Effects of MiR-126 on Myocardial Remodeling and Macrophage Polarization after Acute Myocardial Infarction
-
摘要:
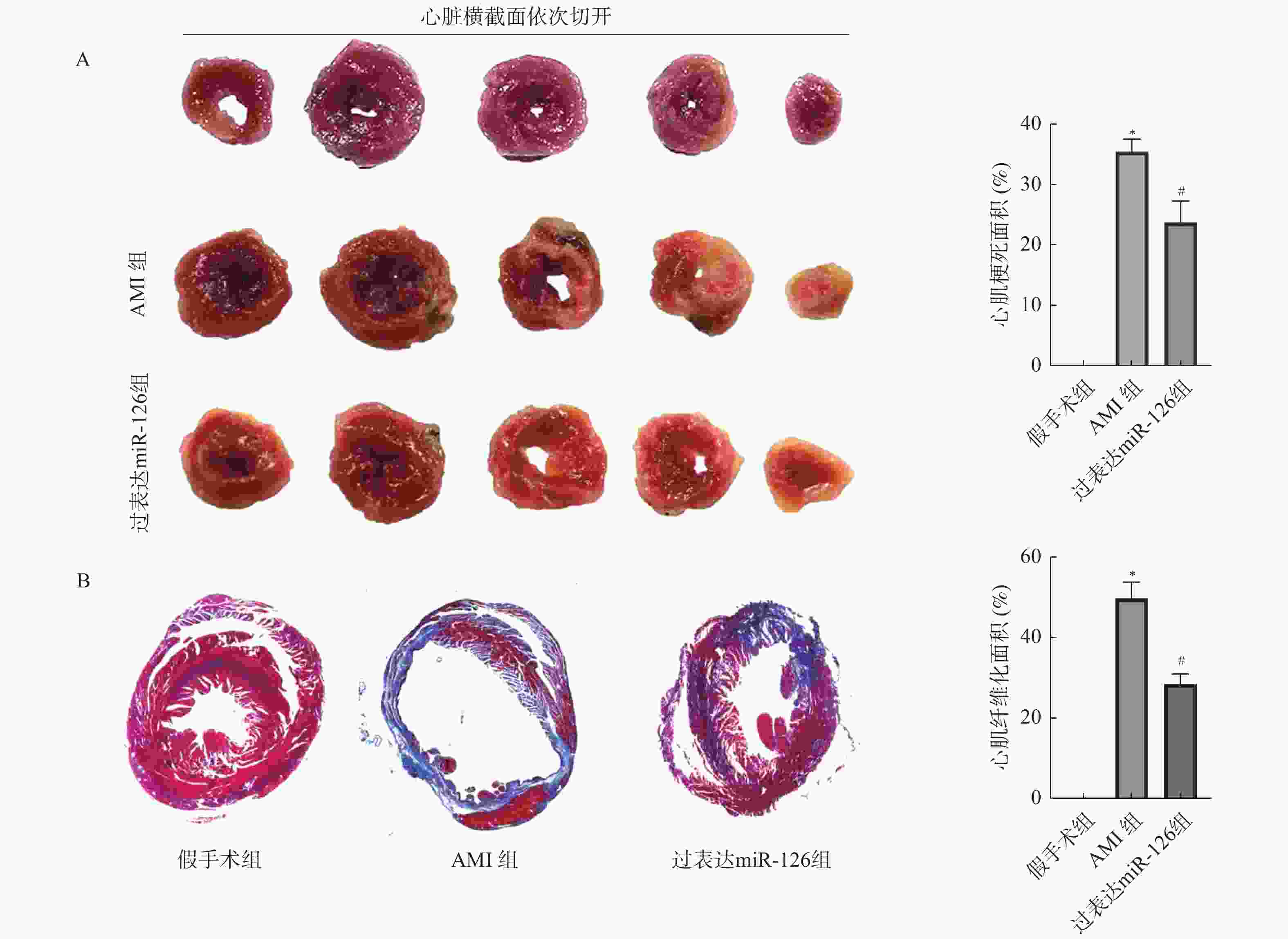
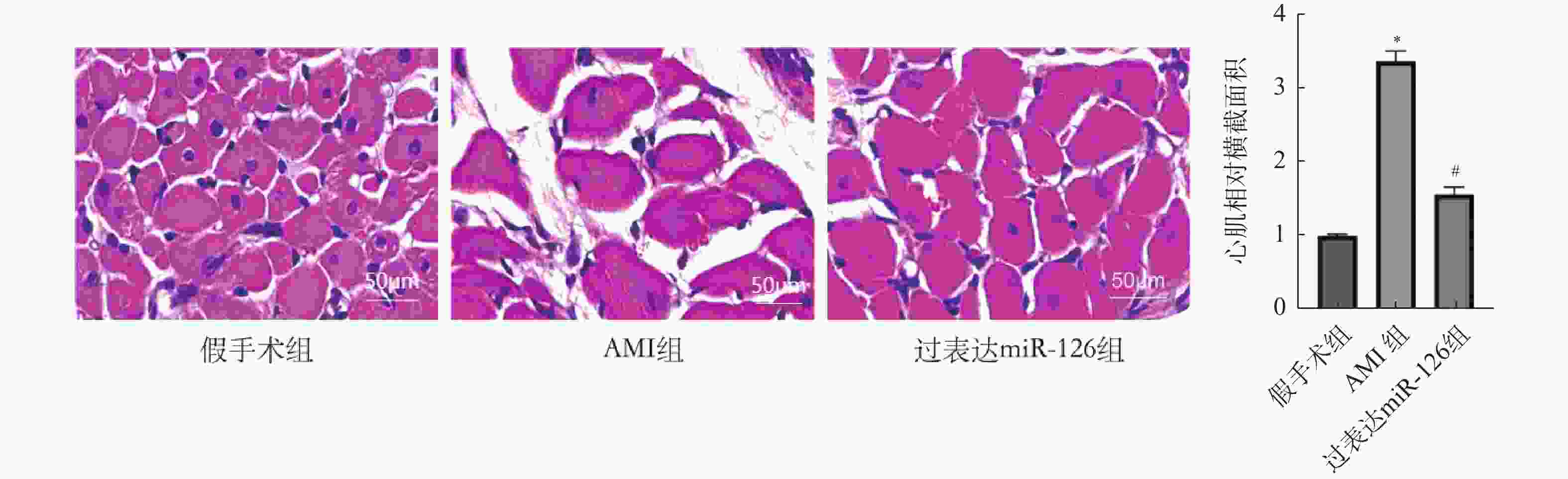
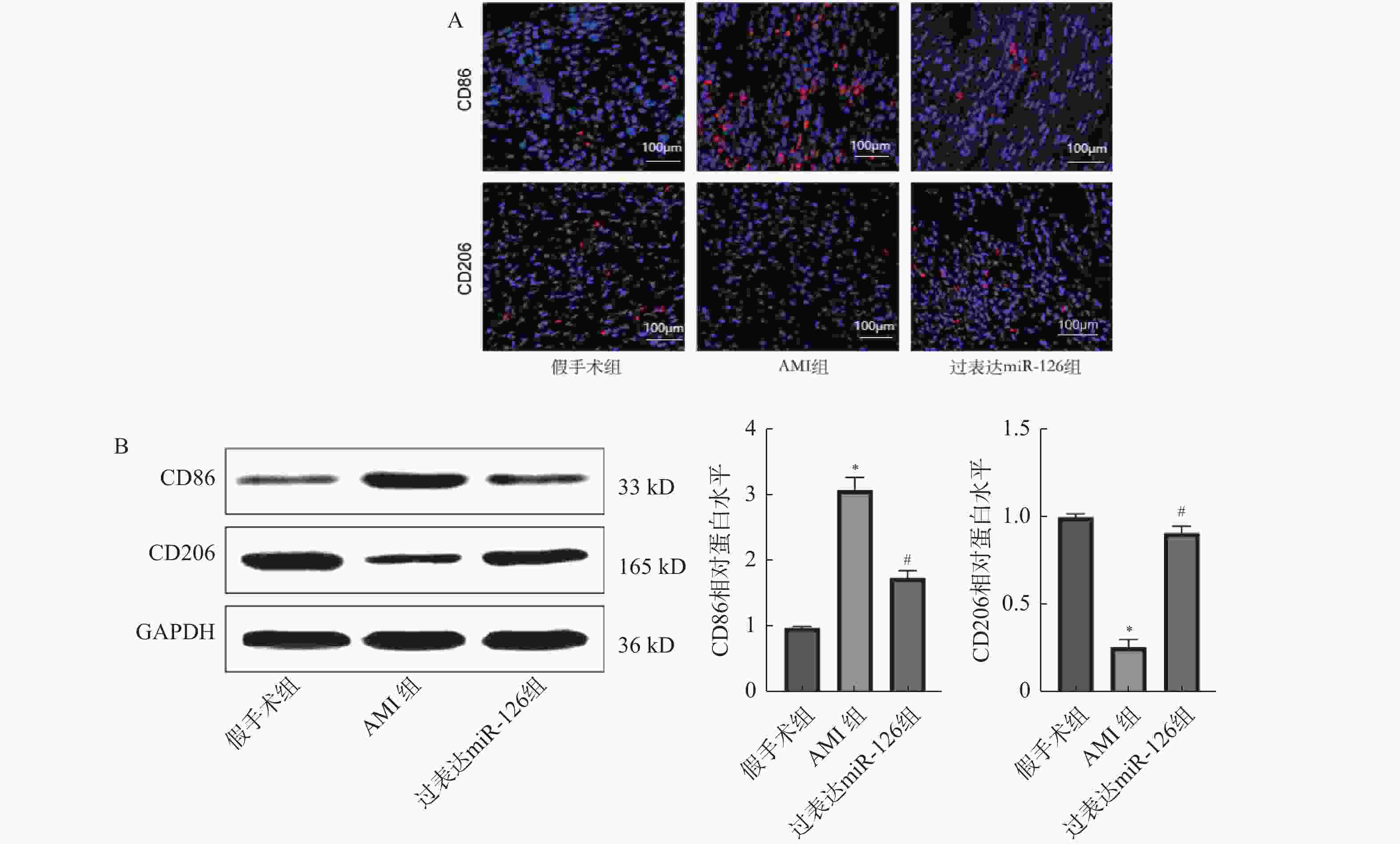
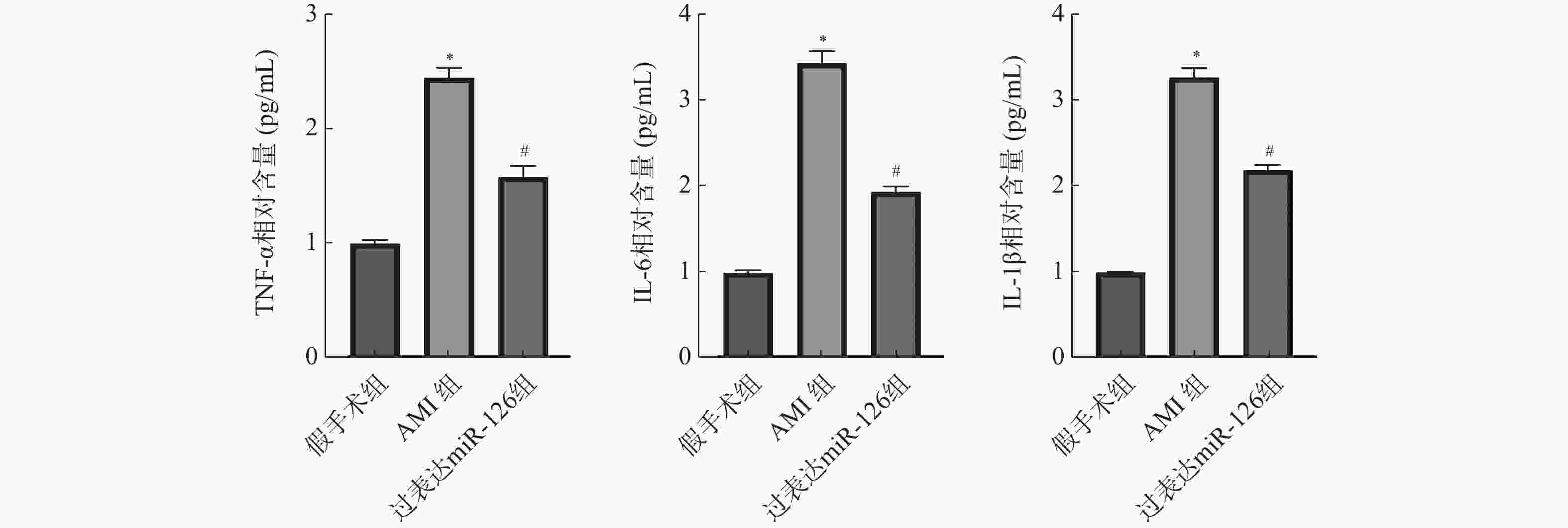
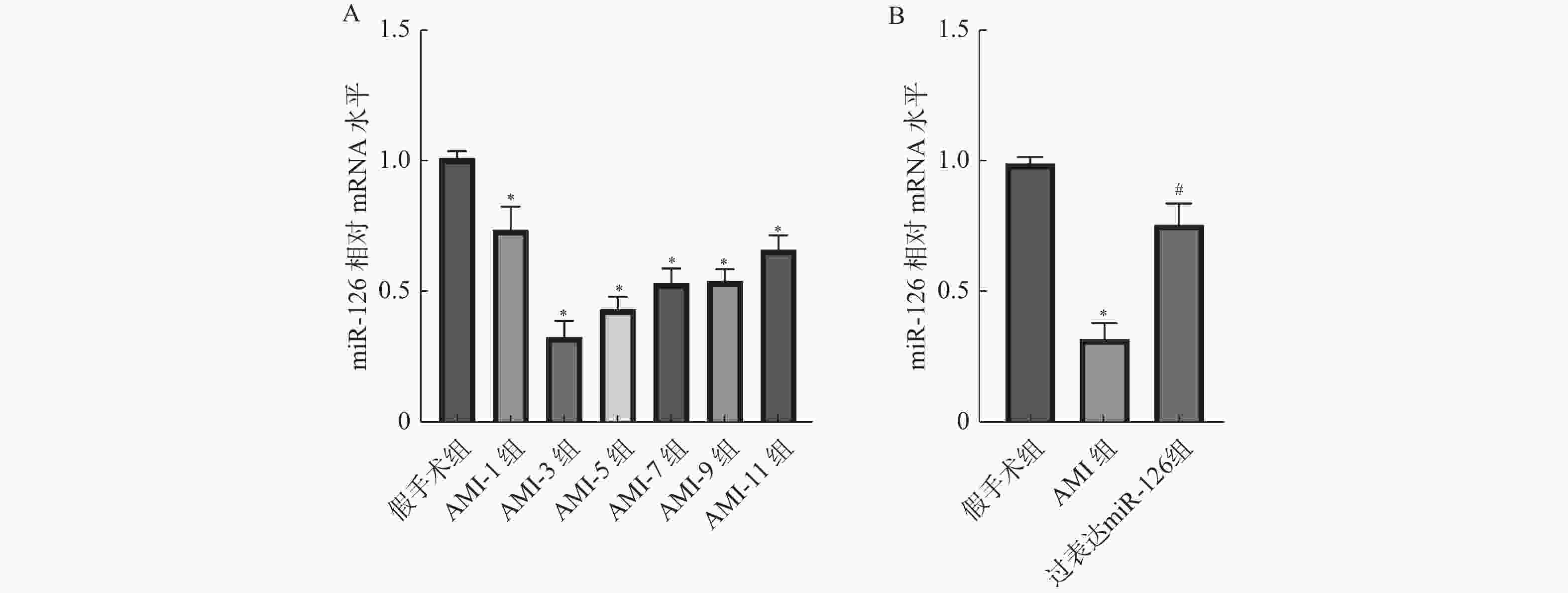
目的 探究miR-126对急性心肌梗死(acute myocardial infarction,AMI)后心肌重塑及巨噬细胞极化的影响。 方法 (1)将21只大鼠分为假手术组与AMI组,其中AMI组大鼠分为术后第1、3、5、7、9、11天(AMI-1、AMI-3、AMI-5、AMI-7、AMI-9、AMI-11),每组3只,筛选出AMI术后第3天进行后续研究。(2)将30只大鼠随机分为:假手术组、AMI组、过表达miR-126组,每组10只。RT-qPCR检测miR-126 mRNA表达水平。2,3,5-氯化三苯基四氮唑(TTC)染色检测心脏梗死面积。Masson染色检测心肌组织纤维化水平。H&E染色检测心肌横截面积。免疫荧光染色检测心肌组织CD86、CD206蛋白水平。Western blot检测心肌组织CD86、CD206蛋白水平。ELISA试剂盒检测血清肿瘤坏死因子α(TNF-α),白细胞介素-1β(IL-1β),IL-6含量。 结果 与假手术组相比,AMI组大鼠心肌组织中miR-126的mRNA表达水平在术后1 d即显著降低(P < 0.05),并在术后3 d降至最低,随后虽有一定程度回升,但在术后11 d仍低于假手术组水平(P < 0.05),后续选择术后第3天进行研究。与假手术组相比,AMI组大鼠心肌组织中miR-126的mRNA表达减少(P < 0.05),心肌纤维化、梗死面积及心肌横截面积增加(P < 0.05),心肌组织CD86蛋白表达增加,CD206蛋白表达减少(P < 0.05),血清TNF-α、IL-6、IL-1β含量增加(P < 0.05);与AMI组相比,过表达miR-126组大鼠心肌组织中miR-126的mRNA表达增加(P < 0.05),大鼠心肌纤维化、梗死面积及心肌横截面积减少(P < 0.05),心肌组织CD86蛋白表达减少,CD206蛋白表达增加(P < 0.05),血清TNF-α、IL-6、IL-1β含量减少(P < 0.05)。 结论 过表达miR-126可改善AMI大鼠心肌重塑,促进M2型巨噬细胞极化。 Abstract:Objective To investigate the effects of miR-126 on myocardial remodeling and macrophage polarization after acute myocardial infarction (AMI). Methods (1) Twenty-one rats were divided into a sham operation group and an AMI group. The AMI group was further divided into postoperative days 1, 3, 5, 7, 9, and 11 days (AMI-1, AMI-3, AMI-5, AMI-7, AMI-9, AMI-11), with 3 rats in each group, and postoperative day 3 was selected for subsequent study. (2) Thirty rats were randomly divided into three groups: sham operation group, AMI group and miR-126 overexpression group, with 10 rats in each group. RT-qPCR was used to detect the expression levels of miR-126 mRNA. 2, 3, 5-Triphenyltetrazole chloride (TTC) staining was used to detect the infarct size. Myocardial fibrosis was detected by Masson staining. The cross-sectional area of the myocardium was determined by H&E staining. Immunofluorescence staining was used to detect the protein levels of CD86 and CD206 in myocardial tissue. Western blotting was used to detect the protein levels of CD86 and CD206 in myocardial tissue. The levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6 in serum were detected by ELISA kit. Results Compared with the sham operation group, the expression level of miR-126 mRNA in myocardial tissue of rats in the AMI group was significantly decreased on postoperative day 1 (P < 0.05), reached the lowest level on postoperative day 3. And then although it rose to a certain extent, it remained lower than that of the sham operation group on postoperative day 11 (P < 0.05), and postoperative day 3 was selected for further study. Compared with the sham operation group, the expression of miR-126 mRNA in myocardial tissue of the AMI group was decreased (P < 0.05), and the myocardial fibrosis, infarct size and myocardial cross-sectional area were increased (P < 0.05). The expression of CD86 protein in myocardial tissue was increased and the expression of CD206 protein was decreased (P < 0.05). The serum levels of TNF-α, IL-6 and IL-1β were increased (P < 0.05). Compared with the AMI group, the rats in the miR-126 overexpression group had a significant increase in the mRNA expression of miR-126 in myocardial tissue (P < 0.05), a significant reduction in myocardial fibrosis, infarct size, and myocardial cross-sectional area (P < 0.05), a significant reduction in the expression of CD86 protein in myocardial tissue, and a significant increase in the expression of CD206 protein (P < 0.05). The serum levels of TNF-α, IL-6 and IL-1β were significantly decreased (P < 0.05). Conclusion Over-expression of miR-126 can improve myocardial remodeling and promote M2 macrophage polarization in AMI rats. -
表 1 miR-126、GAPDH引物序列
Table 1. Primer sequences of miR-126 and GAPDH
基因 上游引物 下游引物 miR-126 GCTAGGAGACGTAGTAGATAGC ATGTTTACAGATAGCATAGACGT GAPDH AACTAGTGATCGAACTAGGCCA CGCATCATACGACGAGTGCTAAC -
[1] Liu W, Li Y, Zhang Y, et al. Identification of biomarkers and immune infiltration in acute myocardial infarction and heart failure by integrated analysis[J]. Biosci Rep, 2023, 43(7): BSR20222552. doi: 10.1042/BSR20222552 [2] Saito Y, Oyama K, Tsujita K, et al. Treatment strategies of acute myocardial infarction: Updates on revascularization, pharmacological therapy, and beyond[J]. J Cardiol, 2023, 81(2): 168-178. doi: 10.1016/j.jjcc.2022.07.003 [3] Kologrivova I, Shtatolkina M, Suslova T, et al. Cells of the immune system in cardiac remodeling: Main players in resolution of inflammation and repair after myocardial infarction[J]. Front Immunol, 2021, 12: 664457. doi: 10.3389/fimmu.2021.664457 [4] Yang L, Yang L, Lu K, et al. 3D chiral self-assembling matrixes for regulating polarization of macrophages and enhance repair of myocardial infarction[J]. Adv Sci (Weinh), 2023, 10(32): e2304627. doi: 10.1002/advs.202304627 [5] Peet C, Ivetic A, Bromage D I, et al. Cardiac monocytes and macrophages after myocardial infarction[J]. Cardiovasc Res, 2020, 116(6): 1101-1112. doi: 10.1093/cvr/cvz336 [6] Sharma A K, Bisht P, Gupta B, et al. Investigating miRNA subfamilies: Can they assist in the early diagnosis of acute myocardial infarction?[J]. Drug Discov Today, 2023, 28(10): 103695. doi: 10.1016/j.drudis.2023.103695 [7] Zhong Z, Wu H, Zhong W, et al. Expression profiling and bioinformatics analysis of circulating microRNAs in patients with acute myocardial infarction[J]. J Clin Lab Anal, 2020, 34(3): e23099. doi: 10.1002/jcla.23099 [8] Shi C C, Pan L Y, Peng Z Y, et al. miR-126 regulated myocardial autophagy on myocardial infarction[J]. Eur Rev Med Pharmacol Sci, 2020, 24(12): 6971-6979. [9] 刘茂林, 樊林花, 尚小森, 等. 麝香保心丸通过抑制TGF-β/Smad/ERK信号通路缓解急性心肌梗死大鼠心肌纤维化[J]. 山西医科大学学报, 2025, 56(6): 636-643. [10] Zuin M, Rigatelli G, Temporelli P, et al. Trends in acute myocardial infarction mortality in the European Union, 2012-2020[J]. Eur J Prev Cardiol, 2023, 30(16): 1758-1771. doi: 10.1093/eurjpc/zwad214 [11] Solomonchuk A, Rasputina L, Didenko D. Prevalence, clinical and functional characteristics of patients with acute myocardial infarction complicated by acute heart failure[J]. Wiad Lek, 2022, 75(7): 1741-1746. doi: 10.36740/WLek202207124 [12] Chen W, Bian W, Zhou Y, et al. Cardiac fibroblasts and myocardial regeneration[J]. Front Bioeng Biotechnol, 2021, 9: 599928. doi: 10.3389/fbioe.2021.599928 [13] Otto C M. Heartbeat: Treatment delays with telephone triage for acute myocardial infarction[J]. Heart, 2022, 108(14): 1075-1077. doi: 10.1136/heartjnl-2022-321491 [14] Ning Y, Huang P, Chen G, et al. Atorvastatin-pretreated mesenchymal stem cell-derived extracellular vesicles promote cardiac repair after myocardial infarction via shifting macrophage polarization by targeting microRNA-139-3p/Stat1 pathway[J]. BMC Med, 2023, 21(1): 96. doi: 10.1186/s12916-023-02778-x [15] Cui T, Feng C, Jiang H, et al. Inhibition of PFKFB3 expression stimulates macrophage-mediated lymphangiogenesis post-acute myocardial infarction[J]. Front Biosci (Landmark Ed), 2023, 28(11): 277. doi: 10.31083/j.fbl2811277 [16] Wang Y, Zhang Y, Li J, et al. Hypoxia induces M2 macrophages to express VSIG4 and mediate cardiac fibrosis after myocardial infarction[J]. Theranostics, 2023, 13(7): 2192-2209. doi: 10.7150/thno.78736 [17] Pan X, Zhang K, Shen C, et al. Astaxanthin promotes M2 macrophages and attenuates cardiac remodeling after myocardial infarction by suppression inflammation in rats[J]. Chin Med J (Engl), 2020, 133(15): 1786-1797. [18] Sun P, Wang C, Mang G, et al. Extracellular vesicle-packaged mitochondrial disturbing miRNA exacerbates cardiac injury during acute myocardial infarction[J]. Clin Transl Med, 2022, 12(4): e779. [19] Diener C, Keller A, Meese E. Emerging concepts of miRNA therapeutics: From cells to clinic[J]. Trends Genet, 2022, 38(6): 613-626. doi: 10.1016/j.tig.2022.02.006 [20] Li J, Liu Y, Lai W, et al. microRNA-126 regulates macrophage polarization to prevent the resorption of alveolar bone in diabetic periodontitis[J]. Arch Oral Biol, 2023, 150: 105686. doi: 10.1016/j.archoralbio.2023.105686 -





 下载:
下载: