The Relationship between Peripheral Blood Immunoglobulin,Erythrocyte Sedimentation Rate,Homocysteine and the Degree of Central Nervous System Vasculitis in Children and Their Influence on Prognosis
-
摘要:
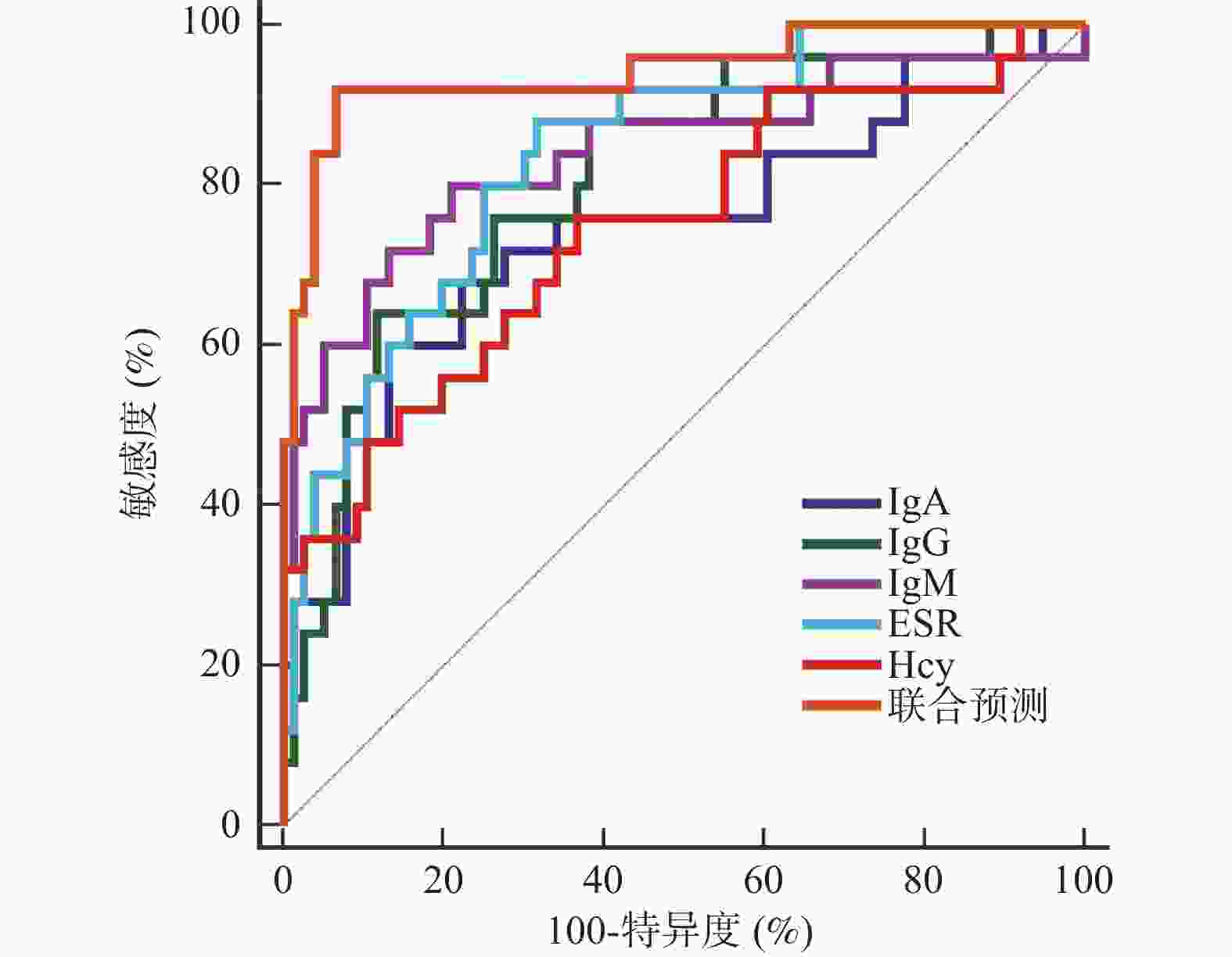
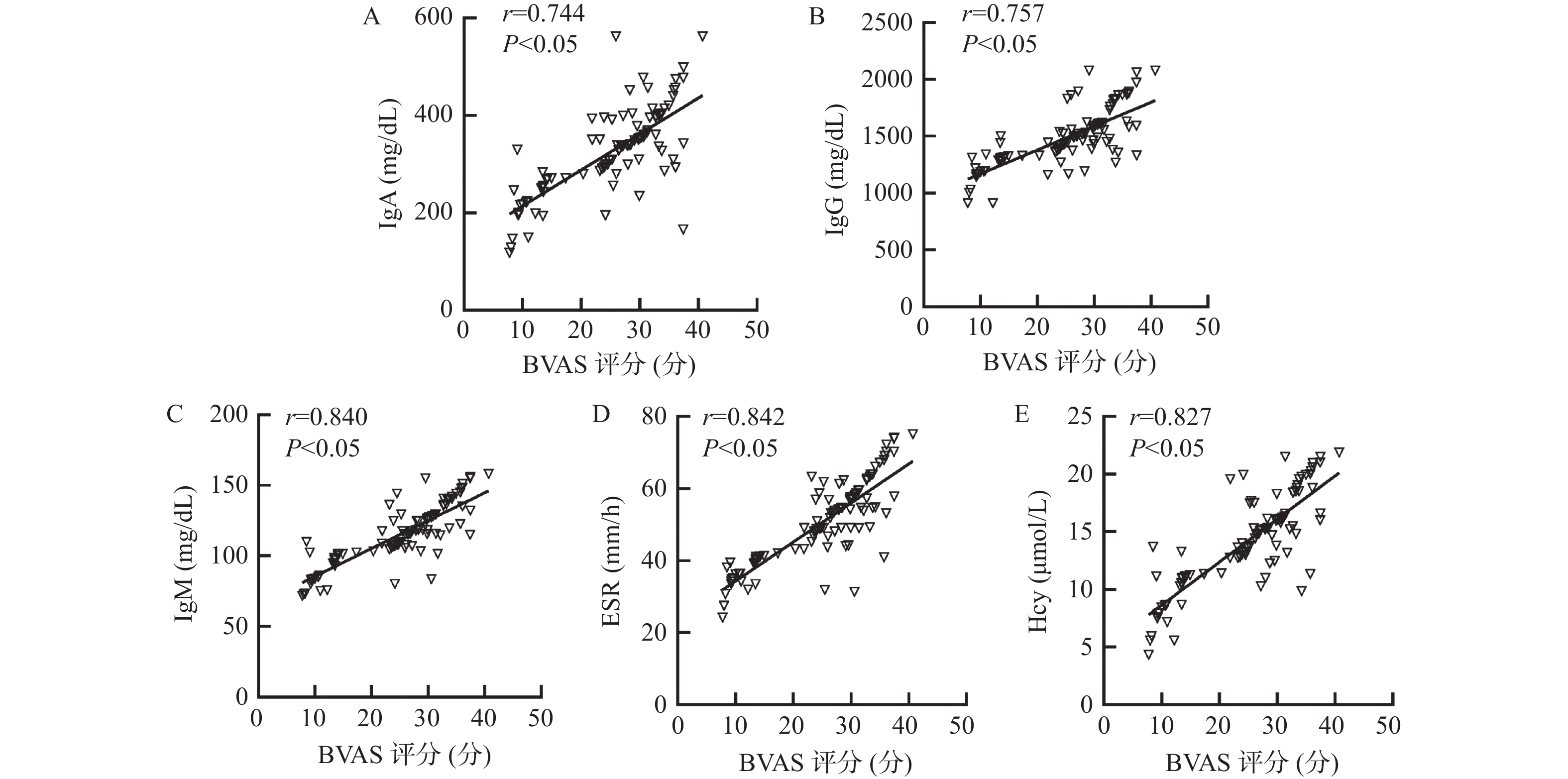
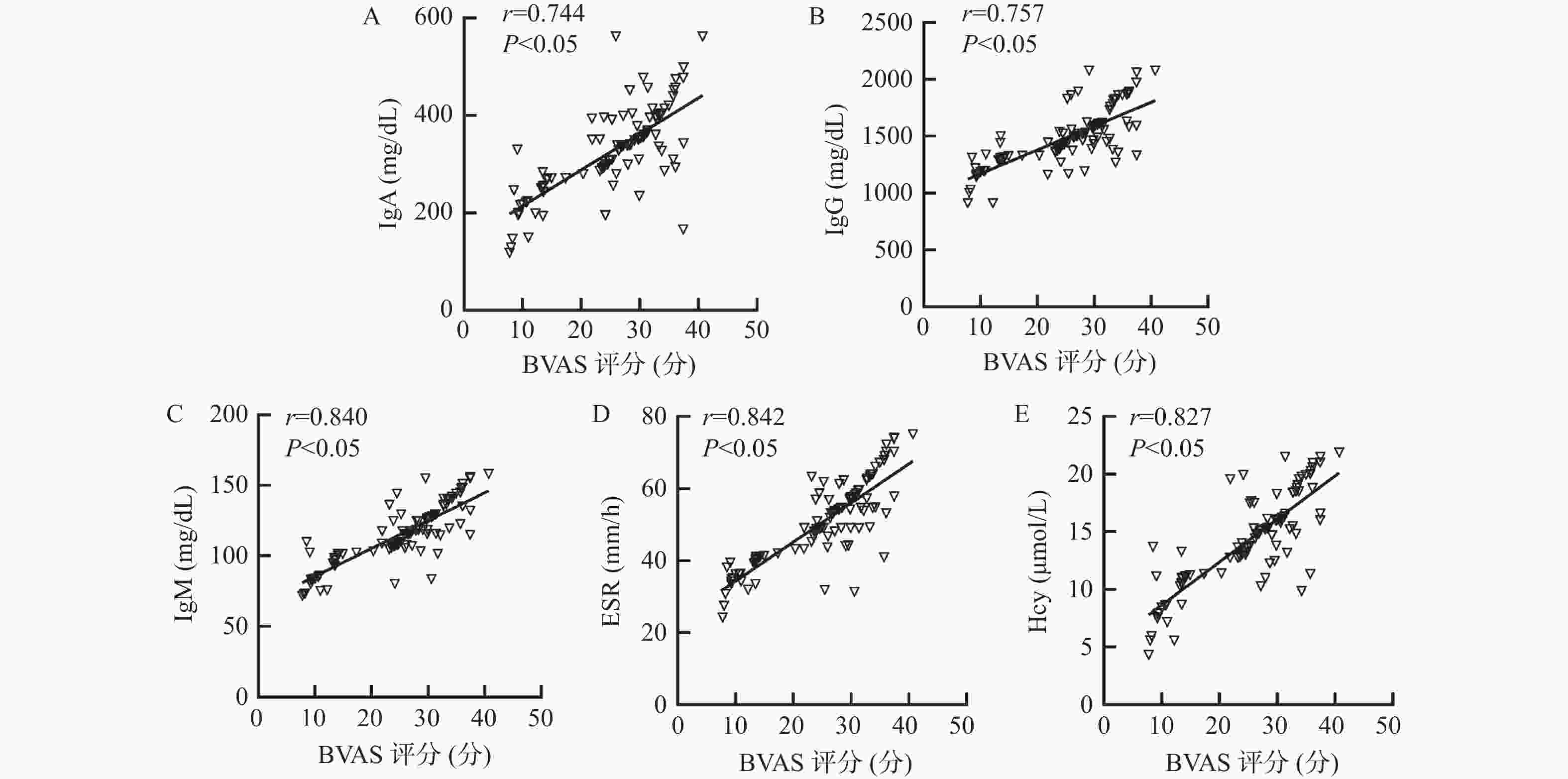
目的 探讨外周血免疫球蛋白、血沉(erythrocyte sedimentation rate,ESR)、同型半胱氨酸(homocysteine,Hcy)与儿童中枢神经系统血管炎(central nervous system vasculitis,CNSV)病情程度的关系及对预后的影响。 方法 选取2018年2月至2023年2月CNSV患儿103例作为研究组,另选取健康体检儿童103例作为对照组。比较2组外周血免疫球蛋白A(immunoglobulin A,IgA)、免疫球蛋白G(immunoglobulin G,IgG)、免疫球蛋白M(immunoglobulin M,IgM)、ESR、Hcy水平,评价各指标与CNSV病情程度[伯明翰血管炎疾病活动性评分(birmingham vasculitis disease activity score,BVAS)]的关系及其对预后的预测价值。 结果 研究组患儿外周血IgA、IgG、IgM、ESR和Hcy水平高于对照组的健康儿童(P < 0.05);疾病活动期患儿BVAS评分、外周血IgA、IgG、IgM、ESR和Hcy水平高于疾病非活动期患儿(P < 0.05);外周血IgA、IgG、IgM、ESR和Hcy与CNSV患儿BVAS评分呈正相关(P < 0.05);随访6个月,失访2例。CNSV患儿中预后良好患儿76例,预后不良患儿25例。预后不良患儿病程长于预后良好患儿,BVAS评分、外周血IgA、IgG、IgM、ESR和Hcy水平高于预后良好患儿(P < 0.05);将其他因素校正前后,外周血IgA、IgG、IgM、ESR和Hcy均是CNSV患儿预后的独立影响因素(P < 0.05);外周血IgA、IgG、IgM、ESR和Hcy预测CNSV患儿预后的的曲线下面积(area under the curve,AUC)分别为0.747、0.808、0.841、0.839、0.746,最佳截断值分别为350.58 mg/dL、 1513.06 mg/dL、124.84 mg/dL、51.22 mm/h、13.66 μmol/L;外周血IgA、IgG、IgM、ESR和Hcy联合预测CNSV患儿预后的AUC为0.943(95%CI:0.878~0.979),敏感度为92.00%,特异度为93.42%,优于各指标单独预测。结论 外周血IgA、IgG、IgM、ESR和Hcy与CNSV病情程度呈正相关,异常高表达会增加预后不良风险,联合预测价值可靠。 Abstract:Objective To investigate the relationship between peripheral blood immunoglobulin, erythrocyte sedimentation rate (ESR), homocysteine (Hcy) and the severity of central nervous system vasculitis (CNSV) in children, as well as its impact on prognosis. Methods A total of 103 children with CNSV from February 2018 to February 2023 were selected as the study group, and 103 healthy children as the control group. The peripheral blood levels of immunoglobulin A (IgA), immunoglobulin G (IgG), immunoglobulin M (IgM), ESR and Hcy were compared between the 2 groups to evaluate the relationship between each index and the degree of CNSV disease [Birmingham vasculitis disease activity score (BVAS)] and its predictive value for prognosis. Results The levels of peripheral blood IgA, IgG, IgM, ESR and Hcy in the study group were higher than those in the control group of healthy children (P < 0.05); the BVAS scores and the levels of peripheral blood IgA, IgG, IgM, ESR and Hcy in children with active disease were higher than those in children with inactive disease (P < 0.05); the levels of peripheral blood IgA, IgG, IgM, ESR and Hcy were positively correlated with the BVAS scores in children with CNSV (P < 0.05); two cases were lost to follow-up after 6 months. Among the children with CNSV, 76 had good prognosis and 25 poor prognosis. The levels of peripheral blood IgA, IgG, IgM, ESR and Hcy in children with poor prognosis were higher than those in children with good prognosis (P < 0.05); before and after correcting for other factors, peripheral blood IgA, IgG, IgM, ESR and Hcy were all independent factors affecting the prognosis of children with CNSV (P < 0.05); the area under curve (AUC) of peripheral blood IgA, IgG, IgM, ESR and Hcy for predicting the prognosis of children with CNSV was 0.747, 0.808, 0.841, 0.839, and 0.746, respectively, with optimal cutoff values of 350.58 mg/dL, 1513.06 mg/dL, 124.84 mg/dL, 51.22 mm/h, and 13.66 μmol/L, respectively; the AUC of peripheral blood IgA, IgG, IgM, ESR and Hcy for jointly predicting the prognosis of children with CNSV was 0.943 (95%CI 0.878-0.979), with a sensitivity of 92.00% and a specificity of 93.42%, which was superior to individual prediction of each indicator.Conclusion Peripheral blood IgA, IgG, IgM, ESR and Hcy are positively correlated with the severity of CNSV. Abnormally high expression increases the risk of poor prognosis, and the combined predictive value is reliable. -
表 1 2组外周血IgA、IgG、IgM、ESR和Hcy水平比较($\bar x \pm s $)
Table 1. Comparison of peripheral blood IgA,IgG,IgM,ESR and Hcy levels between the two groups ($\bar x \pm s $)
组别 n IgA(mg/dL) IgG(mg/dL) IgM(mg/dL) ESR(mm/h) Hcy(μmol/L) 研究组 103 306.25 ± 75.63 1428.19 ± 402.15112.35 ± 26.11 48.67 ± 10.49 13.82 ± 3.58 对照组 103 215.38 ± 48.57 1218.77 ± 236.3884.62 ± 15.74 12.75 ± 3.22 8.16 ± 2.13 t 10.260 4.556 9.231 33.222 13.789 P <0.001* <0.001* <0.001* <0.001* <0.001* *P < 0.05。 表 2 不同病情程度患儿外周血IgA、IgG、IgM、ESR和Hcy水平比较($\bar x \pm s $)
Table 2. Comparison of levels of IgA,IgG,IgM,ESR and Hcy in peripheral blood of children with different disease levels ($\bar x \pm s $)
组别 n IgA(mg/dL) IgG(mg/dL) IgM(mg/dL) ESR(mm/h) Hcy(μmol/L) BVAS评分(分) 疾病活动期患儿 72 332.81±70.13 1476.67 ±212.64121.51±22.38 52.86±10.06 15.42±3.17 27.64±4.82 疾病非活动期患儿 31 244.56±52.60 1315.59 ±175.9491.08±16.29 38.94±8.75 10.10±2.58 11.52±2.41 t 6.280 3.704 6.824 6.688 8.236 17.659 P <0.001* <0.001* <0.001* <0.001* <0.001* <0.001* *P < 0.05。 表 3 不同预后患儿基线资料比较($\bar x \pm s $)
Table 3. Comparison of baseline data in children with different prognoses ($\bar x \pm s $)
组别 n 年龄(岁) 性别(男/女) 体重(kg) 病程(月) CNSV类型 原发性 继发性 预后不良患儿 25 8.21±1.26 16/9 36.27±3.12 10.41±2.31 5(20.00) 20(80.00) 预后良好患儿 76 8.24±1.18 51/25 36.46±3.07 5.28±1.16 17(22.37) 59(77.63) χ2/t 0.108 0.081 0.267 14.603 0.062 P 0.914 0.776 0.790 <0.001* 0.804 *P < 0.05。 表 4 不同预后患儿基线资料、外周血IgA、IgG、IgM、ESR和Hcy水平比较($\bar x \pm s $)
Table 4. Comparison of baseline data and peripheral blood IgA,IgG,IgM,ESR and Hcy levels in children with different prognoses($\bar x \pm s $)
组别 n BVAS评分(分) IgA(mg/dL) IgG(mg/dL) IgM(mg/dL) ESR(mm/h) Hcy(μmol/L) 预后不良患儿 25 30.07±4.12 362.27±73.19 1562.77 ±211.47130.40±25.81 56.68±10.35 16.13±3.20 预后良好患儿 76 20.39±2.20 287.82±52.43 1383.92 ±165.31106.41±20.46 46.04±8.72 13.06±2.14 t 15.051 5.553 4.368 4.756 5.048 5.458 P <0.001* <0.001* <0.001* <0.001* <0.001* <0.001* *P < 0.05。 表 5 外周血IgA、IgG、IgM、ESR和Hcy对预后的影响
Table 5. The impact of peripheral blood IgA、IgG、IgM、ESR and Hcy on prognosis
自变量 校正前 校正后 OR 95%CI P OR 95%CI P 病程 1.849 1.081~3.162 − − − IgA 1.672 1.224~2.285 0.000* 1.904 1.336~2.714 <0.001* IgG 1.572 1.217~2.031 0.000* 1.780 1.258~2.519 <0.001* IgM 1.762 1.159~2.679 0.000* 1.822 1.183~2.806 <0.001* ESR 1.429 1.046~1.952 0.000* 1.525 1.132~2.055 <0.001* Hcy 1.444 1.130~1.846 0.000* 1.599 1.296~1.974 <0.001* *P < 0.05。 -
[1] Rice C M,Scolding N J. The diagnosis of primary central nervous system vasculitis[J]. Pract Neurol,2020,20(2):109-114. doi: 10.1136/practneurol-2018-002002 [2] Ota Y,Srinivasan A,Capizzano A A,et al. Central nervous system systemic lupus erythematosus: Pathophysiologic,clinical,and imaging features[J]. Radiographics,2022,42(1):212-232. doi: 10.1148/rg.210045 [3] Malani Shukla N,Lotze T E,Muscal E. Inflammatory diseases of the central nervous system[J]. Neurol Clin,2021,39(3):811-828. doi: 10.1016/j.ncl.2021.04.004 [4] Neumann T. Update on immunoglobulin a vasculitis[J]. Z Rheumatol,2022,81(4): 305-312. [5] Lapić I,Padoan A,Bozzato D,et al. Erythrocyte sedimentation rate and C-Reactive protein in acute inflammation[J]. Am J Clin Pathol,2020,153(1):14-29. doi: 10.1093/ajcp/aqz142 [6] Tawfik A,Elsherbiny N M,Zaidi Y,et al. Homocysteine and age-Related central nervous system diseases: Role of inflammation[J]. Int J Mol Sci,2021,22(12):6259. doi: 10.3390/ijms22126259 [7] 中国医师协会儿科医师分会风湿免疫学组. 中国儿童血管炎诊断与治疗系列专家共识之一——总论[J]. 中国实用儿科杂志,2023,38(4):241-247. [8] Mukhtyar C,Lee R,Brown D,et al. Modification and validation of the Birmingham vasculitis activity score (version 3)[J]. Ann Rheum Dis,2009,68(12):1827-1832. doi: 10.1136/ard.2008.101279 [9] Nehme A,Boulanger M,Aouba A,et al. Diagnostic and therapeutic approach to adult central nervous system vasculitis[J]. Rev Neurol (Paris),2022,178(10):1041-1054. doi: 10.1016/j.neurol.2022.05.003 [10] Hajj-Ali R A,Calabrese L H. Central nervous system vasculitis: Advances in diagnosis[J]. Curr Opin Rheumatol,2020,32(1):41-46. doi: 10.1097/BOR.0000000000000676 [11] Amin M,Uchino K,Hajj-Ali R A. Central nervous system vasculitis: Primary angiitis of the central nervous system and central nervous system manifestations of systemic vasculitis[J]. Rheum Dis Clin North Am,2023,49(3):603-616. doi: 10.1016/j.rdc.2023.03.011 [12] Marriaga-N ú ñez B,Arellano-Valdez A,Paz J P A,et al. Immunoglobulin-resistant Kawasaki disease[J]. Bol Med Hosp Infant Mex,2023,80(4): 260-264. [13] 丁小娟,倪瑞钟,王玎,等. 抗中性粒细胞细胞质抗体相关性血管炎患者血清IgG4表达水平及临床意义[J]. 现代医药卫生,2021,37(6):906-909. [14] Ito T,Fukui S,Kanie T,et al. Immunoglobulin G4-related coronary periarteritis: A systematic literature review with a case series[J]. Clin Rheumatol,2022,41(8):2281-2295. doi: 10.1007/s10067-022-06179-y [15] Fatima R,Acharya A,Bozorgnia F,et al. Sertraline-associated immunoglobulin a vasculitis[J]. Am J Ther,2022,29(4):484-486. doi: 10.1097/MJT.0000000000001516 [16] Shaikh K J,Osio V A,Leeflang M M,et al. Procalcitonin,C-reactive protein,and erythrocyte sedimentation rate for the diagnosis of acute pyelonephritis in children[J]. Cochrane Database Syst Rev,2020,9(9):CD009185. [17] 何冬,喻杉,陈露. 急性骨髓炎患者血清红细胞沉降率、白细胞、降钙素原和白细胞介素-6水平变化及意义研究[J]. 陕西医学杂志,2023,52(6):709-713. [18] Ying P,Lu T,Xu Y,et al. Preoperative erythrocyte sedimentation rate in patients with rheumatoid arthritis predicts deep vein thrombosis following total knee arthroplasty[J]. Clin Hemorheol Microcirc,2022,81(1):23-31. doi: 10.3233/CH-211286 [19] 杨清,马玉华,何春容,等. 血液常规分析衍生炎性参数指标在抗中性粒细胞胞浆抗体相关性血管炎中的临床意义分析[J]. 标记免疫分析与临床,2022,29(8):1330-1335. [20] 杜莹珏,赖蓓,石婧,等. 系统性血管炎常用指标对疾病活动评估的意义[J]. 中国临床保健杂志,2014,17(2):113-115. [21] Smith A D,Refsum H. Homocysteine - from disease biomarker to disease prevention[J]. J Intern Med,2021,290(4):826-854. doi: 10.1111/joim.13279 [22] Silberstein R B,Pipingas A,Scholey A B. Homocysteine modulates brain functional connectivity in a memory retrieval task[J]. J Alzheimers Dis,2022,90(1):199-209. doi: 10.3233/JAD-220612 [23] 王玉明. 中枢神经系统特发性炎性脱髓鞘疾病患者血清尿酸、同型半胱氨酸水平及其临床意义[J]. 广西医学,2020,42(3):302-304,370. [24] Akahoshi N,Kamichatani W,Ishii I. Homocysteine hypothesis on the impaired peripheral but not central nervous system oxytocin responses in cystathionine γ-lyase-deficient dam mice[J]. Biol Pharm Bull,2020,43(11):1810-1813. doi: 10.1248/bpb.b20-00676 -





 下载:
下载: