Real-World Clinical Efficacy and Safety of Bictegravir/Emtricitabine/Tenofovir Alafenamide (BIC/FTC/TAF) Single-Tablet Regimen
-
摘要:
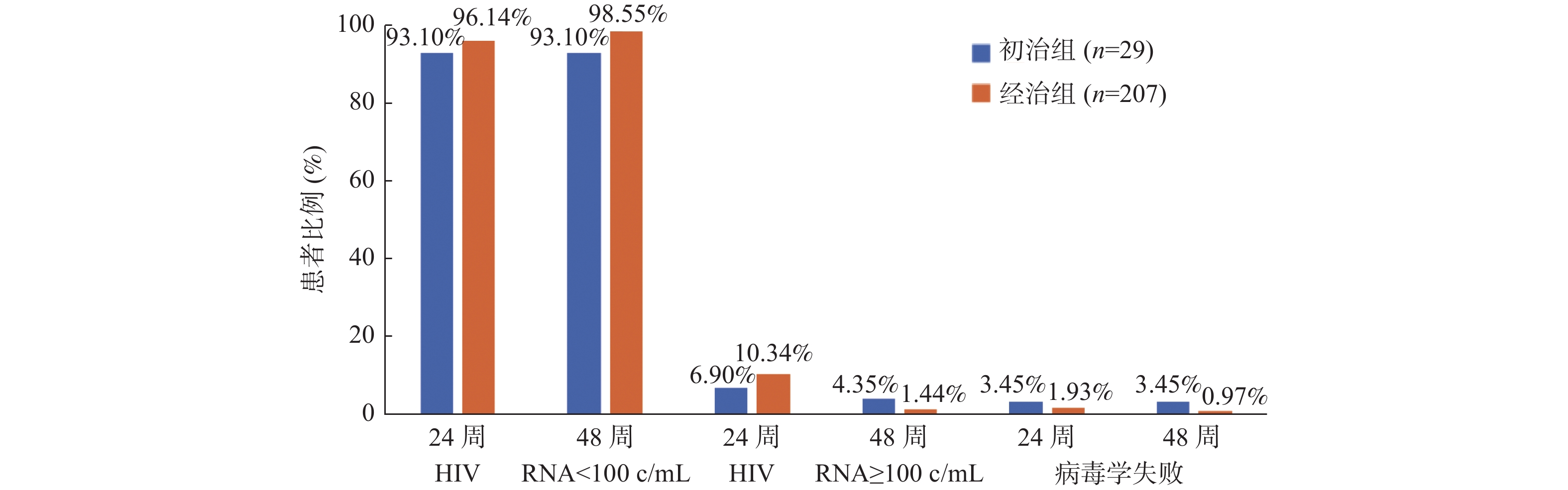
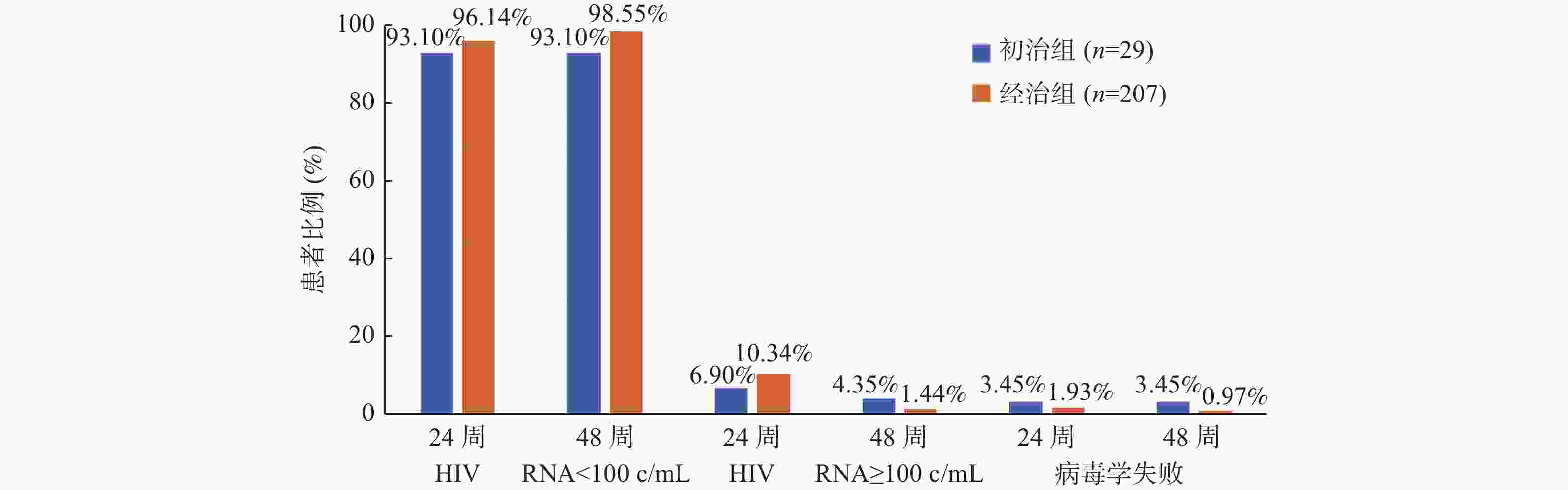
目的 分析比克替拉韦/恩曲他滨/丙酚替诺福韦(bictegravir/emtricitabine/tenofovir alafenamide,BIC/FTC/TAF)三合一单片复方制剂在艾滋病抗病毒治疗患者中的疗效和安全性。 方法 纳入2022 年 2 月 1 日至 2022 年 6 月 1 日期间接受单片复方制剂 (BIC/FTC/TAF) 的初治患者(初治组)和经历过治疗的患者(经治组),并使用前瞻性观察研究的方法对其治疗后的病毒学、免疫学和生化学指标进行了统计分析。 结果 基线共纳入了249名患者,其中经治组220例,初治组29例。48周时符合方案分析结果显示,初治组病毒完全抑制率为93.10%,经治组为98.55%。与基线相比,2组患者治疗48周后的CD4+T淋巴细胞计数和CD4+/CD8+T细胞比值均升高(P < 0.001),血甘油三酯、总胆固醇、总胆红素和血肌酐较基线相比也有所升高,且差异具有统计学意义(P < 0.05),门冬氨酸氨基转移酶和丙氨酸氨基转移酶较基线相比均有所降低,除了初治组丙氨酸氨基转移酶(P > 0.05),其余3个差异均有统计学意义(P < 0.05),而基于血肌酐估算的肾小球滤过率,初治组较基线有所改善(P < 0.001),经治组无明显改变。 结论 BIC/FTC/TAF不论对初治患者还是经治患者均能有效抑制病毒的复制,提高患者的免疫能力,安全性也较好,是可以成为临床某些特定人群的首选治疗方案之一。 -
关键词:
- HIV/AIDS /
- BIC/FTC/TAF /
- 真实世界 /
- 疗效 /
- 安全性
Abstract:Objective To analyze the efficacy and safety of the three-in-one single-tablet regimen of bictegravir/emtricitabine/tenofovir alafenamide(BIC/FTC/TAF) in AIDS patients. Methods Newly treated patients who received a single tablet combination(BIC/FTC/TAF) between February 1, 2022 and June 1, 2022(the initial treatment group) and those who underwent treatment(the treated group) were included. The virological, immunological and biochemical indexes were statistically analyzed by means of prospective observational study. Results 249 patients were included at baseline , with 220 in the treated group and 29 in the newly treated group. At 48 weeks, the analysis showed that the virus suppression rate was 93.10% for the newly treated group and 98.55% for the treated group. Compared to the baseline, both groups showed increased CD4+ T lymphocyte counts and CD4+/CD8+ T cell ratios after 48 weeks of treatment(P < 0.001). There were also significant increases in blood triglycerides, total cholesterol, total bilirubin, and blood creatinine compared to the baseline(P < 0.05). Aspartate aminotransferase and alanine aminotransferase decreased compared to the baseline, with all differences being statistically significant except for alanine aminotransferase in the newly treated group(P > 0.05). Additionally, the estimated glomerular filtration rate based on blood creatinine improved in the newly treated group compared to the baseline(P < 0.001), while there were no significant changes in the treated group. Conclusion BIC/FTC/TAF can effectively inhibit virus replication, improve the immune function of patients, and has good safety. Bic /FTC/TAF can be one of the preferred treatment options for some specific clinical populations. -
Key words:
- HIV/AIDS /
- BIC/FTC/TAF /
- Real l-world /
- Efficacy /
- Safety
-
表 1 249例BIC/FTC/TAF单片治疗方案患者基线资料 [n(%),M(P25,P75)]
Table 1. Baseline data of 249 patients treated with BIC/FTC/TAF single-tablet regimen [n(%),M(P25,P75)]
指标 初治组(n = 29) 经治组(n = 220) 性别 男性 24(82.8) 157(71.4) 女性 5(17.2) 63(28.6) 年龄(岁) 33(29,44.5) 40.5(32,50) 婚姻 未婚 15(51.7) 91(41.4) 已婚 14(48.3) 99(45.0) 离异或丧偶 0 30(13.6) 传播途径 异性性传播 25(86.2) 161(73.2) 同性性传播 4(13.8) 47(21.3) 静脉吸毒 0 12(5.5) 入组前ART治疗时间(a) NA 4.9(2.1,8.0) 体重(Kg) 65(57,70) 62(55,70) VL(copies/mL) < 100 0 148(67.27) 100~100000 6(20.7) 42(19.09) 100000~500000 4(13.8) 8(3.6) > 500000 19(65.5) 22(10) CD4+T细胞(个/μL) 181(50.0,342.0) 308(139.7,509.0) CD4+T细胞 < 200个/μL 16(55.2) 73(33.2) 经治方案 NNRTIs+2NRTIs NA 168(76.4) INSTIs+2NRTIs NA 36(16.4) PIs+2NRTIs NA 9(4.1) NRTIs+INSTIs NA 7(3.2) 换药原因 优化治疗 NA 138(62.7) 副反应 NA 60(27.7) 药物相互作用 NA 4(1.8) 一线治疗失败 NA 1(0.5) 其他 NA 16(7.3) NNRTI:非核苷类反转录酶抑制;NRTI:核苷类反转录酶抑制剂;INSTI:整合酶抑制剂;PIs:蛋白酶抑制剂;NA:无。 表 2 203例CD4+T淋巴细胞数量及CD4+/CD8+T淋巴细胞比值比较[M(P25,P75)]
Table 2. Comparison of CD4+ T-cell counts and CD4+/CD8 +T-cell ratio in 203 cases[M(IQR5)]
组别 n CD4+T淋巴细胞数量/(个/µL) CD4+/CD8+T细胞比值 基线 24周 48周 基线 24周 48周 初治组 29 181.00
(50.00,342.00)324.00
(155.50,526.50)a380.00
(190.89,650.72)a0.17
(0.08,0.37)0.43
(0.19,0.56)a0.54
(0.25,0.72)ab经治组 174 296.50
(136.73,488.75)455.50
(308.00,694.25)a525.00
(379.90,698.25)ac0.35
(0.17,0.67)0.70
(0.40,1.00)a0.75
(0.45,0.92)ab与基线比较,aP < 0.001;与治疗24周比较,bP < 0.05;与治疗24周比较,cP < 0.001。 表 3 2组患者使用BIC/FTC/TAF 后生化指标改变[M(P25,P75)]
Table 3. Changes of biochemical indexes of patients in to groups after BIC/FTC/TAF regimen[M(P25,P75)]
指标 初治组(n = 29) 经治组(n = 207) 基线 48周 Z P 基线 48周 Z P AST
(U/L)25.00
(21.50,37.00)24.00
(19.00,31.50)−2.092 0.036* 26.00
(21.00,36.00)24.00
(21.00,29.00)−3.242 0.001* ALT
(U/L)22.00
(17.00,34.00)19.00
(15.50,33.00)−0.889 0.374 25.00
(16.00,41.00)23.00
(17.00,33.00)−2.150 0.032* TBIL
(μmol/L)11.20
(7.25,15.95)14.00
(10.60,16.75)−2.011 0.044* 11.20
(8.60,15.80)13.60
(10.50,17.30)−4.516 0.001** TC
(mmol/L)4.18
(3.53,4.95)4.87
(4.29,5.52)−2.174 0.030* 4.33
(3.59,5.06)4.71
(4.21,5.42)−6.174 0.001** TG
(mmol/L)1.20
(1.04,2.13)1.89
(1.27,3.14)−2.952 0.003* 1.51
(1.05,2.44)1.72
(1.03,2.51)−2.271 0.023* Scr
(μmol/L)63.00
(53.50,76.50)74.00
(62.00,91.00)−3.064 0.002* 68.00
(56.00,80.00)74.00
(62.00,83.00)−4.658 0.001** eGFR
(mL/min)118.91
(110.06,127.60)93.91
(84.26,112.27)−4.682 < 0.001** 106.86
(95.10,113.06)108.00
(94.55,114.26)−0.860 0.390 血糖
(mmol/L)5.54
(5.07,5.89)5.32
(4.80,5.52)−1.373 0.170 5.28
(4.94,5.65)5.37
(5.02,5.87)−2.099 0.036* *P < 0.05;**P < 0.001。 -
[1] 谭清,周仲辉,严冬梅,等. 成年人类免疫缺陷病毒感染者/艾滋病患者长期抗病毒治疗后免疫功能重建分析[J]. 中国全科医学,2020,23(23):2918-2922. [2] 张福杰,赵燕,马烨,等. 中国免费艾滋病抗病毒治疗进展与成就[J]. 中国艾滋病性病,2022,28(1):6-9. [3] 肖然,付强,杜小莉,等. 多替拉韦用于艾滋病治疗的临床应用[J]. 中国药学杂志,2023,58(6):544-550. [4] Liu P,Tang Z,Lan G,et al. Early antiretroviral therapy on reducing HIV transmission in China: Strengths,weaknesses and next focus of the program[J]. Sci Rep,2018,8(1):3431. doi: 10.1038/s41598-018-21791-2 [5] 卢洪洲,沈银忠. 整合酶抑制剂临床应用专家共识[J]. 上海预防医学,2018,30(10):836-843. [6] Rolle C P,Nguyen V,Patel K,et al. Real-world efficacy and safety of switching to bictegravir/emtricitabine/tenofovir alafenamide in older people living with HIV[J]. Medicine (Baltimore),2021,100(38):e27330. [7] Sandulescu O,Irimia M,Benea O E,et al. Treatment initiation or switch to BIC/FTC/TAF - real-world safety and efficacy data from two HIV centers in Romania[J]. Germs,2021,11(4):512-522. doi: 10.18683/germs.2021.1286 [8] Tabak F,Zerdali E,Altuntas O,et al. Efficacy and safety of co-formulated elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide in HIV-positive patients: Real-world data[J]. Int J STD AIDS,2021,32(6):562-569. doi: 10.1177/0956462420983692 [9] 中华医学会感染病学分会艾滋病丙型肝炎学组,中国疾病预防控制中心. 中国艾滋病诊疗指南(2021年版)[J]. 协和医学杂志,2022,13(2):203-226. [10] Saa G M S,Gandhi R T,Hoy J F,et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the international antiviral society-USA panel[J]. JAMA,2020,324(16):1651-1669. doi: 10.1001/jama.2020.17025 [11] Arribas J, Marzolini C, Mallon P, et al. European AIDS Clinical Society Guidelines, (version 10.1) [EB/OL]. [2021-02-20].https://www.eacsociety.org/files/guidelines-10.1-30032021-1.pdf. [12] D H H S. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV[EB/OL]. [2021-03-12].https://aidsinfo.nih.gov/guidelines on 12/28/2019. [13] Gallant J,Lazzarin A,Mills A,et al. Bictegravir,emtricitabine,and tenofovir alafenamide versus dolutegravir,abacavir,and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): A double-blind,multicentre,phase 3,randomised controlled non-inferiority trial[J]. Lancet,2017,390(10107):2063-2072. doi: 10.1016/S0140-6736(17)32299-7 [14] Sax P E,Pozniak A,Montes M L,et al. Coformulated bictegravir,emtricitabine,and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide,for initial treatment of HIV-1 infection (GS-US-380-1490): A randomised,double-blind,multicentre,phase 3,non-inferiority trial[J]. Lancet,2017,390(10107):2073-2082. doi: 10.1016/S0140-6736(17)32340-1 [15] Molina J M,Ward D,Brar I,et al. Switching to fixed-dose bictegravir,emtricitabine,and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised,double-blind,multicentre,active-controlled,phase 3,non-inferiority trial[J]. Lancet HIV,2018,5(7):e357-e365. doi: 10.1016/S2352-3018(18)30092-4 [16] Daar E S,DeJesus E,Ruane P,et al. Efficacy and safety of switching to fixed-dose bictegravir,emtricitabine,and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised,open-label,multicentre,phase 3,non-inferiority trial[J]. Lancet HIV,2018,5(7):e347-e356. doi: 10.1016/S2352-3018(18)30091-2 [17] Lanoy E,May M,Mocroft A,et al. Prognosis of patients treated with cART from 36 months after initiation,according to current and previous CD4 cell count and plasma HIV-1 RNA measurements[J]. AIDS,2009,23(16):2199-2208. doi: 10.1097/QAD.0b013e3283305a00 [18] 荆凡辉,吕玮,李太生. HIV感染者免疫功能重建新视角: CD4/CD8比值[J]. 中国艾滋病性病,2018,24(6):643-646. [19] Serrano-Villar S,Martínez-Sanz J,Ron R,et al. Effects of first-line antiretroviral therapy on the CD4/CD8 ratio and CD8 cell counts in CoRIS: A prospective multicentre cohort study[J]. Lancet HIV,2020,7(8):e565-e573. doi: 10.1016/S2352-3018(20)30202-2 [20] 陈丽文,高文军,祝达,等. TDF转换为TAF治疗病毒学应答后CHB的疗效和安全性研究[J]. 数理医药学杂志,2021,34(3):384-387. [21] Milinkovic A,Berger F,Arenas-Pinto A,et al. Reversible effect on lipids by switching from tenofovir disoproxil fumarate to tenofovir alafenamide and back[J]. AIDS,2019,33(15):2387-2391. doi: 10.1097/QAD.0000000000002350 [22] Suzuki K,Suda G,Yamamoto Y,et al. Effect of switching from tenofovir disoproxil fumarate to tenofovir alafenamide on lipid profiles in patients with hepatitis B[J]. PLoS One,2022,17(1):e261760. [23] Pottel H,Bjork J,Courbebaisse M,et al. Development and validation of a modified full age spectrum creatinine-based equation to estimate glomerular filtration rate: A cross-sectional analysis of pooled data[J]. Ann Intern Med,2021,174(2):183-191. doi: 10.7326/M20-4366 [24] Hines D M,Ding Y,Wade R L,et al. Treatment adherence and persistence among HIV-1 patients newly starting treatment[J]. Patient Prefer Adherence,2019,13:1927-1939. doi: 10.2147/PPA.S207908 [25] Bangsberg D R,Ragland K,Monk A,et al. A single tablet regimen is associated with higher adherence and viral suppression than multiple tablet regimens in HIV+ homeless and marginally housed people[J]. AIDS,2010,24(18):2835-2840. doi: 10.1097/QAD.0b013e328340a209 -





 下载:
下载: