The Relationship between M1/M2 Macrophage Expression and the Severity of Diabetes Retinopathy
-
摘要:
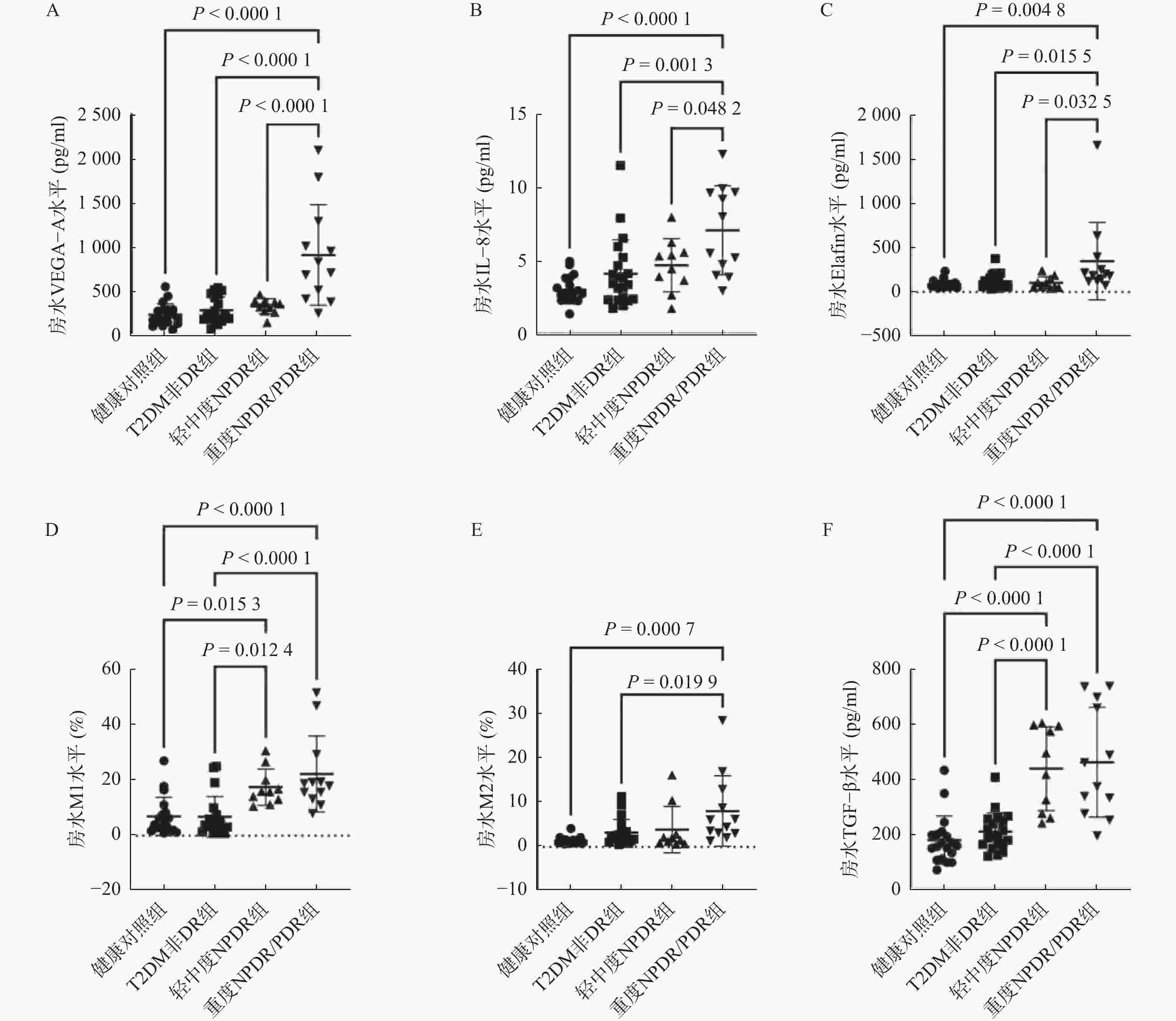
目的 探讨M1和M2型巨噬细胞与糖尿病视网膜病变(diabetic retinopathy,DR)严重程度的关系。 方法 回顾性纳入邯郸市中心医院2022年4月至2023年11月42名患有或不患有DR的2型糖尿病(type 2 diabetes mellitus,T2DM)患者和19名除接受白内障眼内手术外无任何已知系统性或眼部疾病的健康个体作为健康对照组。T2DM患者分为糖尿病伴非显性糖尿病视网膜病变(T2DM非DR组,n = 20)、轻中度非增生性糖尿病视网膜病变(mild to moderate non-proliferative diabetic retinopathy,NPDR)(n = 10)和重度NPDR/增生性糖尿病视网膜病变(proliferative diabetic retinopathy,PDR)(n = 12)。在手术开始时获取了外周血和房水样本,并通过试剂盒分析样本中血管内皮生长因子A(vascular endothelial growth factor A,VEGF-A)、白细胞介素-8(interleukin-8,IL-8)、弹性蛋白酶抑制剂(Elafin)以及转化生长因子-β(transforming growth factor-β,TGF-β)水平,以及流式细胞术分析M1巨噬细胞、M2巨噬细胞的比例。 结果 在T2DM患者中,三组在糖尿病持续时间、胰岛素、二甲双胍使用方面差异有统计学意义(P < 0.05)。各组房水中VEGF-A、IL-8、Elafin、M1比例、M2比例和TGF-β水平差异有统计学意义(F = 17.40、9.492、4.792、11.32、5.768、20.38,均P < 0.01)。重度NPDR/PDR组房水中VEGF-A、IL-8、Elafin、M1比例、VD86和TGF-β水平显著高于健康对照组、T2DM非DR组(P < 0.05),并且重度NPDR/PDR组房水中VEGF-A、IL-8、Elafin水平显著高于轻中度NPDR组(P < 0.05)。轻中度NPDR组房水中M1比例、TGF-β水平显著高于健康对照组、T2DM非DR组(P < 0.05)。房水中M1比例水平与M2比例、Elafin、TGF-β、VEGF-A水平呈显著正相关(r = 0.260、0.411、0.726、0.304,均P < 0.05),M2比例水平与Elafin、TGF-β水平呈显著正相关(r = 0.865、0.552,均P < 0.01)。 结论 房水中IL-8和M1型巨噬细胞的升高可能促进DR的发生,而Elafin和M2型巨噬细胞的增加可能是一种对慢性炎症的代偿性调节,参与局部微血管病变和纤维化的调控。 Abstract:Objective To explore the relationship between the M1/M2 macrophages and severity of diabetic retinopathy (DR). Methods This retrospective study included 42 patients with or without DR who had type 2 diabetes mellitus (T2DM) and 19 healthy individuals undergoing cataract intraocular surgery without systemic or ocular diseases from Handan Central Hospital between April 2022 and November 2023. Patients with T2DM were divided into three groups: diabetes mellitus with non-dominant diabetic retinopathy (T2DM non-DR group, n = 20), mild and moderate non-proliferative diabetic retinopathy (NPDR)(n = 10) and severe NPDR/ proliferative diabetic retinopathy (PDR)(n = 12). Peripheral blood and aqueous humor were obtained at surgery initiation. The levels of VEGF-A, IL-8, Elafin and TGF-β in the samples were analyzed using kits, and the ratio of M1 macrophages to M2 macrophages was analyzed by flow cytometry. Results Among patients with T2DM, there were significant differences in the duration of diabetes, insulin and metformin use among the three groups (P < 0.05). Aqueous humor levels of VEGF-A, IL-8, Elafin, M1 ratio, M2 ratio and TGF-β of each group were significantly different (F = 17.40, 9.492, 4.792, 11.32, 5.768 and 20.38, all P < 0.01). The levels of VEGF-A, IL-8, Elafin, M1 ratio, VD86 and TGF-β in severe NPDR/PDR group were significantly higher than those in healthy control group and T2DM non-DR group (P < 0.05), and the levels of VEGF-A, IL-8 and Elafin in severe NPDR/PDR group were significantly higher than those in mild and moderate NPDR group (P < 0.05). The levels of M1 proportion and TGF-β in aqueous humor of the mild to moderate NPDR group were significantly higher than those in healthy control group and T2DM non-DR group (P < 0.05). The level of M1 in aqueous humor was positively correlated with the levels of M2, Elafin, TGF-β and VEGF-A (r = 0.260, 0.411, 0.726 and 0.304, all P < 0.05), and the level of M2 was positively correlated with the levels of Elafin and TGF-β (r = 0.865, 0.552, all P < 0.01). Conclusion The elevation of IL-8 and M1 macrophages in the aqueous humor may promote the occurrence of DR, while the increase of Elafin and M2 macrophages may be a compensatory regulation to chronic inflammation, which is involved in the regulation of local microvascular lesions and fibrosis. -
Key words:
- Diabetic retinopathy /
- Macrophage /
- Polarization /
- Fibrosis /
- Aqueous humor
-
表 1 患者基线特征[n(%)/($ \bar x \pm s $)]
Table 1. Baseline characteristics of patients [n(%) / ($ \bar x \pm s $)]
特征 健康对照组
(n = 19)T2DM非DR组
(n = 20)轻中度NPDR组
(n = 10)重度NPDR/PDR组
(n = 12)F/χ2 P 95%CI 年龄(岁) 62.58 ± 8.16 62.75 ± 10.09 62.60 ± 7.62 62.00 ± 7.02 0.020 0.996 - 男性 11 (57.9) 10 (50.0) 6 (60.0) 10 (83.3) 3.600 0.308 - BMI(kg/m2) 28.92 ± 2.77 28.54 ± 3.51 29.34 ± 3.91 30.90 ± 3.33 1.355 0.266 - 糖尿病持续时间(年) - 11.25 ± 1.62 13.00 ± 3.37 16.50 ± 4.91 9.690 <0.001* 5.25 (2.31~8.19) 高血压 0 (0) 14 (70.0) 4 (40.0) 8 (66.7) 2.706 0.258 - 治疗药物 胰岛素 0 (0.0) 5 (25.0) 4 (40.0) 10 (83.3) 10.447 0.005* 58.3(26.4~90.1) 二甲双胍 0 (0.0) 16 (80.0) 6 (60.0) 2 (16.7) 12.328 0.002* 63.3(34.3~92.3) 他汀类降血脂药 0 (0.0) 7 (35.0) 4 (40.0) 4 (33.3) 0.114 0.945 - 沙坦酯 0 (0.0) 12 (60.0) 5 (50.0) 9 (75.0) 1.504 0.471 - *P < 0.05。 表 2 房水中的炎症和纤维化标记物的相关性分析(n = 61)
Table 2. Correlation analysis of inflammatory and fibrosis markers in aqueous humor (n = 61)
标记物 相关性 IL-8 VEGF-A Elafin M2比例 M1比例 TGF-β 比例 IL-8 r 1 P VEGF-A r 0.317 1 P 0.013* Elafin r 0.043 0.189 1 P 0.740 0.146 M2比例 r 0.136 0.246 0.865 1 P 0.296 0.056 <0.001** M1比例 r 0.215 0.304 0.260 0.411 1 P 0.096 0.017* 0.043* 0.001** TGF-β r 0.142 0.169 0.437 0.552 0.726 1 P 0.276 0.192 <0.001** <0.001** <0.001** *P < 0.05;**P < 0.01。 -
[1] Grauslund J. Diabetic retinopathy screening in the emerging era of artificial intelligence[J]. Diabetologia, 2022, 65(9): 1415-1423. doi: 10.1007/s00125-022-05727-0 [2] Yang X, Huang Z, Xu M, et al. Autophagy in the retinal neurovascular unit: New perspectives into diabetic retinopathy[J]. J Diabetes, 2023, 15(5): 382-396. doi: 10.1111/1753-0407.13373 [3] Fu H, Liu H. Deletion of toll-like receptor 4 ameliorates diabetic retinopathy in mice[J]. Arch Physiol Biochem, 2023, 129(2): 519-525. doi: 10.1080/13813455.2020.1841795 [4] Wang Z, An H, Tang J, et al. Elevated number and density of macrophage-like cell as a novel inflammation biomarker in diabetic macular edema[J]. Sci Rep, 2023, 13(1): 5320. doi: 10.1038/s41598-023-32455-1 [5] Yao Y, Li J, Zhou Y, et al. Macrophage/microglia polarization for the treatment of diabetic retinopathy[J]. Front Endocrinol (Lausanne), 2023, 14: 1276225. doi: 10.3389/fendo.2023.1276225 [6] Hu Q, Zhang X, Peng H, et al. A new modulator of neuroinflammation in diabetic retinopathy: USP25[J]. Inflammation, 2024, 47(4): 1520-1535. [7] Pan Z, Zhao Y, Zhou S, et al. CD44 drives M1 Drives M1 macrophage polarization in diabetic retinopathy[J]. Curr Eye Res, 2023, 48(8): 770-780. doi: 10.1080/02713683.2023.2210273 [8] Wilkinson C P, Ferris F L 3rd, Klein R E, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales[J]. Ophthalmology, 2003, 110(9): 1677-1682. [9] American Diabetes Association Professional Practice Committee. 2. classification and diagnosis of diabetes: Standards of medical care in diabetes-2022[J]. Diabetes Care, 2022, 45(suppl 1): S17-S38. [10] Sun J K, Aiello L P, Abràmoff M D, et al. Updating the staging system for diabetic retinal disease[J]. Ophthalmology, 2021, 128(4): 490-493. doi: 10.1016/j.ophtha.2020.10.008 [11] Tabatabaei-Malazy O, Peimani M, Mohseni S, et al. Therapeutic effects of dietary antioxidative supplements on the management of type 2 diabetes and its complications; umbrella review of observational/trials meta-analysis studies[J]. J Diabetes Metab Disord, 2022, 21(2): 1833-1859. doi: 10.1007/s40200-022-01069-1 [12] Zhang X, Nie Y, Gong Z, et al. Plasma apolipoproteins predicting the occurrence and severity of diabetic retinopathy in patients with type 2 diabetes mellitus[J]. Front Endocrinol (Lausanne), 2022, 13: 915575. doi: 10.3389/fendo.2022.915575 [13] Banete A, Barilo J, Whittaker R, et al. The activated macrophage-a tough fortress for virus invasion: How viruses strike back[J]. Front Microbiol, 2021, 12: 803427. doi: 10.3389/fmicb.2021.803427 [14] Wang J, Lu S, Yang F, et al. The role of macrophage polarization and associated mechanisms in regulating the anti-inflammatory action of acupuncture: A literature review and perspectives[J]. Chin Med, 2021, 16(1): 56. doi: 10.1186/s13020-021-00466-7 [15] Sui A, Chen X, Demetriades A M, et al. Inhibiting NF-κB signaling activation reduces retinal neovascularization by promoting a polarization shift in macrophages[J]. Invest Ophthalmol Vis Sci, 2020, 61(6): 4. doi: 10.1167/iovs.61.6.4 [16] Zhu Y, Zhang L, Lu Q, et al. Identification of different macrophage subpopulations with distinct activities in a mouse model of oxygen-induced retinopathy[J]. Int J Mol Med, 2017, 40(2): 281-292. doi: 10.3892/ijmm.2017.3022 [17] Hu Z, Mao X, Chen M, et al. Single-cell transcriptomics reveals novel role of microglia in fibrovascular membrane of proliferative diabetic retinopathy[J]. Diabetes, 2022, 71(4): 762-773. doi: 10.2337/db21-0551 [18] Burger F, Baptista D, Roth A, et al. Single-cell RNA-seq reveals a crosstalk between hyaluronan receptor LYVE-1-expressing macrophages and vascular smooth muscle cells[J]. Cells, 2022, 11(3): 411. doi: 10.3390/cells11030411 [19] Kheir S, Villeret B, Garcia-Verdugo I, et al. IL-6-elafin genetically modified macrophages as a lung immunotherapeutic strategy against Pseudomonas aeruginosa infections[J]. Mol Ther, 2022, 30(1): 355-369. doi: 10.1016/j.ymthe.2021.08.007 [20] Lodyga M, Hinz B. TGF-β1–A truly transforming growth factor in fibrosis and immunity[J]. Semin Cell Dev Biol, 2020, 101: 123-139. doi: 10.1016/j.semcdb.2019.12.010 [21] Rodriguez R, Lowe K, Keniry M, et al. Involvement of TGF-βsignaling pathway in oxidative stress and diabetic retinopathy[J]. Arch Clin Exp Ophthalmol, 2021, 3(2): 23-28. [22] Fan J, Shen W, Lee S R, et al. Targeting the notch and TGF-β signaling pathways to prevent retinal fibrosis in vitro and in vivo[J]. Theranostics, 2020, 10(18): 7956-7973. doi: 10.7150/thno.45192 [23] Chen L, Cao Y, Shen Y, et al. Downregulation of PIK3IP1 in retinal microglia promotes retinal pathological neovascularization via PI3K-AKT pathway activation[J]. Sci Rep, 2023, 13(1): 12754. doi: 10.1038/s41598-023-39473-z -





 下载:
下载: