.77 4 3(25.0) 7(36.8) 入院评分 90(82.5,90) 90(80,90) -0.279 0.78 表 2 2组患者经过药物治疗后的近期疗效比较[n(%)]
Table 2. Comparison of short-term efficacy after drug treatment between the two groups [n(%)]
组别 n CR PR SD PD ORR DCR 对照组 12 0(0.00) 2(16.67) 7(58.33) 3(25.00) 2(16.67) 9(75.00) 治疗组 19 0(0.00) 12(63.16) 5(26.32) 2(10.53) 12(63.16) 17(89.47) χ2 − − − − 6.419 0.320 P − − − − 0.011* 0.571 对照组:BEV单用治疗;治疗组:BEV联合TMZ治疗。“−”为无数据;*P < 0.05。 表 3 影响31例复发性高级别脑胶质瘤OS的单因素分析
Table 3. Single factor analysis of OS affecting 31 cases of recurrent high-grade brain glioma
项目 HR 95%CI P 组别 1.467 0.612~3.515 0.390 性别 0.931 0.373~2.329 0.879 年龄 1.018 0.987~1.050 2.252 疾病分级 1.950 0.807~4.712 0.138 入院评分 0.008 0.866~0.979 0.008* 组织病理型分类 0.001* 组织病理型分类1 9.615 2.765~33.431 组织病理型分类2 1.828 0.587~5.696 组织病理型分类1:GBM、ACM;组织病理型分类2:ODG。*P < 0.05。 表 4 影响31例复发性高级别脑胶质瘤OS的多因素分析
Table 4. Multifactorial analysis of OS affecting 31 cases of recurrent high-grade glioma
项目 B SE HR 95%CI P 入院评分 −0.033 0.037 0.968 0.900~1.040 0.368 组织病理型分类 0.019* 组织病理型分类1 −1.318 0.735 0.268 0.063~1.129 0.070 组织病理型分类2 −2.002 0.713 0.135 0.033~0.546 0.050 组织病理型分类1:GBM、ACM;组织病理型分类2:ODG。*P < 0.05。 表 5 2组患者接受药物治疗后发生的ADR比较[n(%)]
Table 5. Comparison of ADR after drug treatment between the two groups [n(%)]
组别 n 恶心、呕吐 乏力 腹泻 对照组 12 6(50.00) 2(16.67) 1(8.33) 治疗组 19 7(36.84) 5(26.32) 2(10.53) χ2 − 0.523 0.034 0.00 P − 0.470 0.853 1.000 对照组:BEV单用治疗;治疗组:BEV联合TMZ治疗。“−”为无数据。 表 6 2组接受治疗后血小板及白细胞降低比较[n(%)]
Table 6. Comparison of platelet and white blood cell decreases between two groups after treatment [n(%)]
组别 n 血小板降低 白细胞降低 对照组 12 3(25.00) 1(8.33) 治疗组 19 13(68.42) 11(57.89) χ2 − 5.552 5.669 P − 0.018* 0.017* 对照组:BEV单用治疗;治疗组:BEV联合TMZ治疗。“−”为无数据。*P < 0.05。 -
[1] Miller K D,Ostrom Q T,Kruchko C,et al. Brain and other central nervous system tumor statistics[J]. CA:A Cancer Journal for Clinicians,2021,71(5):381-406. doi: 10.3322/caac.21693 [2] Louis D N,Perry A,Wesseling P,et al. The 2021 WHO classification of tumors of the central nervous system: A summary[J]. Neuro-Oncology,2021,23(8):1231-1251. doi: 10.1093/neuonc/noab106 [3] Gritsch S,Batchelor T T,Gonzalez Castro L N. Diagnostic,therapeutic,and prognostic implications of the 2021 world health organization classification of tumors of the central nervous system[J]. Cancer,2022,128(1):47-58. doi: 10.1002/cncr.33918 [4] Ostrom Q T,Cioffi G,Waite K,et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the united states in 2014-2018[J]. Neuro-Oncology,2021,23(12 Suppl 2):iii1-iii105. doi: 10.1093/neuonc/noab200 [5] Sahebjam S,Forsyth P A,Tran N D,et al. Hypofractionated stereotactic re-irradiation with pembrolizumab and bevacizumab in patients with recurrent high-grade gliomas: Results from a phase I study[J]. Neuro-Oncology,2021,23(4):677-686. doi: 10.1093/neuonc/noaa260 [6] Gimbrone M A,Leapman S B,Cotran R S,et al. Tumor dormancy in vivo by prevention of neovascularization[J]. Journal Experimental Medicine,1972,136(2):261-276. doi: 10.1084/jem.136.2.261 [7] Wick W,Gorlia T,Bendszus M,et al. Lomustine and bevacizumab in progressive glioblastoma[J]. The New England Journal of Medicine,2017,377(20):1954-1963. doi: 10.1056/NEJMoa1707358 [8] Wen P Y,Macdonald D R,Reardon D A,et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group[J]. Journal Clinical Oncology,2010,28(11):1963-1972. doi: 10.1200/JCO.2009.26.3541 [9] 张伟,王政. 中国抗癌协会脑胶质瘤整合诊治指南(精简版)[J]. 中国肿瘤临床,2022,49(16):811-818. [10] Ameratunga M,Pavlakis N,Wheeler H,et al. Anti-angiogenic therapy for high-grade glioma[J]. Cochrane Database Syst Rev,2018,11(11):CD008218. [11] Xu Y A,Guan H J,Yu K F,et al. Efficacy and safety of pharmacotherapy for recurrent high-grade glioma: A systematic review and network meta-analysis.[J]. Fronts in Pharmacology,2023,14:1191480. doi: 10.3389/fphar.2023.1191480 [12] Chen Y L,Guo L B,Li X Z,et al. Reduced-dose bevacizumab vs. standard-dose bevacizumab in recurrent high-grade glioma: Which one is better? A meta-analysis[J]. Clinical Neurology and Neurosurgery,2020,198:106239. doi: 10.1016/j.clineuro.2020.106239 [13] 汪思亮,盛晓波,韦忠红,等. 肿瘤血管正常化与肿瘤治疗[J]. 肿瘤,2013,33(07):653-657. [14] Bergers G, Hanahan D, Modes of resistance to anti-angiogenic therapy [J]. Nature Reviews Cancer, 2008, 8(8): 592-603. [15] Pàez-Ribes M,Allen E,Hudock J,et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis[J]. Cancer Cell,2009,15(3):220-231. doi: 10.1016/j.ccr.2009.01.027 [16] Jain R K. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy[J]. Nature Medicine,2001,7(9):987-989. doi: 10.1038/nm0901-987 [17] 张静,张文超,钱子君,等. 血管正常化提高肿瘤治疗疗效[J]. 中国癌症杂志,2016,26(2):188-192. [18] Macdonald D R, Temozolomide for recurrent high-grade glioma [J]. Seminars in Oncology, 2001, 28(Suppl 13): 3-12. [19] 崔润,郭琤琤,郭颖,等. 贝伐珠单抗治疗复发胶质母细胞瘤疗效和预后分析[J]. 中国肿瘤临床,2022,49(21):1088-1093. [20] Armstrong T S,Cao Y,Scheurer M E,et al. Risk analysis of severe myelotoxicity with temozolomide: The effects of clinical and genetic factors[J]. Neuro Oncology,2009,11(6):825-32. doi: 10.1215/15228517-2008-120 [21] Saran F,Chinot O L,Henriksson R,et al. Bevacizumab,temozolomide,and radiotherapy for newly diagnosed glioblastoma: Comprehensive safety results during and after first-line therapy[J]. Neuro Oncology,2016,18(7):991-1001. doi: 10.1093/neuonc/nov300 -






 下载:
下载: