Relationship between Serum PON-1,NDRG4 and SFRP4 Expression,Peripheral Blood Immune Cell Distribution,and Prognosis in Gastric Cancer Patients
-
摘要:
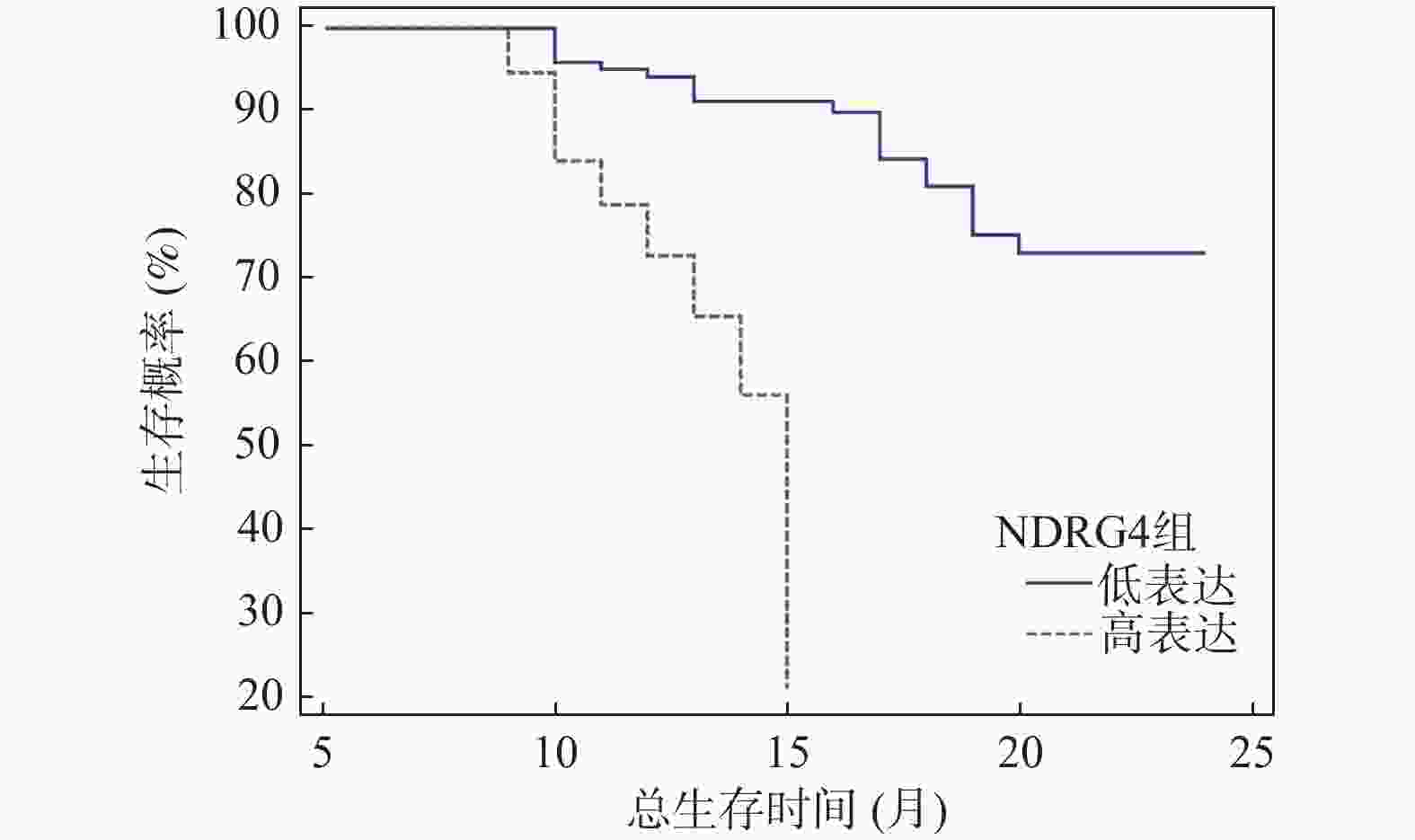
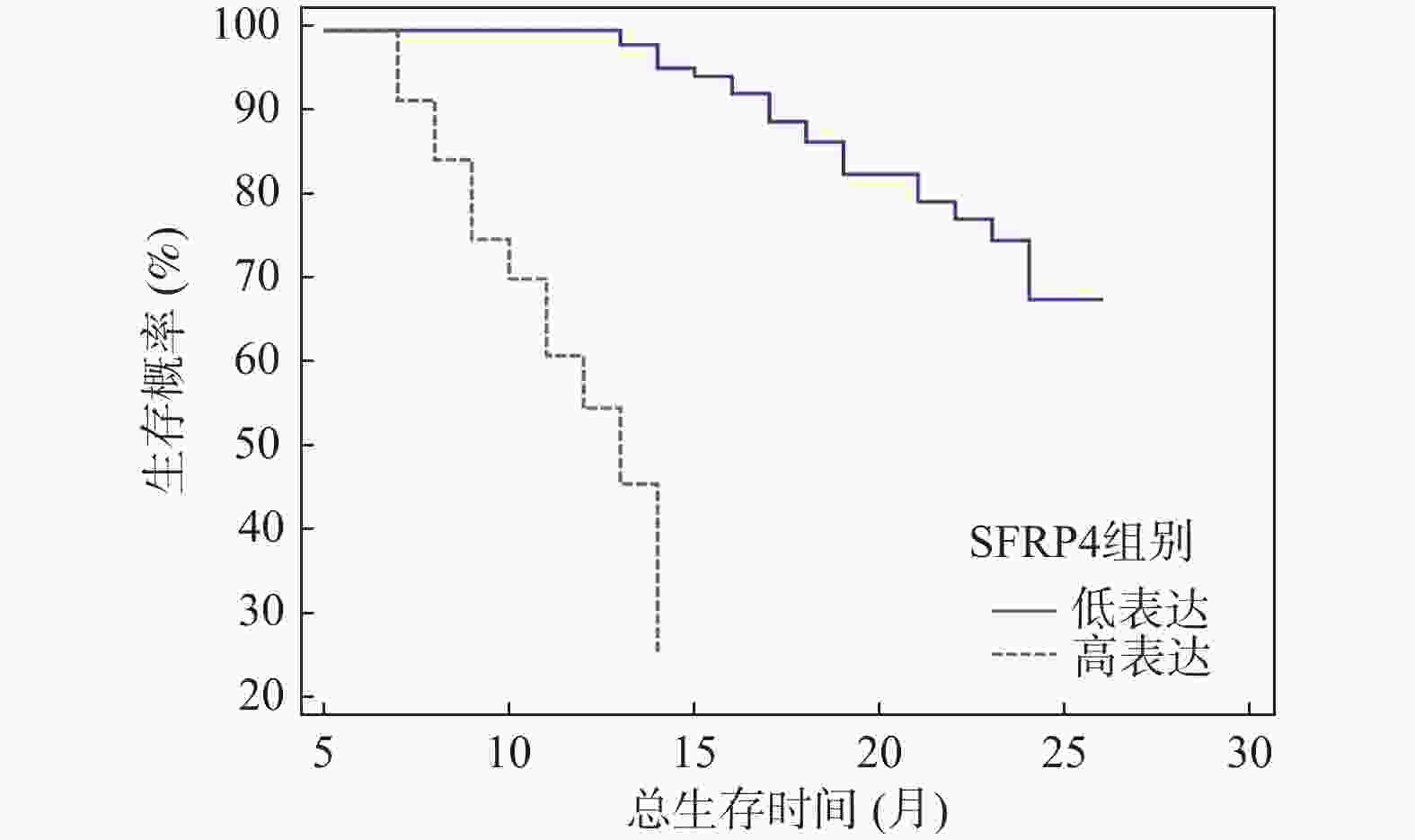
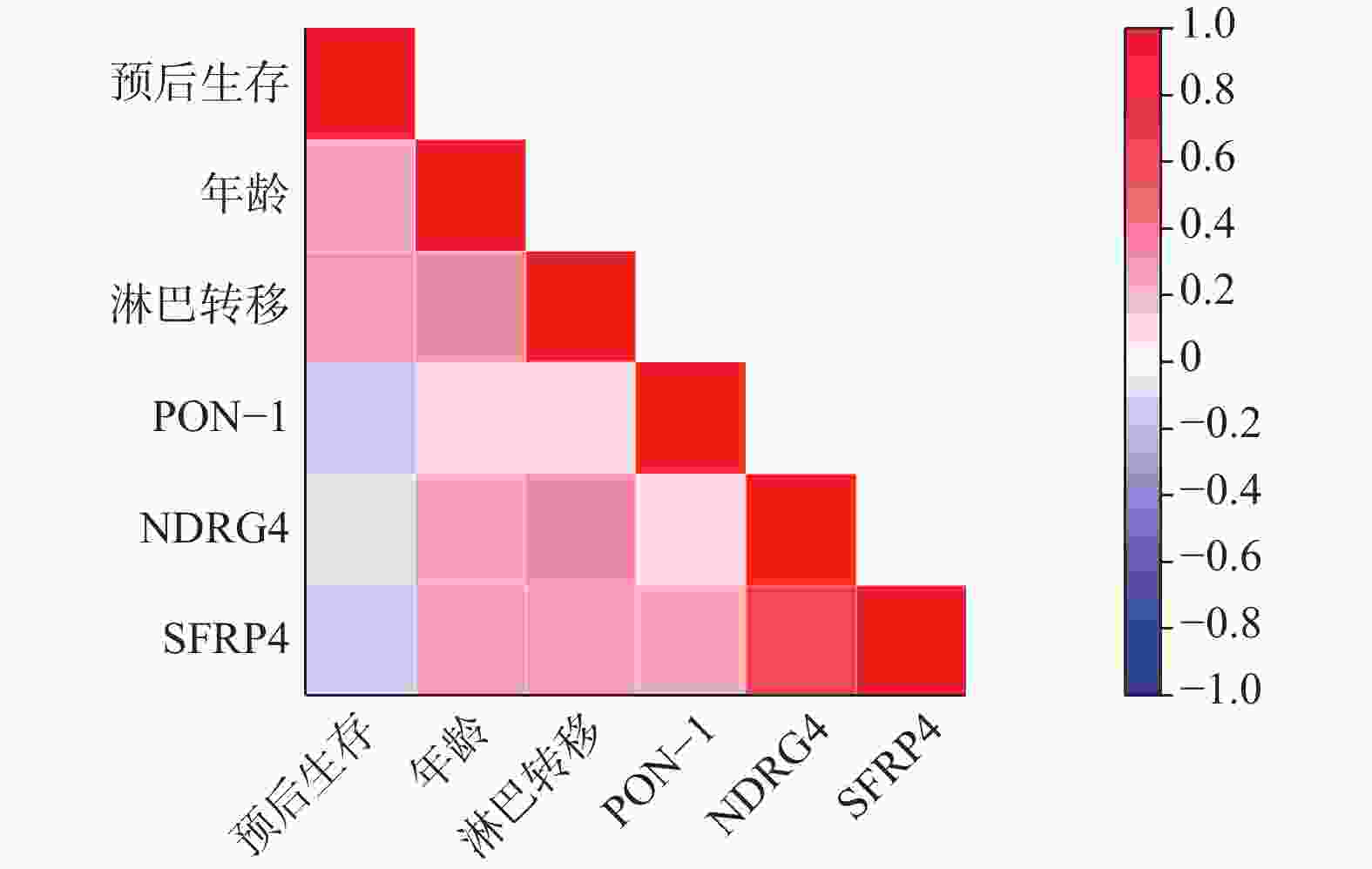
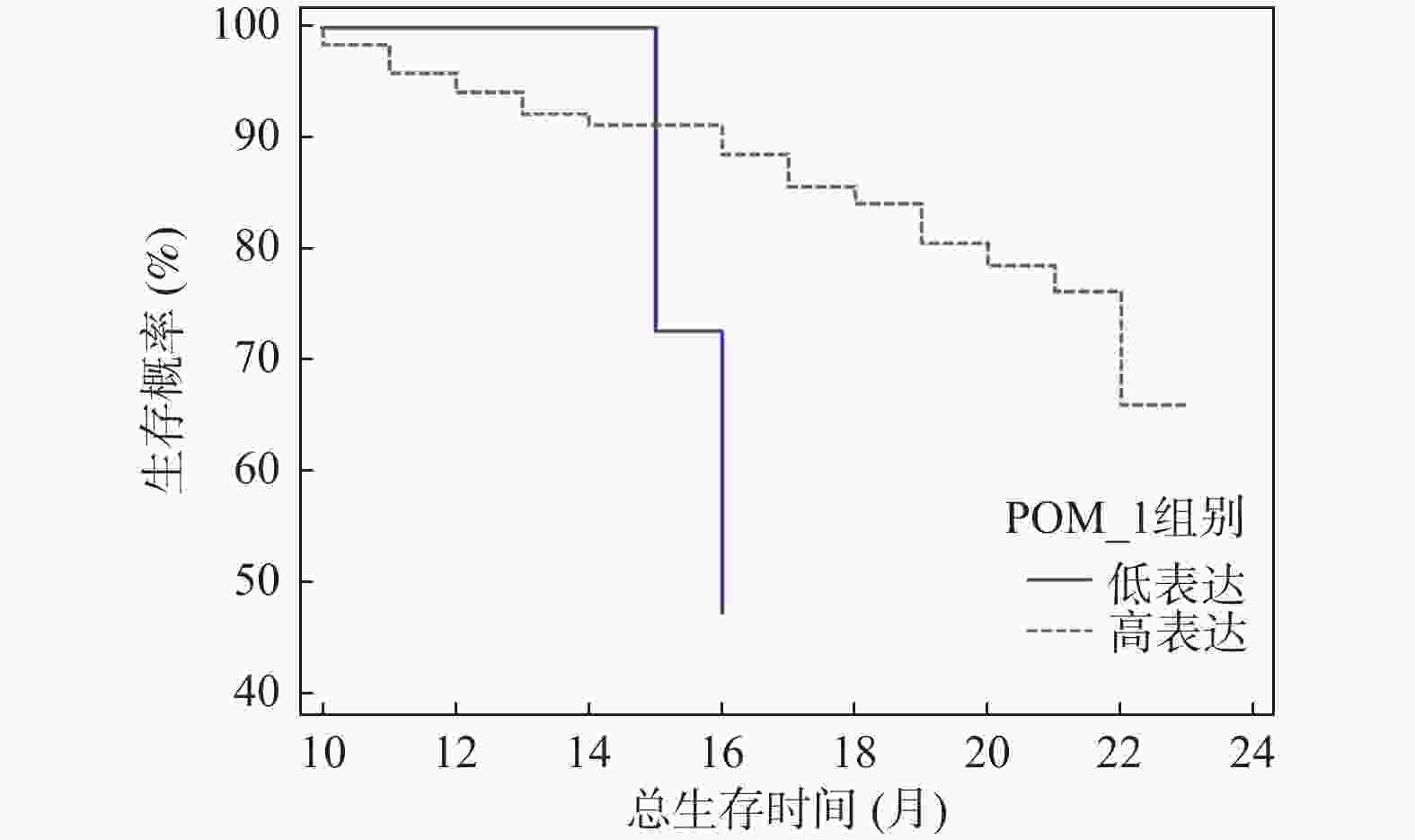
目的 探究胃癌患者血清对氧磷酶 1 (paraoxonase-1,PON-1)、N-Myc 下游调节基因 4 (N-Myc downstream-regulated gene 4,NDRG4)、分泌型卷曲相关蛋白4(secreted frizzled-related protein 4,SFRP4)表达水平与外周血免疫细胞分布及预后之间的内在联系。 方法 选取2020年4月至 2023年8月期间于日照市岚山区人民医院确诊并接受治疗的187 例胃癌患者临床资料设为病例组,同时选取同期可疑180例患者作为对照组。采集所有研究对象的空腹静脉血,运用酶联免疫吸附试验(enzyme-linked immunosorbent assay,ELISA)测定血清中 PON-1、NDRG4、SFRP4的表达水平。借助流式细胞术检测病例组患者外周血中各类免疫细胞的占比情况,以此综合评估外周血免疫细胞分布。随访收集病例组生存信息,记录总生存期(overall survival,OS)。按血清标志物表达中位数将病例组分高 / 低表达组,对比组间免疫状态与预后差异;用 Kaplan-Meier 法、Cox 比例风险回归模型分析标志物与预后相关性,Spearman 分析指标与生存预后关联。 结果 与对照组相比,病例组年龄、性别、BMI差异无统计学意义(P > 0.05),但PON-1、SFRP4及CD8+T细胞表达升高,NDRG4、CD3+T、CD4+T、B淋巴细胞和NK细胞表达降低(P < 0.05)。以各指标中位数分组后发现,PON-1高表达组CD8+T细胞占比低于低表达组(P < 0.05),NDRG4高表达组CD4+T细胞占比高于低表达组(P < 0.05),SFRP4高表达组CD4+T和NK细胞占比低于低表达组(P < 0.05)。单因素COX回归分析结果显示,年龄、PON-1、NDRG4、SFRP4 表达、淋巴转移等与胃癌患者预后相关(P < 0.05);Cox回归分析表明,NDRG4≥0.85 ng/mL、淋巴转移、PON-1<120.56 pg/mL、SFRP4≥210.32 pg/mL及年龄≥60岁是胃癌患者死亡的危险因素(P < 0.05)。截至随访结束,187例胃癌患者中61例死亡,中位生存期14.5个月(3~24个月)。Kaplan-Meier分析显示,血清PON-1高表达组中位生存期16.00(12.75,21.00)个月,显著长于低表达组的15.00(15.00,16.00)个月(P < 0.01);NDRG4高表达组中位生存期12.00(10.00,14.00)个月,显著短于低表达组的17.50(13.00,20.00)个月(P < 0.01);SFRP4高表达组中位生存期9.00(8.00,12.00)个月,显著短于低表达组的19.00(15.00,22.00)个月(P < 0.01)。Spearman分析表明,年龄、淋巴转移、NDRG4、SFRP4与预后生存正相关(P < 0.05),PON-1与预后生存负相关(P < 0.05)。 结论 胃癌患者血清 PON-1、NDRG4、SFRP4 表达水平不仅与外周血免疫细胞分布密切相关,且相互之间存在明确的相关性,同时对患者预后有着关键影响,为胃癌预后评估提供了潜在的参考指标。 -
关键词:
- 胃癌 /
- 对氧磷酶 1 /
- N-Myc 下游调节基因 4 /
- 分泌型卷曲相关蛋白 4 /
- 外周血免疫细胞分布 /
- 预后
Abstract:Objective To explore the intrinsic relationship between serum Paraoxonase-1 (PON-1), N-Myc downstream-regulated gene 4 (NDRG4), secreted frizzled-related protein 4 (SFRP4) expression levels, peripheral blood immune cell distribution, and prognosis in patients with gastric cancer. Methods Clinical data from 187 gastric cancer patients diagnosed and treated at Rizhao Lanshan District People's Hospital between April 2020 and August 2023 were selected as the case group, with 180 suspected patients from the same period serving as the control group. Fasting venous blood was collected from all study subjects, and enzyme-linked immunosorbent assay (ELISA) was utilized to measure serum PON-1, NDRG4, and SFRP4 expression levels. Flow cytometry was employed to detect the proportions of various immune cells in the peripheral blood of the case group, providing a comprehensive evaluation of peripheral blood immune cell distribution. Survival information was gathered through follow-up, and overall survival (OS) was recorded. The case group was divided into high and low expression groups based on the median serum biomarker expression, allowing for a comparison of immune status and prognostic differences between groups. The Kaplan-Meier method and Cox proportional hazards regression model were used to analyze the correlation between markers and prognosis, while Spearman analysis was employed to assess the association between indicators and survival prognosis. Results Compared to the control group, the case group showed no statistically significant differences in age, gender, and BMI (P > 0.05), but exhibited increased expression of PON-1, SFRP4, and CD8+ T cells, alongside decreased expression of NDRG4, CD3+ T, CD4+ T, B lymphocytes, and NK cells (P < 0.05). After grouping by median values, the PON-1 high expression group demonstrated a lower proportion of CD8+ T cells compared to the low expression group (P < 0.05), while the NDRG4 high expression group had a higher proportion of CD4+ T cells than the low expression group (P < 0.05). Additionally, the SFRP4 high expression group exhibited a lower proportion of CD4+ T and NK cells compared to the low expression group (P < 0.05). Univariate Cox regression analysis revealed that age, PON-1, NDRG4, SFRP4 expression, and lymph node metastasis were associated with the prognosis of gastric cancer patients (P < 0.05). Cox regression analysis indicated that NDRG4≥0.85ng/mL, lymph node metastasis, PON-1<120.56 pg/mL, SFRP4≥210.32 pg/mL, and age≥60 years were risk factors for mortality in gastric cancer patients (P < 0.05). By the end of follow-up, 61 out of 187 gastric cancer patients had died, with a median survival of 14.5 months (3~24 months). Kaplan-Meier analysis showed that the PON-1 high expression group had a median survival of 16.00 (12.75, 21.00) months, significantly longer than the low expression group’ s 15.00 (15.00, 16.00) months (P < 0.01); the NDRG4 high expression group had a median survival of 12.00 (10.00, 14.00) months, significantly shorter than the low expression group's 17.50 (13.00, 20.00) months (P < 0.01); the SFRP4 high expression group had a median survival of 9.00 (8.00, 12.00) months, significantly shorter than the low expression group's 19.00 (15.00, 22.00) months (P < 0.01). Spearman analysis revealed that age, lymph node metastasis, NDRG4, and SFRP4 were positively correlated with survival prognosis (P < 0.05), while PON-1 was negatively correlated (P < 0.05). Conclusion Serum PON-1, NDRG4, and SFRP4 expression levels in gastric cancer patients are closely related to peripheral blood immune cell distribution, exhibit clear interrelationships, and critically impact patient prognosis, providing potential reference indicators for gastric cancer prognosis assessment. -
表 1 两组患者基线资料、血清指标和免疫状态指标比较[($\bar x \pm s $)/n]
Table 1. Comparison of baseline data, serum indicators and immune status indicators of the two groups of patients [ ($\bar x \pm s $)/n]
项目 病例组(n=187) 对照组(n=180) t/χ2 P 年龄(岁) 58.71 ± 7.55 57.62 ± 8.39 1.309 0.191 性别(男/女) 105/82 88/92 1.939 0.164 BMI(kg/m2) 21.86 ± 2.43 21.92 ± 2.67 0.225 0.822 PON-1(pg/mL) 125.67 ± 32.54 189.76 ± 45.21 15.631 <0.001** NDRG4(ng/mL) 0.89 ± 0.23 1.56 ± 0.34 22.185 <0.001** SFRP4(pg/mL) 215.34 ± 56.78 123.45 ± 32.10 18.987 <0.001** CD3+T(%) 45.66 ± 5.48 68.59 ± 4.22 44.791 <0.001** CD4+T(%) 26.33 ± 3.24 40.29 ± 5.63 29.248 <0.001** CD8+T(%) 40.28 ± 3.31 23.66 ± 2.48 54.278 <0.001** B 淋巴细胞(%) 6.38 ± 2.44 15.37 ± 3.25 30.041 <0.001** NK 细胞(%) 6.62 ± 2.38 15.61 ± 4.28 24.989 <0.001** **P < 0.001。 表 2 PON-1 高表达组与低表达组免疫状态指标的比较($\bar x \pm s $)
Table 2. Comparison of immune status indicators between the PON-1 high expression group and the low expression group($\bar x \pm s $)
指标 高表达组(n = 93) 低表达组(n =94) t P CD3+T 细胞占比(%) 65.23 ± 7.89 66.12 ± 8.23 0.755 0.451 CD4+T 细胞占比(%) 32.56 ± 4.56 33.21 ± 4.87 0.942 0.347 CD8+T 细胞占比(%) 25.67 ± 3.45 28.98 ± 3.78 6.252 <0.001** B 淋巴细胞占比(%) 12.34 ± 2.34 12.56 ± 2.56 0.613 0.541 NK 细胞占比(%) 15.67 ± 3.21 16.12 ± 3.45 0.923 0.357 **P < 0.001。 表 3 NDRG4 高表达组与低表达组免疫状态指标的比较($\bar x \pm s $)
Table 3. Comparison of immune status indicators between the NDRG4 high expression group and the low expression group ($\bar x \pm s $)
指标 高表达组(n = 94) 低表达组(n = 93) t P CD3+T 细胞占比(%) 65.89 ± 8.12 65.45 ± 7.98 0.374 0.709 CD4+T 细胞占比(%) 34.56 ± 4.78 31.23 ± 4.56 4.873 <0.001** CD8+T 细胞占比(%) 26.78 ± 3.67 27.56 ± 3.89 1.410 0.160 B 淋巴细胞占比(%) 12.56 ± 2.45 12.45 ± 2.34 0.314 0.754 NK 细胞占比(%) 15.89 ± 3.34 15.98 ± 3.45 0.181 0.856 **P < 0.001。 表 4 SFRP4 高表达组与低表达组免疫状态指标的比较($\bar x \pm s $)
Table 4. Comparison of immune status indicators between the high expression group and the low expression group of SFRP4($\bar x \pm s $)
指标 高表达组(n =94) 低表达组(n = 93) t P D3+T 细胞占比(%) 64.98 ± 7.98 66.34 ± 8.21 1.149 0.252 CD4+T 细胞占比(%) 31.89 ± 4.67 33.56 ± 4.89 2.388 0.018* CD8+T 细胞占比(%) 27.67 ± 3.89 27.98 ± 3.76 0.554 0.580 B 淋巴细胞占比(%) 12.45 ± 2.34 12.67 ± 2.56 0.613 0.540 NK 细胞占比(%) 14.56 ± 3.12 17.89 ± 3.45 6.924 <0.001** *P < 0.05;**P < 0.001。 表 5 胃癌患者预后的单因素分析[($\bar x \pm s $)/n(%)]
Table 5. Univariate analysis of prognosis in patients with gastric cancer [($\bar x \pm s $)/n (%)]
项目 死亡组(n=61) 存活组(n=126) t/χ2 P 年龄(岁) ≥60 45(73.77) 65(51.59) 8.350 0.004* <60 16(26.23) 61(48.41) 性别 男 35(57.38) 80(63.49) 0.649 0.420 女 26(42.62) 46(36.51) BMI(kg/m2) 22.56 ± 1.62 22.48 ± 1.57 0.323 0.747 病程(月) 5.84 ± 1.38 5.92 ± 1.44 0.361 0.719 糖尿病史 有 30(49.18) 65(51.59) 0.095 0.758 无 31(50.82) 61(48.41) TNM 分期 Ⅰ-Ⅱ 期 35(57.38) 55(43.65) 3.102 0.078 Ⅲ-Ⅳ期 26(42.62) 71(56.35) 淋巴转移 有 42(68.85) 50(39.68) 13.993 <0.001** 无 19(31.15) 76(60.32) 肿瘤直径(mm) 3.44 ± 1.38 3.45 ± 1.37 0.047 0.963 PON-1表达(pg/mL) <120.56 38(62.30) 55(43.65) 5.715 0.017* ≥120.56 23(37.70) 71(56.35) NDRG4 表达(ng/mL) ≥0.85 40(65.57) 54(42.86) 8.484 0.004* <0.85 21(34.43) 72(57.14) SFRP4表达(pg/mL) ≥210.32 39(63.93) 55(43.65) 6.764 0.009* <210.32 22(36.07) 71(56.35) FPG(mmol/L) 8.50 ± 1.46 8.41 ± 1.47 0.393 0.694 TG(mmol/L) 1.27 ± 0.22 1.22 ± 0.29 1.190 0.235 LDL-C(mmol/L) 3.58 ± 2.24 3.65 ± 2.41 0.190 0.849 Cr(μmol/L) 101.84 ± 55.48 100.92 ± 19.47 0.167 0.868 BMI:体质量指数;PON-1 :对氧磷酶 1;NDRG4:N-Myc 下游调节基因 4;SFRP4:分泌型卷曲相关蛋白 4;FPG:空腹血糖;TG :甘油三酯;LDL-C :低密度脂蛋白胆固醇;Cr :肌酐;*P < 0.05;**P < 0.001。 表 6 影响胃癌患者预后的单因素Cox分析
Table 6. Univariate Cox analysis affecting the prognosis of patients with gastric cancer
指标 P HR 95%CI 下限 上限 年龄 0.004* 2.683 1.352 5.324 性别 0.420 0.812 0.465 1.416 BMI 0.747 1.035 0.821 1.306 病程 0.719 0.972 0.815 1.159 糖尿病史 0.758 1.082 0.632 1.868 TNM 分期 0.078 1.825 0.958 3.476 淋巴转移 <0.001** 3.256 1.789 5.927 肿瘤直径 0.963 0.996 0.802 1.235 PON-1表达 0.017* 2.158 1.162 3.998 NDRG4 表达 0.004* 2.732 1.398 5.338 SFRP4表达 0.009* 2.385 1.228 4.632 FPG 0.694 1.042 0.857 1.265 TG 0.235 1.486 0.782 2.823 LDL-C 0.849 0.988 0.805 1.212 Cr 0.868 1.002 0.995 1.009 *P < 0.05;**P < 0.001。 表 7 变量赋值
Table 7. Variable assignment
变量 编码 赋值方式 因变量 预后生存 Y 存活=0;死亡=1 自变量 NDRG4 X1 <0.85ng/mL=0;≥0.85ng/mL=1 淋巴转移 X2 无=0;有=1 PON-1 X3 ≥120.56 pg/mL=0;<120.56 pg/mL=1 SFRP4 X4 <210.32 pg/mL=0;≥210.32 pg/mL=1 年龄 X5 <60岁=0;≥60岁=1 表 8 胃癌患者死亡的Cox比例风险回归分析
Table 8. Cox proportional hazards regression analysis of mortality in patients with gastric cancer
影响因素 β SE Waldχ2 P HR 95%CI 下限 上限 NDRG4 0.857 0.085 9.155 0.003* 1.292 1.095 1.526 淋巴转移 1.017 0.277 13.507 <0.001** 2.764 1.607 4.753 PON-1 −0.780 0.346 5.089 0.024* 0.458 0.233 0.903 SFRP4 0.929 0.238 15.235 <0.001** 2.531 1.588 4.035 年龄 0.927 0.291 10.123 <0.001** 2.526 1.427 4.470 *P < 0.05;**P < 0.001。 表 9 相关指标与胃癌患者预后生存相关性分析(r)
Table 9. Correlation analysis (r) between relevant indicators and prognosis and survival of gastric cancer patients
变量 预后生存 年龄 淋巴转移 PON-1 NDRG4 SFRP4 预后生存 1 − − − − − 年龄 0.611** 1 − − − − 淋巴转移 0.635** 0.455** 1 − − − PON-1 ~0.518** 0.194** 0.271* 1 − − NDRG4 0.562** 0.380** 0.277** 0.320* 1 − SFRP4 0.486** 0.306** 0.400** 0.224** 0.183** 1 *P < 0.05;**P < 0.001。 -
[1] Guan W L, He Y, Xu R H. Gastric cancer treatment: Recent progress and future perspectives[J]. J Hematol Oncol, 2023, 6(1): 57-63. [2] Ajani J A, D'Amico T A, Bentrem D J, et al. 2022, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2022, 20(2): 167-192. doi: 10.6004/jnccn.2022.0008 [3] Yang W J, Zhao H P, Yu Y, et al. Updates on global epidemiology, risk and prognostic factors of gastric cancer[J]. World J Gastroenterol, 2023, 29(16): 2452-2468. doi: 10.3748/wjg.v29.i16.2452 [4] 杨为彬, 史经汉, 朱硕, 等. 卡瑞利珠单抗结合DSOX方案对晚期胃癌患者血清BARD1、TAP表达的影响[J]. 中国老年学杂志, 2024, 44(1): 31-34. [5] 唐嘉黛, 杨静, 宋红莉, 等. 318例原发性胃癌转移及预后影响因素的相关分析[J]. 昆明医科大学学报, 2023, 44(2): 9-12. [6] 中国抗癌协会胃癌专业委员会, 中国抗癌协会肿瘤胃肠病学专业委员会. 胃癌根治术标本的规范化外科处理中国专家共识(2022版)[J]. 中华胃肠外科杂志, 2022, 25(2): 93-103. [7] Amin M B, Edge S B, Greene F L, et al. AJCC cancer staging manual. 8th ed. new york[J]Springer, 2017, 8(6): 517-535. [8] 黄奕龙, 罗强, 黄爱茹. 胃癌患者血清中miR-373和miR-592的表达变化及意义[J]. 山东医药, 2022, 62(4): 82-85. [9] 卢顺利, 李洪涛, 于建平, 等. 血清甲胎蛋白阳性胃癌患者临床病理特征与预后关系的研究[J]. 中国肿瘤临床, 2023, 50(1): 30-36. [10] Yasuda T, Wang Y A. Gastric cancer immunosuppressive microenvironment heterogeneity: Implications for therapy development[J]. Trends Cancer, 2024, 10(7): 627-642. doi: 10.1016/j.trecan.2024.03.008 [11] Wu X, Chen L, Cheng J, et al. Effect of dietary salt intake on risk of gastric cancer: A systematic review and meta-analysis of case-control studies[J]. Nutrients, 2022, 14(20): 4260. doi: 10.3390/nu14204260 [12] Conti C B, Agnesi S, Scaravaglio M, et al. Early gastric cancer: Update on prevention, diagnosis and treatment[J]. Int J Environ Res Public Health, 2023, 20(3): 2149-2166. [13] Hou W, Zhao Y, Zhu H. Predictive biomarkers for immunotherapy in gastric cancer: Current status and emerging prospects[J]. Int J Mol Sci, 2023, 24(20): 15321-15331. doi: 10.3390/ijms242015321 [14] Xia J Y, Aadam A A. Advances in screening and detection of gastric cancer[J]. J Surg Oncol, 2022, 125(7): 1104-1109. doi: 10.1002/jso.26844 [15] Duan Y, Xu Y, Dou Y, et al. Helicobacter pylori and gastric cancer: Mechanisms and new perspectives[J]. J Hematol Oncol, 2025, 18(1): 10-13. doi: 10.1186/s13045-024-01654-2 [16] Lordick F, Carneiro F, Cascinu S, et al. Esmo guidelines committee electronic address: Clinicalguidelines@esmo. org. gastric cancer: Esmo clinical practice guideline for diagnosis, treatment and follow-up[J]. Ann Oncol, 2022, 33(10): 1005-1020. doi: 10.1016/j.annonc.2022.07.004 [17] Shah R, Khaitan P G, Pandita T K, et al. Gastric cancer in Jammu and Kashmir, India: A review of genetic perspectives[J]. J Cancer Res Ther, 2022, 18(4): 873-879. doi: 10.4103/jcrt.JCRT_12_19 [18] Zeng Y, Jin R U. Molecular pathogenesis, targeted therapies, and future perspectives for gastric cancer[J]. Semin Cancer Biol, 2022, 86(Pt 3): 566-582. [19] 谢欣媛, 牛晓辰, 孙建辉, 等. 胃腺癌患者铁死亡相关LncRNA预后模型的构建[J]. 昆明医科大学学报, 2025, 46(4): 36-38. [20] Rao X, Zhang C, Luo H, et al. Targeting gastric cancer stem cells to enhance treatment response[J]. Cells, 2022, 11(18): 2828-2835. doi: 10.3390/cells11182828 -





 下载:
下载: