Studies on the Chemical Epigenetic Modification of Fungus Samsoniella Hepiali CDB9-31
-
摘要:
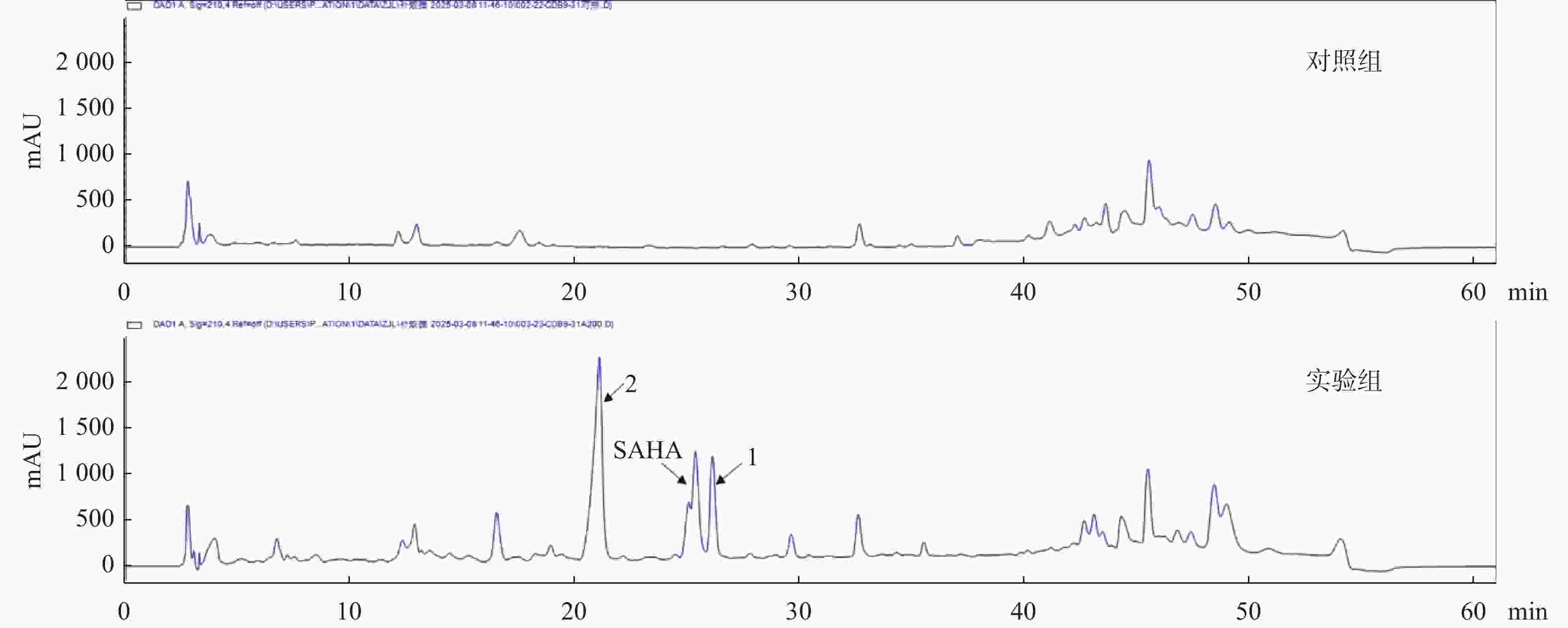
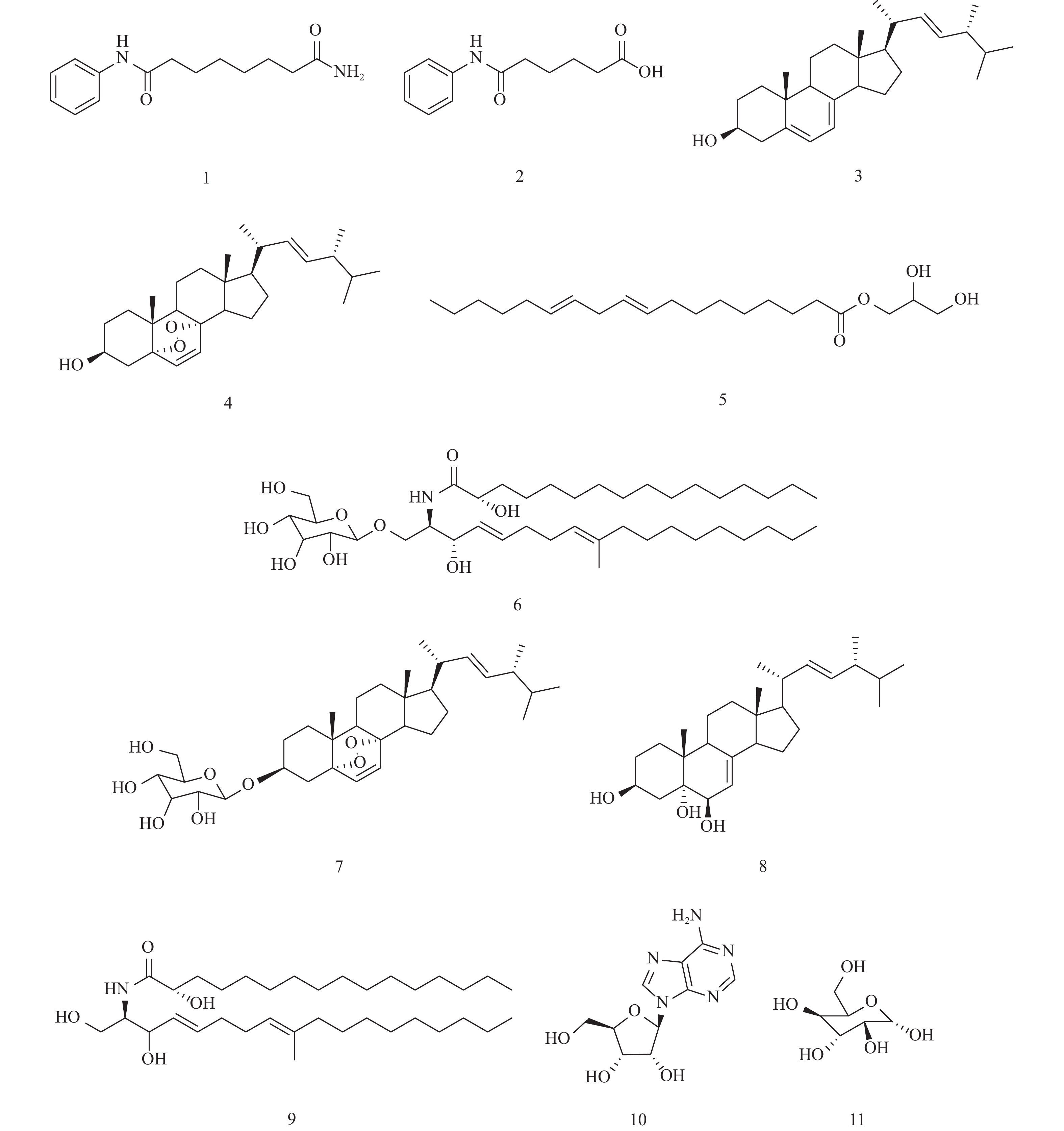
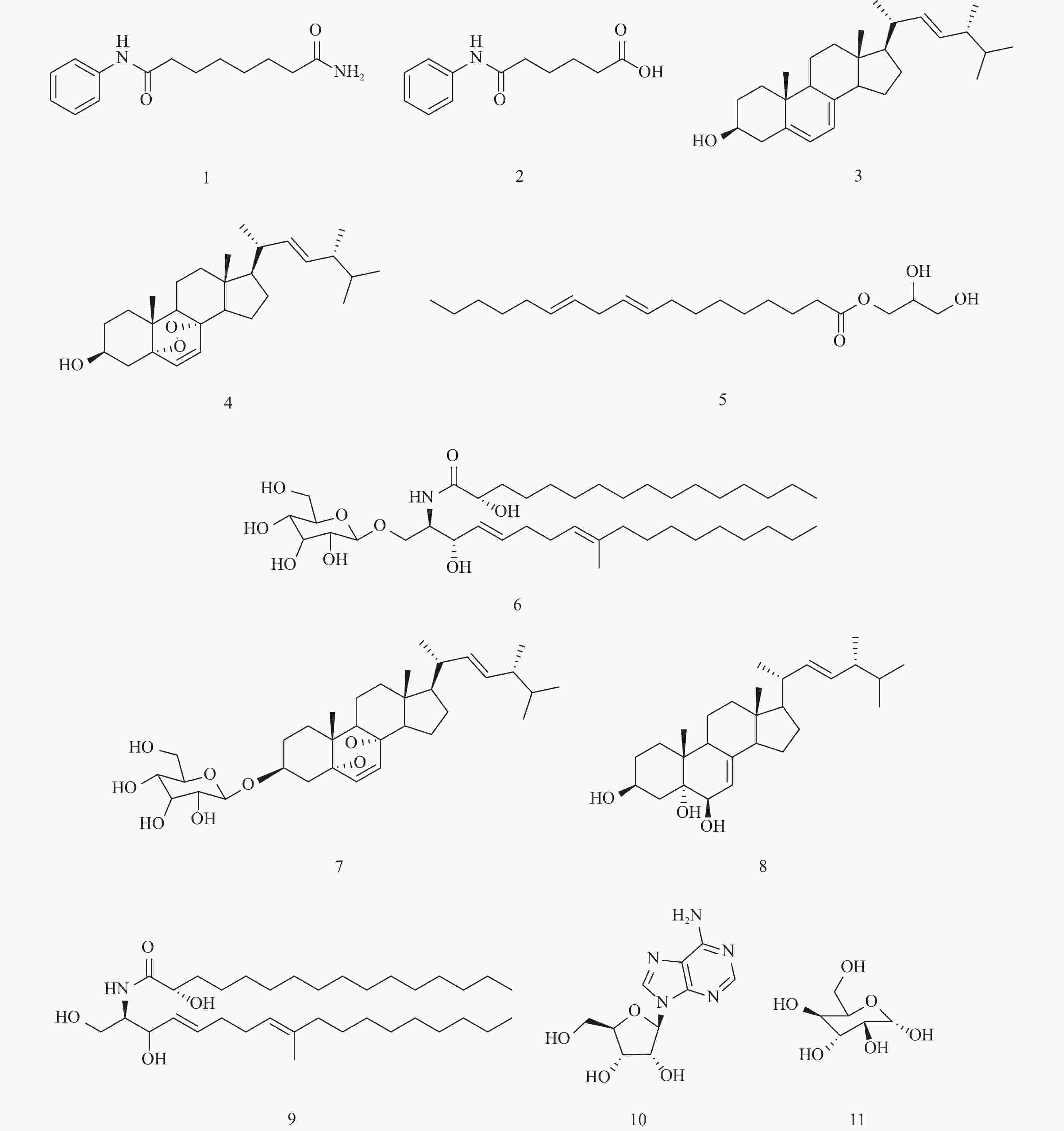
目的 采用薄层色谱法(thin-layer chromatography,TLC)和高效液相色谱法(high-performance liquid chromatography,HPLC)分析组蛋白去乙酰化酶抑制剂辛二酰苯胺异羟肟酸(suberoylanilide hydroxamic acid,SAHA)对虫生真菌Samsoniella hepiali CDB9-31次生代谢产物的影响。 方法 利用硅胶柱层析、葡聚糖凝胶柱、反相柱层析等方法对添加了表观遗传试剂的Samsoniella hepiali CDB9-31发酵产物进行分离、纯化,并利用现代波谱分析方法对化合物进行结构鉴定,采用滤纸片扩散法测定所得单体化合物的抗菌活性。 结果 对其发酵提取物进行TLC和HPLC分析,发现SAHA可以诱导该菌株产生丰富的次生代谢产物,并分离得到11个单体化合物,分别鉴定为:N'-phenyloctanediamide(1)、5-Phenylcarbamoyl-pentanoic(2)、ergosterol(3)、5,8-Epidioxy-5α,8α-ergosta-6,22E-diene-3β-ol(4)、1-monolinolein(5)、(4E,8E)-2-N-(2-Hydroxypalmitoyl)-1-O-(β-D-glucopyranosyl)-9-methyl-4,8-sphingadienine(6)、Ergosterol peroxide 3-O-β-D-glucopyranoside(7)、(22E,24R)-7,22-diene-3β,5α,6β-ergostatriol(8)、(2S,2'R,3R,4E,8E)-N-2'-Hydroxyhexadecanoyl-2-amion-9-methyl-4,8-octadecadiene-1,3-diol(9)、Adenosine(10)、D-Glulopyranose(11)。化合物1和2是SAHA的衍生物,推测是CDB9-31特殊的代谢环境引起了SAHA的生物转化,除了化合物3,其余化合物均为首次从该属中分离得到。抗菌活性结果显示,其中有6个化合物至少对一种病原菌表现出了不同程度的抑制作用。 结论 本研究丰富了虫生真菌Samsoniella hepiali次生代谢产物化学多样性。 -
关键词:
- 表观遗传修饰 /
- 虫生真菌 /
- Samsoniella hepiali /
- 次生代谢产物 /
- 抗菌活性
Abstract:Objective To analyze the effects of the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) on the secondary metabolites of the entomopathogenic fungus Samsoniella hepiali CDB9-31 using thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC). Methods The fermentation products of Samsoniella hepiali CDB9-31 treated with epigenetic modifiers were separated and purified using methods such as silica gel column chromatography, Sephadex column chromatography and reversed-phase column chromatography. The structures of the compounds were elucidated using modern spectroscopic analysis methods. The antimicrobial activity of the obtained monomeric compounds was determined using the filter paper disc diffusion method. Results The TLC and HPLC analyses of its fermentation extracts revealed that SAHA could induce the strain to produce more diverse array of secondary metabolites, and 11 monomeric compounds were isolated and identified as follows: N'-phenyloctanediamide (1), 5-Phenylcarbamoyl-pentanoic (2), ergosterol (3), 5, 8-Epidioxy-5α, 8α-ergosta-6, 22E-diene-3β-ol (4), 1-monolinolein (5), (4E, 8E)-2-N-(2-Hydroxypalmitoyl)-1-O-(β-D-glucopyranosyl)-9-methyl-4, 8-sphingadienine (6), Ergosterol peroxide 3-O-β-D-glucopyranoside (7), (22E, 24R)-7, 22-diene-3β, 5α, 6β-ergostatriol (8), (2S, 2'R, 3R, 4E, 8E)-N-2'-Hydroxyhexadecanoyl-2-amion-9-methyl-4, 8-octadecadiene-1, 3-diol (9), Adenosine (10), D-Glulopyranose (11). Compounds 1 and 2 were derivatives of SAHA, and it was speculated that the special metabolic environment of CDB9-31 caused the biotransformation of SAHA. Except for compound 3, all other compounds were isolated from this genus for the first time. The antibacterial activity results showed that six of these compounds exhibited varying degrees of inhibitory effects against at least one pathogenic bacterium. Conclusion This study has enriched the chemical diversity of secondary metabolites from the entomopathogenic fungus Samsoniella hepiali. -
表 1 Samsoniella hepiali CDB9-31代谢产物的抑菌活性
Table 1. Antimicrobial activities of secondary metabolites isolated from Samsoniella hepiali CDB9-31
指示菌 抑菌圈直径 (mm) 1 2 4 5 7 8 Kan Van Amp AmB B. subtilis ATCC 9372 - - - - - - # # 35.20 # S. aureus ATCC 6538 - - - - - - # # 20.00 # S. pneumoniae BNCC 338425 - - - - - 13.00 # # 21.20 # E. coli ATCC 25922 - - - - - - 16.30 # # # P. aeruginosa ATCC 15442 - - - - - - 25.00 # # # A. baumanii ATCC 19606 - - 8.00 - - - 18.30 # # # K. pneumoniae ATCC 13883 - 6.50 - - - - 15.10 # # # H. influenza ATCC 10211 - - 8.10 - - - 18.00 # # # S. typhimurium ATCC 14028 10.00 8.00 10.20 - 7.00 - 15.00 # # # MRSA 43300 - - - - - - # 16.00 # # C. albicans ATCC 14053 - - - 8.00 - - # # # 16.00 Drug-resistant C. albicans 23# - - - - - - # # # 10.00 注:抑菌圈直径包含滤纸片直径(6.00 mm);“-”代表在给药剂量下无抗菌活性;“#”表示未进行该项测试;Kan:卡那霉素;Van:万古霉素;Amp:氨苄青霉素;AmB:两性霉素B。 表 2 化合物1~2、4~5和7~8的最小抑菌浓度
Table 2. The minimum inhibitory concentrations of compounds 1~ 2,4~ 5,and 7~ 8
指示菌 化合物MIC(μg/mL) 1 2 4 5 7 8 Kan Amp AmB S. pneumoniae BNCC 338425 # # # # # 128 # 8 # K. pneumoniae ATCC 13883 # 512 # # # # 64 # # H. influenza ATCC 10211 # # 128 # # # 32 # # S. typhimurium ATCC 14028 512 > # # > # 8 # # C. albicans ATCC 14053 # # # 512 # # # # 16 注:“>”代表MIC大于512 μg/mL;“#”表示未进行该项测试;Kan:卡那霉素;Amp:氨苄青霉素;AmB:两性霉素B。 -
[1] 王记祥, 马良进. 虫生真菌在农林害虫生物防治中的应用[J]. 浙江林学院学报, 2009, 26(2): 286-291. [2] 邵颖, 丁婷, 葛飞, 等. 40 株虫生真菌发酵液对白色念珠菌的抑制作用[J]. 安徽农业大学学报, 2005, 32(3): 328-331. doi: 10.3969/j.issn.1672-352X.2005.03.013 [3] 赵璇, 田雨航, 庞道然, 等. 蛹虫草的化学成分、药理作用及产业化研究进展[J]. 中草药, 2024, 55(7): 2413-2422. [4] Keller N P. Fungal secondary metabolism: regulation, function and drug discovery[J]. Nat Rev Microbiol, 2019, 17(3): 167-180. doi: 10.1038/s41579-018-0121-1 [5] Bharatiya P, Rathod P, Hiray A, et al. Multifarious elicitors: Invoking biosynthesis of various bioactive secondary metabolite in fungi[J]. Appl Biochem Biotechnol, 2021, 193(3): 668-686. doi: 10.1007/s12010-020-03423-6 [6] Ma X, Wei X, Xing S, et al. Polyketide production in a mangrove-associated fungus Diaporthe goulteri, induced by chemical epigenetic modification[J]. Nat Prod Res, 2024, 38(1): 1-9. doi: 10.1080/14786419.2022.2102627 [7] Teigo A, Tohru T, Takashi Y, et al. Structures of spiroindicumides A and B, unprecedented carbon skeletal spirolactones, and determination of the absolute configuration by vibrational circular dichroism exciton approach[J]. Org Lett, 2013, 15(17): 4320-4323. doi: 10.1021/ol401741z [8] 王晓洋, 龚倩玉, 许言超, 等. 5-氮杂胞苷诱导内生真菌Penicillium sp. GZWMJZ-042代谢抗α-葡萄糖苷酶活性产物研究[J]. 中国抗生素杂志, 2023, 48(10): 1118-1125. doi: 10.3969/j.issn.1001-8689.2023.10.004 [9] Wang Y, Wang Z Q, Thanarut C, et al. Phylogeny and species delimitations in the economically, medically, and ecologically important genus Samsoniella (Cordycipitaceae, Hypocreales)[J]. MycoKeys, 2023, 99(99): 227-250. [10] Toshe R, Khalid J S, Kemkuignou M B, et al. Antibiofilm and cytotoxic metabolites from the entomopathogenic fungus Samsoniella aurantia.[J]. Beilstein J Org Chem, 2025, 21(23): 327-339. [11] Zhang K T, Huang Z P, Xu X R, et al. Two new diketopiperazines from the Cordyceps fungus Samsoniella sp. XY4[J]. The Journal of Antibiotics, 2023, 76(12): 735-740. doi: 10.1038/s41429-023-00662-7 [12] 邵颖, 陈宏伟, 柴文波, 等. 蛹拟青霉发酵液中抑菌活性成分的分离纯化[J]. 食品科学, 2012, 33(23): 116-120. [13] Ma J T, Dong X Y, Li Z H, et al. Antibacterial metabolites from kiwi endophytic fungus Fusarium tricinctum, a potential biocontrol strain for kiwi canker disease[J]. J Agric Food Chem, 2023, 71(20): 7679-7688. doi: 10.1021/acs.jafc.3c00233 [14] 崔乃菠, 马境谦, 余河水, 等. 基于刃天青微平板法检测金黄色葡萄球菌对天然产物的敏感性[J]. 天津中医药大学学报, 2022, 41(6): 774-779. [15] Shi X, Li X, He X, et al. Chemical epigenetic regulation secondary metabolites derived from Aspergillus sydowii DL1045 with inhibitory activities for protein tyrosine phosphatases[J]. Molecules, 2024, 29(3): 670. doi: 10.3390/molecules29030670 [16] Alektiar S N, Han J, Dang Y, et al. Radical hydrocarboxylationof unactivated alkenes via photocatalytic formate activation[J]. J Am Chem Soc, 2023, 145(20): 10991-10997. doi: 10.1021/jacs.3c03671 [17] 丁允章, 孔黎春, 张奕嘉, 等. 紫色红曲霉Mp-21次级代谢产物抗氧化及抑制α-葡萄糖苷酶活性的研究[J]. 微生物学报, 2022, 62(1): 103-118. [18] Sonfack F H D, Nanfack D A R, Tchuente T M A, et al. A new ceramide and other constituents from the fruits of Ficus lutea Vahl (Moraceae) and their chemotaxonomic significance[J]. Biochem Syst Ecol, 2023, 106(1): 104573. [19] Zhang M M, Wang D, Lu F, et al. Identification of the active substances and mechanisms of ginger for the treatment of colon cancer based on network pharmacology and molecular docking[J]. BioData Min, 2021, 14(1): 1-16. doi: 10.1186/s13040-020-00232-9 [20] 吴杰, 陈丹丹, 邓小林, 等. 海南红树林内生真菌Endomelanconiopsis endophytica次级代谢产物及其体外生物活性[J]. 广东海洋大学学报, 2023, 43(4): 27-33. [21] Li X, Gao J, Chen H, et al. Toxins from a symbiotic fungus, leptographium qinlingensis associated with dendroctonus armandi and their in vitro toxicitiesto pinus armandi seedlings[J]. Eur J Plant Pathol, 2012, 134(2): 239-247. doi: 10.1007/s10658-012-9981-9 [22] 陈招莉, 符大天, 张蕾, 等. 暗褐网柄牛肝菌化学成分及其抗肿瘤细胞毒活性[J]. 中成药, 2023, 45(10): 3296-3301. [23] Qiu Y Z, Zhu Y Q, Lu H, et al. Secondary metabolites from the marine-derived fungus Penicillium chrysogenum Y20-2, and their pro-angiogenic activity[J]. Z Naturforsch C J Biosci, 2023, 8(9-10): 345-352. [24] 季宇彬, 郑婷, 尹姣定. 中国南海海绵Cinachyrella sp. 的化学成分研究[J]. 中国海洋药物, 2018, 37(2): 7-12. [25] Jie F, Danyang Z, Qiannan X, et al. A new phenolic glycoside from Trollius chinensis Bunge with anti-inflammatory and antibacterial activities[J]. Nat Prod Res, 2020, 36(13): 1-8. [26] Singh V R. Enzymatic catalysts for hydroxamic acid formation: A mini‐review[J]. ChemBioEng Revi, 2023, 11(2): 339-347. [27] Bhatiya R K, Bhatiya S K, Meta P K, et al. Production and characterization of acyl transfer activity of amidase from Alcaligenes sp. MTCC 10674 for synthesis of hydroxamic acids[J]. J Microbiol Biotechnol, 2013, 40(1): 21-27. [28] Vikram R S, Hitesh S, Ananta G, et al. Novel amidase catalyzed process for the synthesis of vorinostat drug[J]. J Appl Microbiol, 2020, 129(6): 1589-1597. doi: 10.1111/jam.14753 [29] Takei T, Yoshida M, Ohnishi Kameyama M, et al. Ergosterol peroxide, an apoptosis-inducing component isolated from sarcodon aspratus (Berk.) S. Ito[J]. Biosci Biotechnol Biochem, 2005, 69(1): 212-215. doi: 10.1271/bbb.69.212 [30] Shi X W, Li X J, Gao J M, et al. Fasciculols H and I, two lanostane derivatives from chinese mushroom naematoloma fasciculare[J]. Chem Biodivers, 2011, 8(10): 1864-1870. doi: 10.1002/cbdv.201000203 [31] Bok J W, Lermer L, Chilton J, et al. Antitumor sterols from the mycelia of cordyceps sinensis[J]. Phytochemistry, 1999, 51(7): 891-898. doi: 10.1016/S0031-9422(99)00128-4 [32] 汪煜. 广义虫草分离鉴定及腺苷与虫草素的含量研究 [D]. 扬州: 扬州大学, 2024. -





 下载:
下载: