Research Progress on the Correlation between Serum Uric Acid and Head and Neck Tumors
-
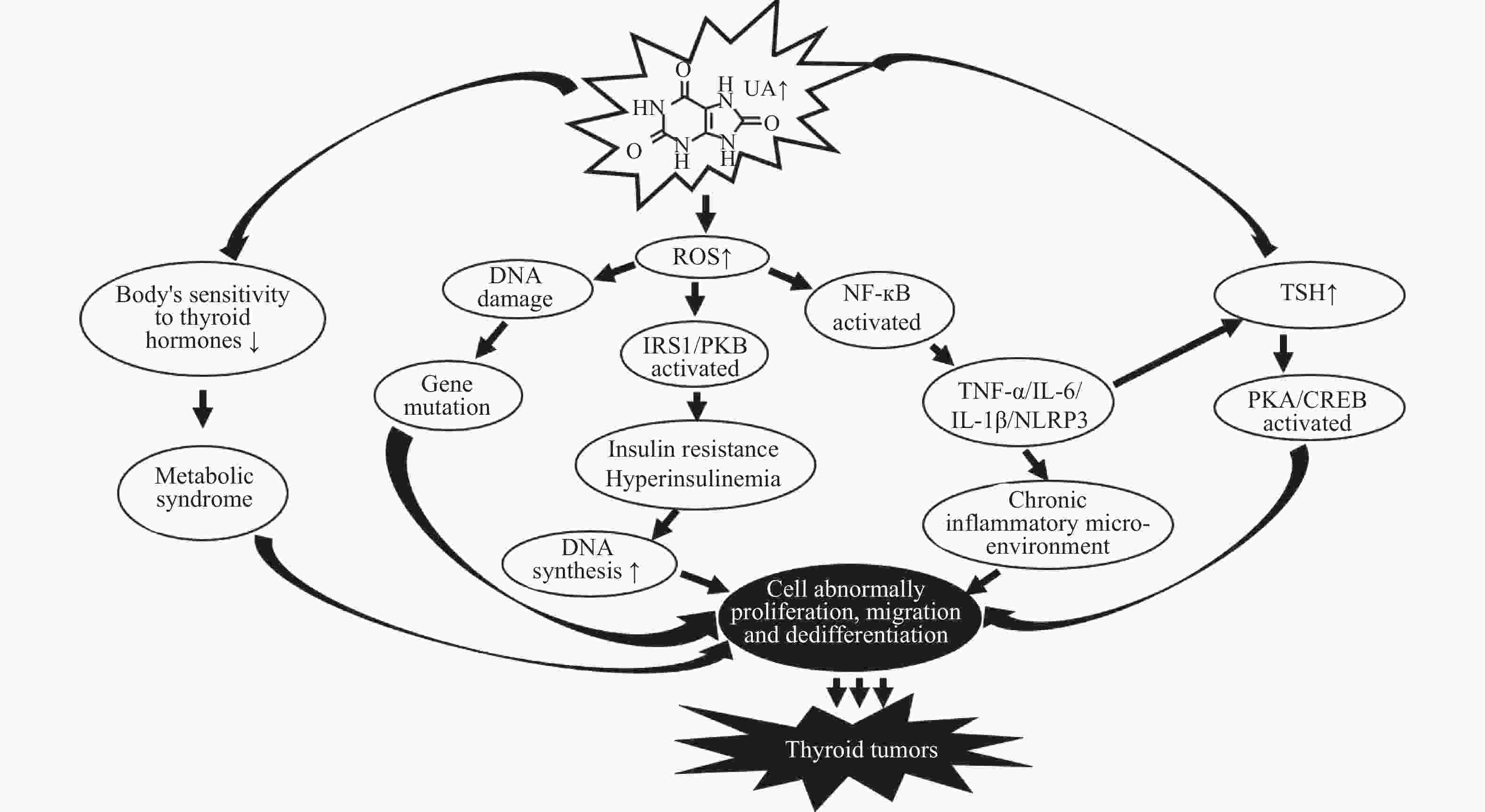
摘要: 尿酸是嘌呤代谢的最终产物,也是重要的内源性抗氧化剂,尿酸通过其抗氧化和促氧化作用在不同恶性肿瘤中发挥不同作用并与多种恶性肿瘤的发生、发展和预后密切相关。血清尿酸与头颈肿瘤如口腔癌、喉癌、鼻咽癌、甲状腺肿瘤等的相关性研究报道较少,本文对血清尿酸与头颈肿瘤发生、发展及预后的相关性进行综述以期揭示尿酸在头颈肿瘤预防、诊断和治疗中的价值。Abstract: Uric acid is the final product of purine metabolism and an important endogenous antioxidant. Uric acid plays different roles in different malignant tumors through its antioxidant and pro-oxidative effects, and is closely related to the occurrence, development and prognosis of various malignant tumors. There are few reports on the correlation between serum uric acid and head and neck tumors, such as oral cancer, larynx cancer, nasopharyngeal cancer, thyroid cancer, etc. In this paper, the correlation between serum uric acid and the occurrence, development and prognosis of head and neck tumors is reviewed to reveal the value of uric acid in the prevention, diagnosis and treatment of head and neck tumors.
-
Key words:
- Uric acid /
- Head and neck tumors /
- Thyroid tumor /
- Etiology
-
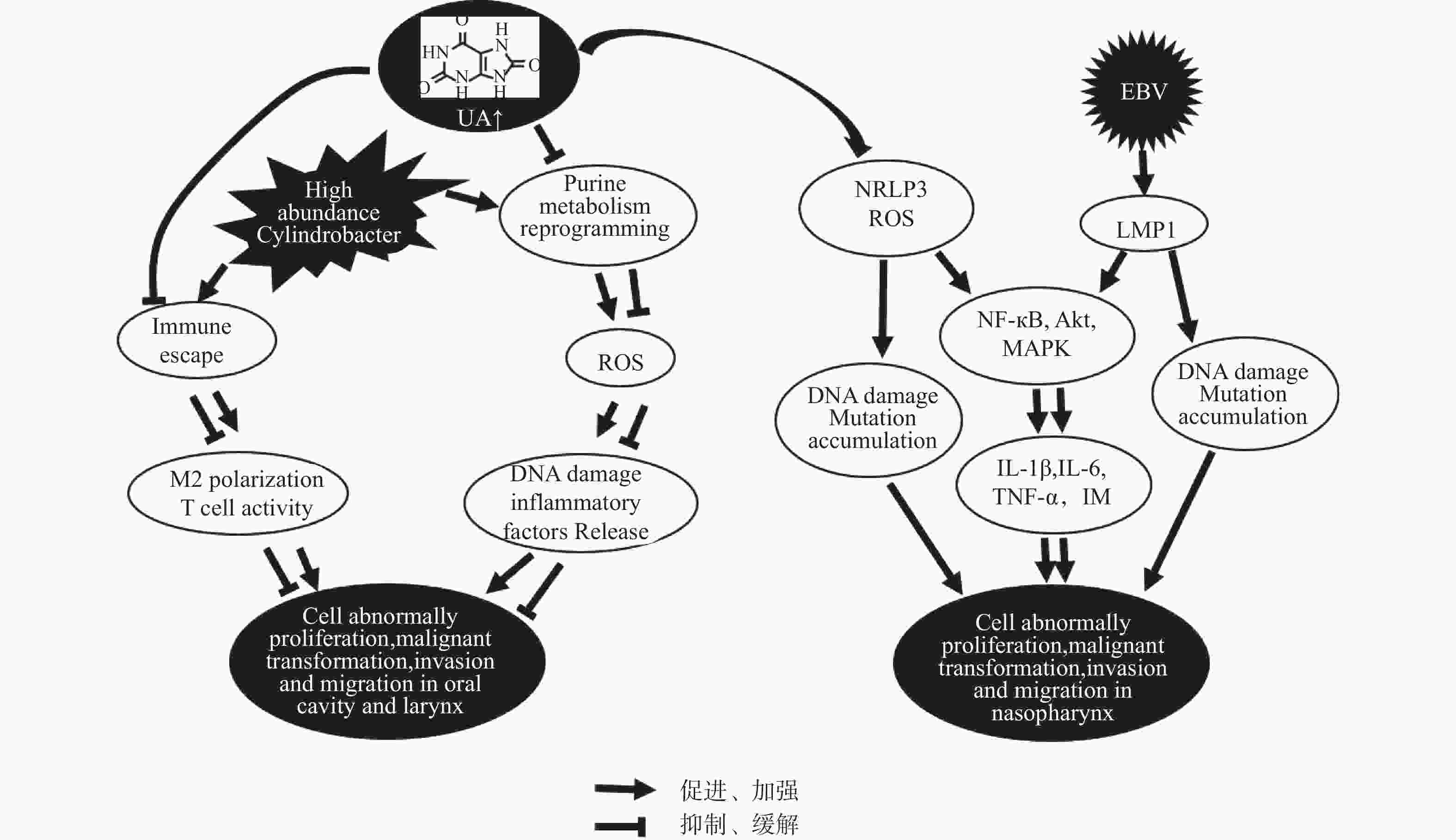
图 2 口腔癌、喉癌、鼻咽癌“尿酸-微生物-免疫”交互作用可能机制
Akt:丝氨酸/苏氨酸激酶;IM:炎症微环境;IL-1β:白介素-1β;IL-6:白介素-6;LMP1:EB病毒潜伏膜蛋白1;MAPK:丝裂原激活蛋白激酶;M2:巨噬细胞(M2型);NF-κB:转录因子-κB;NLRP3:核苷酸结合寡聚化结构域样受体蛋白3炎症小体;ROS:活性氧族;TNF-α:肿瘤坏死因子-α;UA:尿酸。
Figure 2. The “uric acid-microbiota-immunity” interaction mechanism in oral cancer,laryngeal cancer and nasopharyngeal carcinoma
表 1 高尿酸血症与头颈部主要肿瘤类型发病风险和/或预后的关系
Table 1. The relationship between hyperuricemia and the risk of and/or prognosis of the main tumor types in the head and neck region
肿瘤类型 发病风险/预后 相关研究纳入的
最大病例数甲状腺结节
(良性)发病风险增加 698.6万例 甲状腺癌 发病风险增加、预后较差 139例 鼻咽癌 预后较差 341例 喉癌 预后较好 814例 口腔癌 发病风险降低 145例 食管癌 预后较差 209例 -
[1] Borghi C, Agabiti-Rosei E, Johnson R J, et al. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease[J]. Eur J Intern Med., 2020, 80(10): 1-11. [2] Zhao L, Guo R, Zhao Z, et al. Linking hyperuricemia to cancer: emerging evidence on risk and progression[J]. Curr Oncol Rep., 2025, 27(6): 703-716. doi: 10.1007/s11912-025-01677-z [3] Rodriguez-Navarro C, Elert K, Ibañez-Velasco A, et al. Unraveling the pathological biomineralization of monosodium urate crystals in gout patients[J]. Commun Biol., 2024, 7(1): 828-838. doi: 10.1038/s42003-024-06534-6 [4] Li Z, Su Y, Su H, et al. Serum uric acid and its metabolism-a vital factor in the inflammatory transformation of cancer[J]. J Adv Res, 2025, (25)8: 646-658 [5] Kim Y R, Choi C K, Lee Y H, et al. Association between albumin, total bilirubin, and uric acid serum levels and the risk of cancer: A prospective study in a Korean population[J]. Yonsei Med J, 2021, 62(9): 792-798. doi: 10.3349/ymj.2021.62.9.792 [6] Liu Y, Chen W, Yang R, et al. Effect of serum uric acid and gout on the incidence of colorectal cancer: A meta-analysis[J]. Am J Med Sci, 2024, 367(2): 119-127. doi: 10.1016/j.amjms.2023.11.013 [7] Ihira H, Nakano S, Yamaji T, et al. Plasma albumin, bilirubin, and uric acid and the subsequent risk of cancer: A case-cohort study in the japan public health center-based prospective study [J]. Am J Epidemiol, 2024. [8] Yadav K D, Patil B A, Raheel S A, et al. Serum uric acid levels in patients with oral cancer, leukoplakia and submucous fibrosis: A cross-sectional study[J]. Transl Cancer Res, 2020 Apr; 9(4): 3084-3091. [9] Dharmana L, Pottam A, Kollabathula S R, et al. Comparative evaluation of serum urea, uric acid, and creatine kinase levels in oral cancer and potentially malignant disorders of the oral cavity: A clinico-biochemical study[J]. Cureus, 2023, 15(5): e39123. [10] Caruntu A, Moraru L, Ciubotaru D A, et al. Assessment of serum urea, creatinine and uric acid in oral cancer[J]. J Clin Med, 2022, 11(12): 3459. doi: 10.3390/jcm11123459 [11] Anitha G, Kumar K V, Deshpande G, et al. Utility of serum and salivary lactate dehydrogenase and uric acid levels as a diagnostic profile in oral squamous cell carcinoma patients[J]. J Oral Maxillofac Pathol, 2022, 26(2): 218-227. doi: 10.4103/jomfp.jomfp_26_22 [12] Manifar S, Rahimzamani A, Shirkhoda M, et al. Role of serum uric acid as a protective biomarker in patients with different histopathological grades of oral squamous cell carcinoma: A case-control study[J]. Biomed Res Int, 2020: 5185423. [13] Du X, Chen L, Li W, et al. Use of pretreatment serum uric acid level to predict metastasis in locally advanced nasopharyngeal carcinoma[J]. Head Neck, 2017, 39(3): 492-497. doi: 10.1002/hed.24631 [14] Guo J, Yang Q, Jiang Q, et al. Integrating baseline nutritional and inflammatory parameters with post-treatment EBV DNA level to predict outcomes of patients with de novo metastatic nasopharyngeal carcinoma receiving chemotherapy combination PD-1 inhibitor[J]. Nutrients, 2023, 15(19): 4262. doi: 10.3390/nu15194262 [15] Xu Y, Wu Z, Ye W, et al. Prognostic value of serum uric acid and tumor response to induction chemotherapy in locally advanced nasopharyngeal carcinoma[J]. BMC Cancer, 2021, 21(1): 519. doi: 10.1186/s12885-021-08285-7 [16] Lee S, Li X, Kim J H, et al. Prognostic value of uric acid in predicting metastasis following definitive radiotherapy in patients with head and neck cancer. In Vivo, 2025, 39(4): 2464-2473. [17] Hsueh C, Shao M, Cao W, et al. Pretreatment serum uric acid as an efficient predictor of prognosis in men with laryngeal squamous cell cancer: A retrospective cohort study[J]. Oxid Med Cell Longev, 2019, 1821969. [18] Chen Y, Li Q, Chen D T, et al. Prognostic value of pre-operative serum uric acid levels in esophageal squamous cell carcinoma patients who undergo R0 esophagectomy[J]. Cancer Biomark, 2016, 17(1): 89-96. doi: 10.3233/CBM-160621 [19] Tran N Q, Le B H, Hoang C K, et al. Prevalence of thyroid nodules and associated clinical characteristics: findings from a large sample of people undergoing health checkups at a university hospital in Vietnam[J]. Risk Manag Healthc Policy, 2023, 16: 899-907. doi: 10.2147/RMHP.S410964 [20] Li Y, Jin C, Li J, et al. Prevalence of thyroid nodules in China: A health examination cohort-based study[J]. Front Endocrinol (Lausanne), 2021, 12: 676144. doi: 10.3389/fendo.2021.676144 [21] Xu L, Zeng F, Wang Y, et al. Prevalence and associated metabolic factors for thyroid nodules: A cross-sectional study in Southwest of China with more than 120 thousand populations[J]. BMC Endocr Disord, 2021, 21(1): 175. doi: 10.1186/s12902-021-00842-2 [22] Huang Y, Li Z, Yang K, et al. The association of uric acid with the development of thyroid nodules: A retrospective cohort study[J]. BMC Endocr Disord, 2022, 22(1): 197. doi: 10.1186/s12902-022-01119-y [23] Bao F, Shi YJ, Cong H, et al. Study on the correlation between thyroid nodule and metabolic index in physical examination population[J]. Zhonghua Yu Fang Yi Xue Za Zhi, 2023, 57(12): 2110-2116. [24] Zhang F, Teng D, Tong N, et al. Gender-specific associations between metabolic disorders and thyroid nodules: A cross-sectional population-based study from China[J]. Thyroid, 2022, 32(5): 571-580. doi: 10.1089/thy.2021.0686 [25] Liu Y, Lin Z, Sheng C, et al. The prevalence of thyroid nodules in northwest China and its correlation with metabolic parameters and uric acid[J]. Oncotarget, 2017, 8(25): 41555-41562. doi: 10.18632/oncotarget.14720 [26] Hu J, Luo Y, Lin X. A systematic review and meta-analysis of the correlation between hyperuricemia and thyroid nodules in adults[J]. Gland Surg, 2021, 10(12): 3324-3333. doi: 10.21037/gs-21-722 [27] Gu J, Xie R, Zhao Y, et al. A machine learning-based approach to predicting the malignant and metastasis of thyroid cancer[J]. Front Oncol, 2022, 12: 938292. doi: 10.3389/fonc.2022.938292 [28] Guo X, Chen X, Zhang C, et al. Hyperinsulinemia and thyroid peroxidase antibody in Chinese patients with papillary thyroid cancer[J]. Endocr J, 2019, 66(8): 731-737. doi: 10.1507/endocrj.EJ18-0358 [29] 那日苏. 甲状腺良性结节和分化型甲状腺癌与肾功能的相关性研究[D]. 青海大学硕士学位论文, 2023. [30] Li H, Zhang L, Wang Y, et al. The feasibility of using a multivariate regression model incorporating ultrasound findings and serum markers to predict thyroid cancer metastasis[J]. Front Endocrinol, 2024, 15: 1461865. doi: 10.3389/fendo.2024.1461865 [31] Yang L, Li A, Yu W, et al. Blockade of purine metabolism reverses macrophage immunosuppression and enhances anti-tumor immunity in non-small cell lung cancer[J]. Drug Resist Updat., 2025, 78: 101175. doi: 10.1016/j.drup.2024.101175 [32] Zhao J, Fu Y, Qiu H. Effect and mechanism of Plantaginis Semen polysaccharides on intestinal microecology in rats with hyperuricemia[J]. Front Microbiol., 2025, 16: 1555734. doi: 10.3389/fmicb.2025.1555734 [33] Li F, Huang H, Xu J, et al. Fusobacterium nucleatum-triggered purine metabolic reprogramm-ing drives tumorigenesis in head and neck carcinoma[J]. Discov Oncol, 2023, 14(1): 120. doi: 10.1007/s12672-023-00727-x [34] Li F, Huang H, Xu J, et al. Fusobacterium nucleatum-triggered purine metabolic reprogramming drives tumorigenesis in head and neck carcinoma[J]. Discov Oncol., 2023, 14(1): 120. doi: 10.1007/s12672-023-00727-x [35] Mukhopadhyay P, Ghosh S, Pandit K, et al. Uric acid and its correlation with various metabolic parameters: A population-based study[J]. Indian J Endocrinol Metab., 2019, 23(1): 134-139. doi: 10.4103/ijem.IJEM_18_19 [36] Chao G, Zhu Y, Fang L. Retrospective Analysis of the Correlation between Uric Acid and Thyroid Hormone in People with Normal Thyroid Function[J]. J Diabetes Res. 2019, 5904264. [37] APizzoni A, Zhang X, Naim N, et al. Soluble cyclase-mediated nuclear cAMP synthesis is sufficient for cell proliferation[J]. Proc Natl Acad Sci, 2023, 120(4): e2208749120. doi: 10.1073/pnas.2208749120 [38] Lu Y, Wang J, An Y, et al. Impaired sensitivity to thyroid hormones is associated with hyperuricemia in a Chinese euthyroid population[J]. Front Endocrinol, 2023, 14: 1132543. doi: 10.3389/fendo.2023.1132543 -





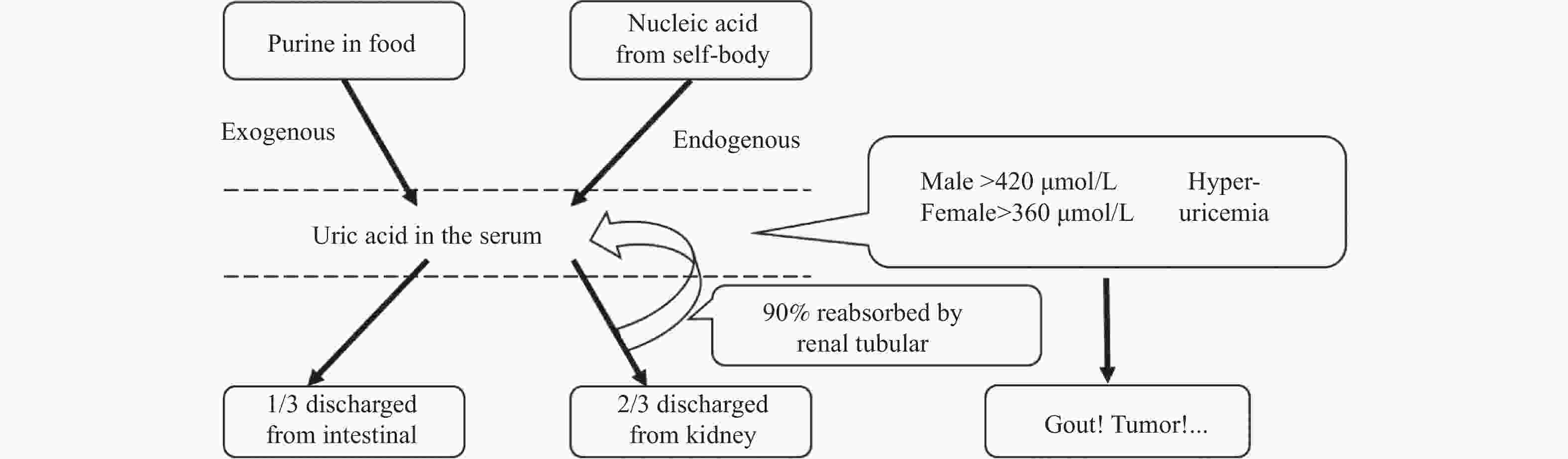
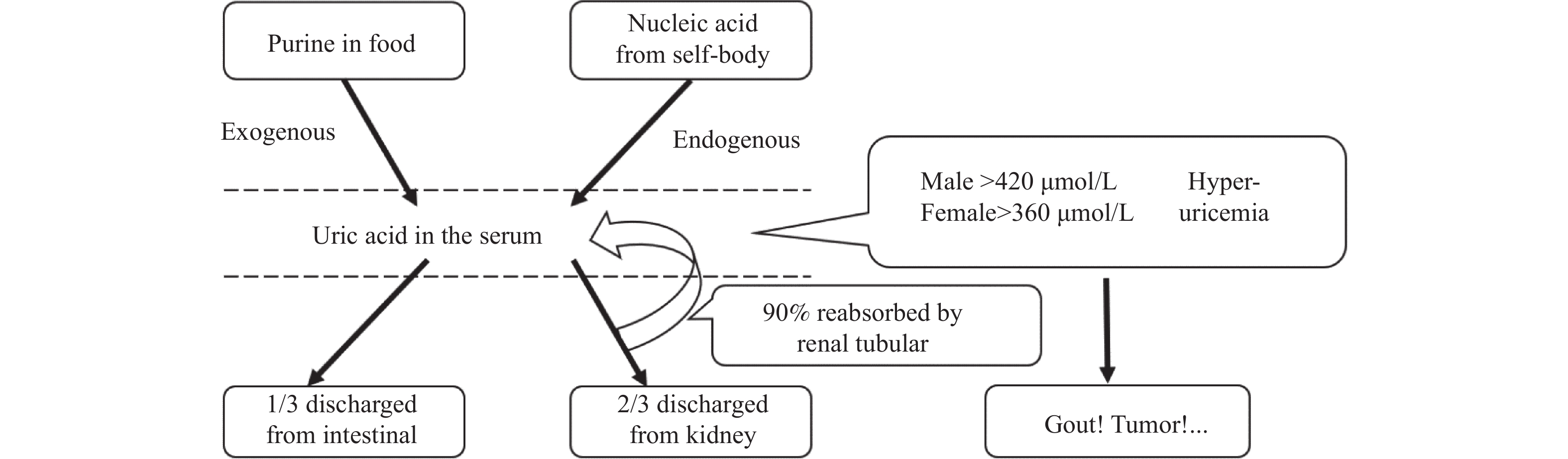
 下载:
下载: