Expression of LMO2 and CAPRIN1 in Pancreatic Cancer Tissues and Their Correlation with Prognosis
-
摘要:
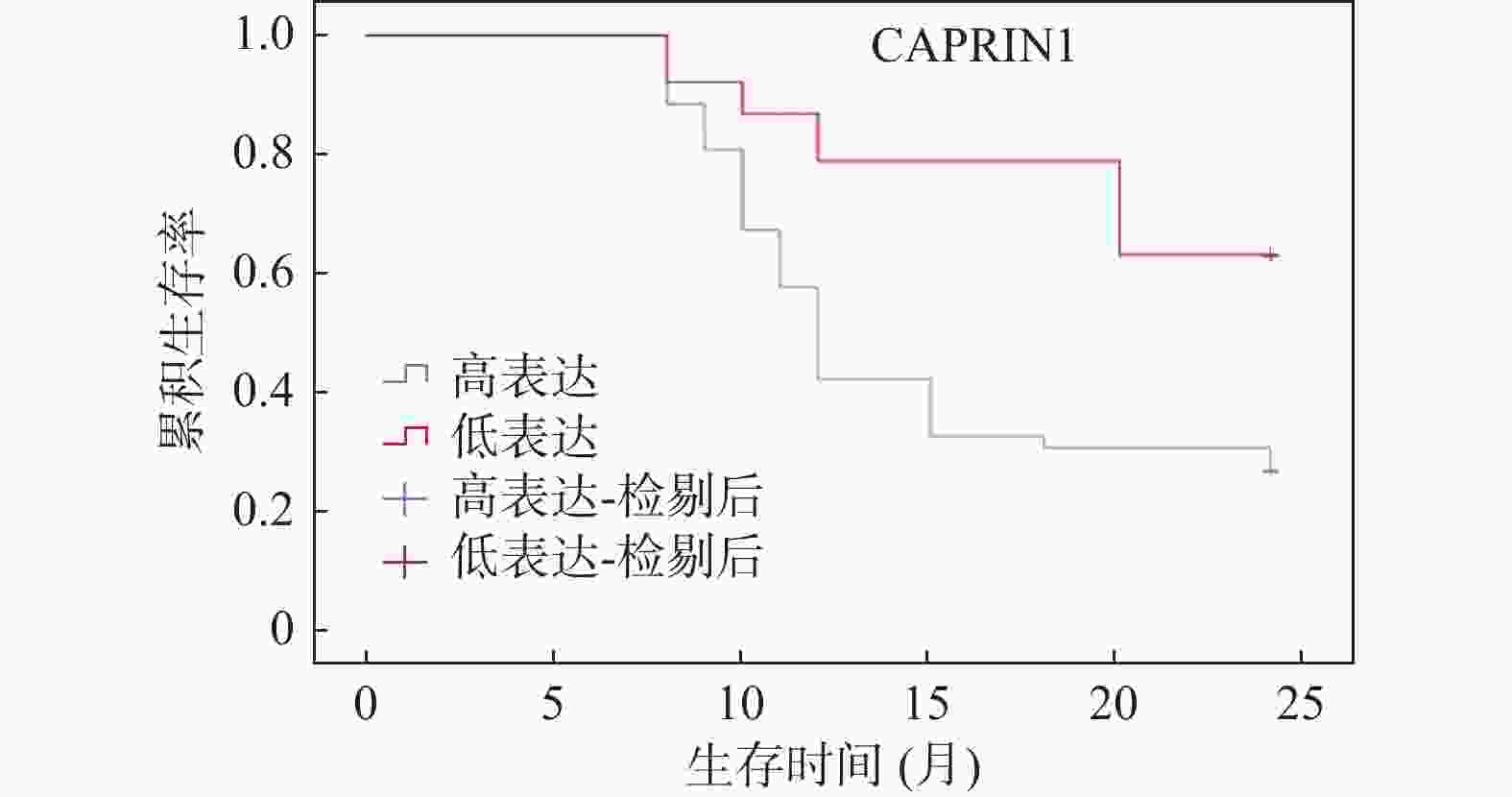
目的 探讨胰腺癌组织中LIM-only蛋白2(LIM domain only 2,LMO2)和细胞周期相关蛋白1(cell cycle associated protein 1,CAPRIN1)的表达特点及其与临床病理特征和预后的关系,同时通过体外敲低实验验证其对胰腺癌细胞功能的影响。 方法 选取2015年8月至2018年3月邢台市中心医院收治并经病理确诊的90例胰腺癌患者,采集癌组织标本作为病例组,同时以癌旁组织(距癌缘>2 cm,经病理学确认无癌浸润及异常病变)作为对照组。采用免疫组织化学方法检测LMO2和CAPRIN1的表达水平,并结合临床资料分析其与分化程度、淋巴结转移及TNM分期等临床病理特征的关系。随访2年,利用Kaplan-Meier方法分析生存情况,并采用Cox比例风险回归模型评估独立预后因素。体外实验中,选取PANC-1和BxPC-3细胞系,通过Lipofectamine 3000转染特异性siRNA敲低LMO2、CAPRIN1或联合敲低,采用qPCR和Western blot验证敲低效率,并通过CCK-8、EdU、划痕及Transwell实验评估细胞增殖和迁移能力。 结果 免疫组织化学结果显示,LMO2和CAPRIN1在胰腺癌组织中阳性表达率显著高于癌旁组织(P = 0.009;P = 0.012)。LMO2高表达与分化程度(P = 0.026)、淋巴结转移(P = 0.007)相关;CAPRIN1高表达与TNM分期(P = 0.019)、分化程度(P = 0.018)、淋巴结转移(P = 0.004)相关。相关性分析显示LMO2与CAPRIN1表达呈正相关(P = 0.013)。随访结果显示,LMO2高表达组和CAPRIN1高表达组患者生存率均显著低于低表达组(P = 0.015;P = 0.021)。多因素Cox回归分析提示,LMO2高表达(P = 0.031)、CAPRIN1高表达(P = 0.019)、TNMⅢ+Ⅳ期(P = 0.005)、低/中分化(P = 0.047)及淋巴结转移(P = 0.027)均为胰腺癌患者预后的独立危险因素。体外实验结果显示,敲低LMO2或CAPRIN1可显著抑制PANC-1和BxPC-3细胞增殖与迁移能力,联合敲低效果更显著(P < 0.001)。 结论 LMO2和CAPRIN1在胰腺癌组织中高表达,与不良临床病理特征及较差生存相关。体外功能实验进一步验证其在胰腺癌细胞增殖和迁移中的关键作用,提示LMO2和CAPRIN1可作为胰腺癌潜在预后标志物及治疗靶点。 Abstract:Objective To investigate the expression of LIM-only protein 2 (LMO2) and cell cycle-associated protein 1 (CAPRIN1) in pancreatic cancer tissues, and their correlation with clinicopathological features and prognosis, and to validate their effects on pancreatic cancer cell function through in vitro knockdown experiments. Methods Ninety pancreatic cancer patients admitted to Xingtai Central Hospital between August 2015 and March 2018 and pathologically confirmed were enrolled. Cancer tissue specimens were collected as the case group, while adjacent non-tumor tissue (>2 cm from the cancer margin, pathologically confirmed without cancer infiltration or abnormal lesions) served as the control group. Immunohistochemistry (IHC) was used to detect the expression levels of LMO2 and CAPRIN1, and their relationships with clinicopathological features including differentiation degree, lymph node metastasis, and TNM staging were analyzed in conjunction with clinical data. A two-year follow-up was conducted, and survival was analyzed using the Kaplan-Meier method, while independent prognostic factors were assessed with the Cox proportional hazards regression model.In vitro experiments, PANC-1 and BxPC-3 cell lines were selected, and specific siRNA targeting LMO2, CAPRIN1, or combined knockdown was performed using Lipofectamine 3000. Knockdown efficiency was verified by qPCR and Western blot, and cell proliferation and migration were assessed using CCK-8, EdU, wound healing, and Transwell assays. Results IHC results showed that the positive expression rates of LMO2 and CAPRIN1 were significantly higher in pancreatic cancer tissues than in adjacent tissues (P = 0.009; P = 0.012). High LMO2 expression was associated with differentiation degree (P = 0.026) and lymph node metastasis (P = 0.007), while high CAPRIN1 expression was significantly correlated with TNM staging (P = 0.019), differentiation degree (P = 0.018), and lymph node metastasis (P = 0.004). Correlation analysis revealed a positive correlation between LMO2 and CAPRIN1 expression (P = 0.013). Follow-up results showed that survival rates in patients with high LMO2 and high CAPRIN1 expression were significantly lower than in those with low expression (P = 0.015; P = 0.021). Multivariate Cox regression analysis indicated that high LMO2 expression (P = 0.031), high CAPRIN1 expression(P = 0.019), TNM stage III+IV (P = 0.005), poor/moderate differentiation (P = 0.047), and lymph node metastasis (P = 0.027) were all independent risk factors for prognosis in pancreatic cancer patients. In vitro experimental results showed that knockdown of LMO2 or CAPRIN1 significantly inhibited PANC-1 and BxPC-3 cell proliferation and migration ability, with combined knockdown showing more pronounced effects (P < 0.001). Conclusion High expression of LMO2 and CAPRIN1 in pancreatic cancer tissue is associated with adverse clinicopathological features and poor survival outcomes. In vitro functional experiments further verified their critical roles in pancreatic cancer cell proliferation and migration, suggesting that LMO2 and CAPRIN1 may serve as potential prognostic biomarkers and therapeutic targets for pancreatic cancer. -
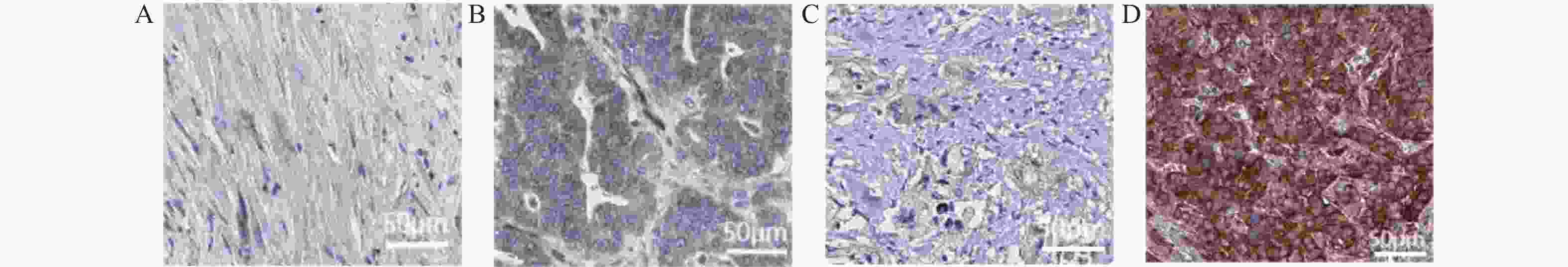
表 1 胰腺癌组织与癌旁组织中LMO2和CAPRIN1蛋白表达比较[n(%)]
Table 1. Comparison of LMO2 and CAPRIN1 protein expression in pancreatic cancer tissue and adjacent tissues [n(%)]
指标 组别 n - + ++ +++ 阳性率 χ2 P LMO2蛋白表达 病例组 90 25(27.78) 18(20.00) 27(30.00) 20(22.22) 65(72.22) 9.351 0.009** 对照组 90 42(46.67) 26(28.89) 13(14.44) 9(10.00) 48(53.33) CAPRIN1蛋白表达 病例组 90 23(25.56) 15(16.67) 31(34.44) 21(23.33) 67(74.44) 6.299 0.012* 对照组 90 39(43.33) 28(31.11) 13(14.44) 10(11.11) 51(56.67) *P < 0.05;**P < 0.01。 表 2 胰腺癌组织与癌旁组织中LMO2和CAPRIN1mRNA表达比较[M(P25,P75)]
Table 2. Comparison of LMO2 and CAPRIN1mRNA expression in pancreatic cancer tissue and adjacent normal tissue [M(P25,P75)]
指标 组别 n 含量 Z P LMO2 mRNA表达 病例组 90 4.85 (4.15,5.36) −11.593 <0.001*** 对照组 90 1.75 (1.63,2.29) CAPRIN1mRNA表达 病例组 90 4.62 (4.16,5.12) −11.595 <0.001*** 对照组 90 1.78 (1.48,2.18) ***P < 0.001。 表 3 胰腺癌细胞中LMO2和CAPRIN1 mRNA表达比较($ \bar x \pm s $,n = 3)
Table 3. Comparison of LMO2 and CAPRIN1 mRNA expression in pancreatic cancer cells ($ \bar x \pm s $,n = 3)
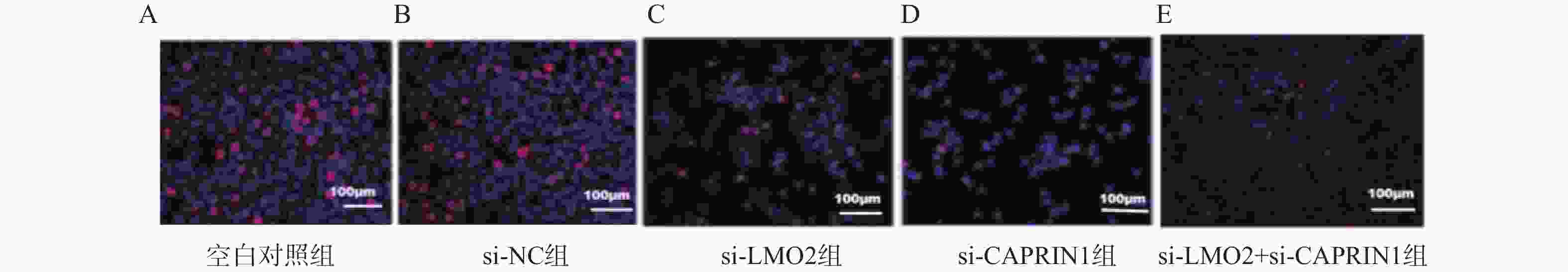
组别 LMO2 mRNA表达 CAPRIN1mRNA表达 空白对照组 1.03±0.02 1.02±0.01 si-NC组 1.00±0.02 1.00±0.04 si-LMO2组 0.57±0.03ΔΔΔ 1.00±0.02 si-CAPRIN1组 0.98±0.03 0.66±0.03ΔΔΔ si-LMO2 + si-CAPRIN1组 0.56±0.04ΔΔΔ 0.64±0.02ΔΔΔ F 206.30 185.31 P <0.001*** <0.001*** ***P < 0.001;与空白对照组相比,ΔΔΔP < 0.001 表 4 各组胰腺癌细胞增殖率($ \bar x \pm s $,n = 3)
Table 4. Proliferation rates of pancreatic cancer cells in different groups ($ \bar x \pm s $,n = 3)
组别 24 h OD450 48 h OD450 72 h OD450 EdU阳性率(%) 空白对照组 0.45±0.03 0.84±0.02 1.14±0.02 71.43±0.78 si-NC组 0.43±0.01 0.83±0.02 1.09±0.03 70.50±0.70 si-LMO2组 0.32±0.02ΔΔΔ 0.64±0.01ΔΔΔ 0.84±0.02ΔΔΔ 51.43±0.85ΔΔΔ si-CAPRIN1组 0.31±0.01ΔΔΔ 0.62±0.01ΔΔΔ 0.83±0.02ΔΔΔ 51.27±0.50ΔΔΔ si-LMO2 + si-CAPRIN1组 0.29±0.01ΔΔΔ 0.56±0.02ΔΔΔ 0.67±0.03ΔΔΔ 40.50±0.89ΔΔΔ F 65.96 203.22 248.14 952.81 P <0.001*** <0.001*** <0.001*** <0.001*** 与空白对照组相比,ΔΔΔP < 0.001;***P < 0.001。 表 5 各组胰腺癌细胞迁移能力比较($ \bar x \pm s $,n = 3)
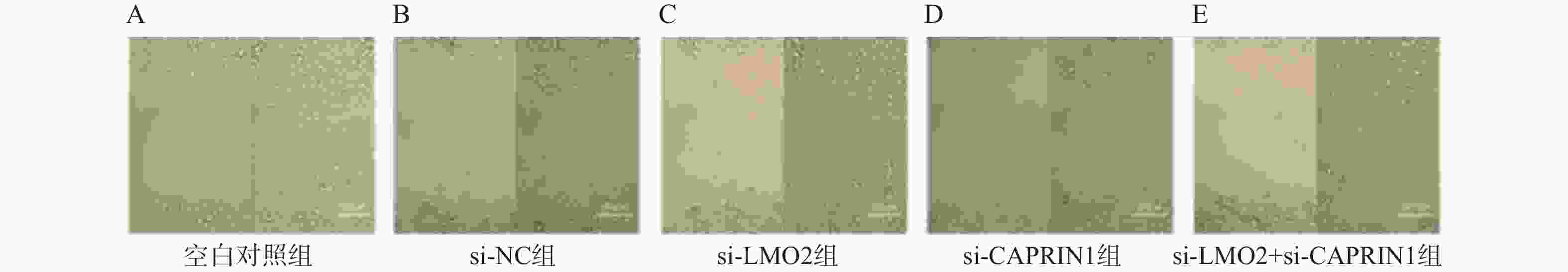
Table 5. Comparison of pancreatic cancer cell migration capacity among groups ($ \bar x \pm s $,n = 3)
组别 划痕愈合率(%) 迁移细胞数
(个/视野)空白对照组 84.43±0.90 168.67±3.06 si-NC组 84.23±0.65 161.67±2.52 si-LMO2组 57.67±1.31ΔΔΔ 105.33±3.06ΔΔΔ si-CAPRIN1组 57.20±1.51ΔΔΔ 101.67±2.52ΔΔΔ si-LMO2 + si-CAPRIN1组 41.57±0.95ΔΔΔ 81.67±3.06ΔΔΔ F 864.30 558.17 P <0.001*** <0.001*** 与空白对照组相比,ΔΔΔP < 0.001;***P < 0.001。 表 6 癌组织中LMO2、CAPRIN1表达水平与临床病理因素的关系[n(%)]
Table 6. Correlation between LMO2 and CAPRIN1 expression levels in cancer tissues and clinical-pathological factors [n(%)]
特征 n LMO2 χ2 P CAPRIN1 χ2 P 高表达(n = 47)
(++/+++)低表达(n = 43)
(-/+)高表达(n = 52)
(++/+++)低表达(n = 38)
(-/+)性别 男 47 25(53.19) 22(46.81) 0.037 0.847 29(61.70) 18(38.30) 0.621 0.431 女 43 22(51.16) 21(48.84) 23(53.49) 20(46.51) 年龄(岁) <60 38 18(47.37) 20(52.63) 0.621 0.431 20(52.63) 18(47.37) 0.714 0.398 ≥60 52 29(55.77) 23(44.23) 32(61.54) 20(38.46) 肿瘤位置 腺头 68 38(55.88) 30(44.12) 1.494 0.222 41(60.29) 27(39.71) 0.722 0.395 腺尾 22 9(40.91) 13(59.09) 11(50.00) 11(50.00) 肿瘤直径(cm) <5 51 30(58.82) 21(44.18) 1.734 0.188 32(62.75) 19(37.25) 1.190 0.275 ≥5 39 17(44.74) 21(55.26) 20(51.28) 19(48.72) TNM分期 Ⅰ+Ⅱ 51 28(54.90) 23(45.10) 0.339 0.561 24(47.06) 27(52.94) 5.523 0.019* Ⅲ+Ⅳ 39 19(48.72) 20(51.28) 28(71.79) 11(28.21) 分化程度 低分化 20 7(35.00) 13(65.00) 4.910 0.026* 10(50.00) 10(50.00) 5.530 0.018* 中分化 43 27(62.90) 16(37.21) 28(51.85) 15(34.88) 高分化 27 12(44.44) 15(55.56) 14(65.12) 13(48.15) 淋巴结是否转移 否 41 15(36.59) 26(63.41) 7.380 0.007ΔΔ 17(41.46) 24(58.54) 8.216 0.004## 是 49 32(65.31) 17(34.69) 35(71.43) 14(28.57) 与LMO2高表达组相比,ΔΔP < 0.01;与CAPRIN1高表达组相比,##P < 0.01;*P < 0.05。 表 7 癌组织中 LMO2和CAPRIN1表达相关性分析(n)
Table 7. Correlation analysis of LMO2 and CAPRIN1 expression in cancer tissues (n)
LMO2 CAPRIN1 总计 阴性 阳性 阴性 11 14 25 阳性 12 53 65 总计 23 67 90 表 8 Cox比例风险回归分析胰腺癌患者预后影响因素
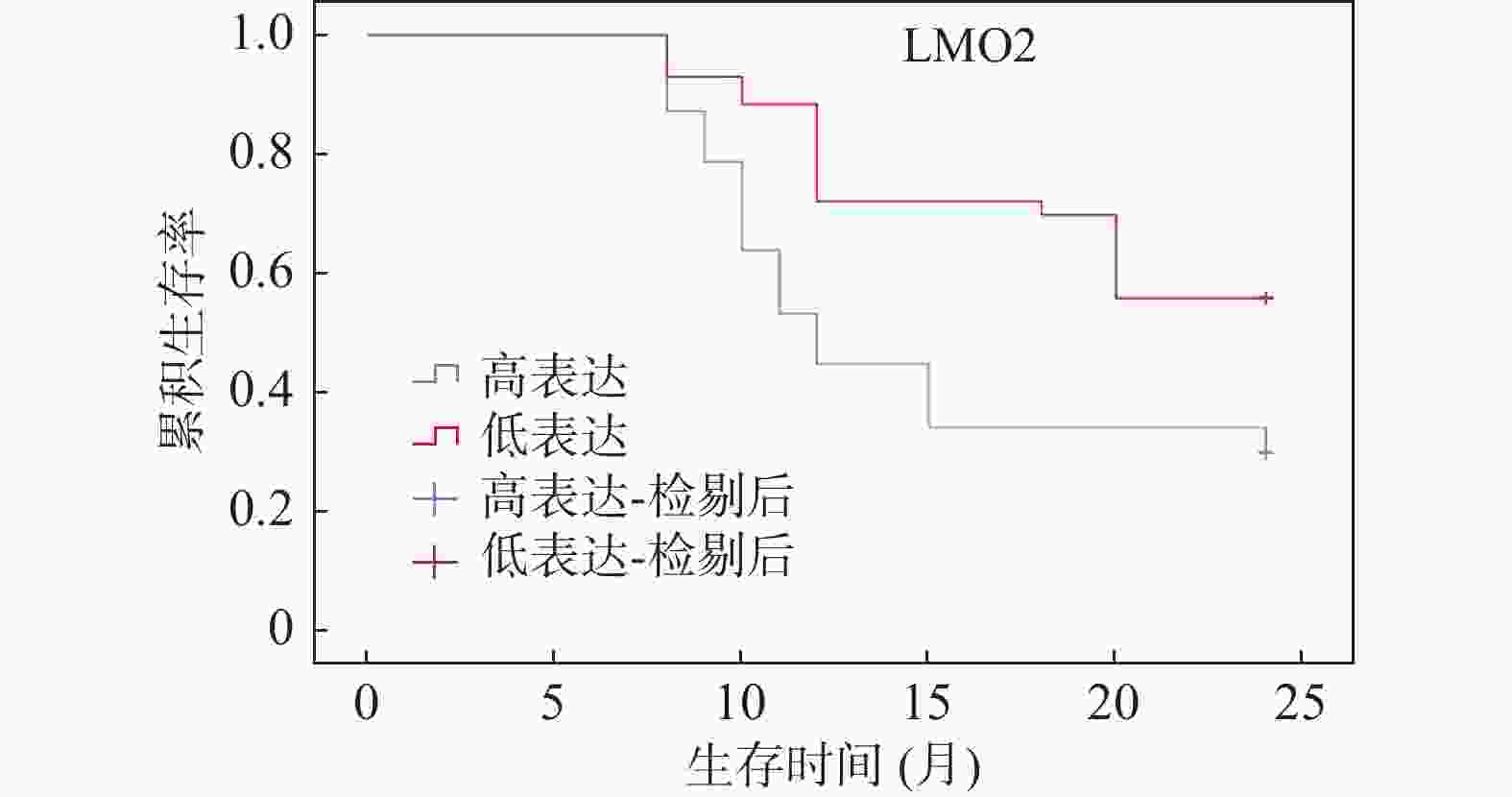
Table 8. Cox proportional hazards regression analysis of prognostic factors in pancreatic cancer patients
变量 β HR 95%CI Wald P LMO2高表达 0.576 1.78 1.05~3.01 4.67 0.031* CAPRIN1高表达 0.668 1.95 1.12~3.41 5.47 0.019* TNM分期(III+IV) 0.808 2.24 1.28~3.91 7.89 0.005** 分化程度(低/中) 0.525 1.69 1.01~2.85 3.96 0.047* 淋巴结转移(有) 0.602 1.83 1.07~3.15 4.89 0.027* 性别(男/女) 0.083 1.09 0.64~1.87 0.09 0.762 年龄(≥60岁/<60岁) 0.135 1.14 0.69~1.90 0.21 0.647 肿瘤位置(腺头/腺尾) 0.182 1.2 0.70~2.07 0.38 0.537 肿瘤直径(≥5 cm/<5 cm) 0.224 1.25 0.74~2.10 0.56 0.454 *P < 0.05;**P < 0.01。 -
[1] 王文婷, 彭钊, 邓祖亮, 等. 二甲双胍联合吉西他滨对胰腺癌细胞增殖、迁移及上皮间质转化的影响[J]. 山东医药, 2021, 61(10): 41-44. [2] 沈俊, 周锐, 王建祥. 性别决定相关基因簇9对胰腺癌干细胞增殖、侵袭的影响及其与吉西他滨耐药性的关系[J]. 中华实验外科杂志, 2020, 37(7): 1255-1258. [3] 董芳, 拜海涛, 王晓芳, 等. p18INK4C缺失通过激活LMO2加快Notch-1诱导的急性T淋巴细胞白血病进展[J]. 中国科学: 生命科学, 2017, 47(12): 1386-1396. [4] 吴鹏程, 李太平, 晋涛, 等. O~6-甲基鸟嘌呤-DNA-甲基转移酶、细胞周期相关蛋白1、F框/WD-40域蛋白7在脑胶质瘤组织中的表达情况及临床意义[J]. 癌症进展, 2020, 18(16): 1649-1652. [5] 倪泉兴, 虞先濬, 刘亮. 中国胰腺癌临床诊断标准的探讨[J]. 中国癌症杂志, 2012, 22(2): 81-87. [6] 张泉东, 金政锡, 郝迪斯. 磷脂酰肌醇蛋白多糖-1与趋化因子受体CXCR7在胰腺癌中的表达及临床意义[J]. 国际免疫学杂志, 2014, 37(2): 157-160. [7] Tseng Y A, Tamayo S, Abada E, et al. Interobserver agreement in scoring HER2-negative and HER2-low immunohistochemistry in breast cancer: Reasons for discordance and impact of a single training session[J]. Am J Clin Pathol, 2025, 164(2): 244-256. doi: 10.1093/ajcp/aqaf043 [8] Yuan M, Yu Y, Meng Y, et al. A dissected LMO2 functional analysis and clinical relevance in brain gliomas[J]. Biochem Biophys Rep, 2022, 33: 101406. [9] Latchmansingh K A, Wang X, Verdun R E, et al. LMO2 expression is frequent in T-lymphoblastic leukemia and correlates with survival, regardless of T-cell stage[J]. Mod Pathol, 2022, 35(9): 1220-1226. doi: 10.1038/s41379-022-01063-1 [10] Sikandar S S, Gulati G S, Antony J, et al. Identification of a minority population of LMO2(+) breast cancer cells that integrate into the vasculature and initiate metastasis[J]. Sci Adv, 2022, 8(45): eabm3548. doi: 10.1126/sciadv.abm3548 [11] Yu Y, Yuan M, Zhang Z, et al. Analysis of the effect of LMO2 on indicating CD8+ T-lymphocyte infiltration in pan-cancers[J]. Biochem Biophys Rep, 2024, 41: 101890. [12] Yang T, Huang L, Qin H, et al. STRESS granule-associated RNA-binding protein CAPRIN1 drives cancer progression and regulates treatment response in nasopharyngeal carcinoma[J]. Med Oncol, 2022, 40(1): 47. doi: 10.1007/s12032-022-01910-w [13] Yuan X, Yang X. CAPRIN1 transcriptionally activated PLPP4 to inhibit DOX sensitivity and promote breast cancer progression[J]. Cell Biochem Biophys, 2025, 83(2): 2035-2045. [14] Okano F, Saito T, Minamida Y, et al. Identification of membrane-expressed CAPRIN-1 as a novel and universal cancer target, and generation of a therapeutic anti-CAPRIN-1 antibody TRK-950[J]. Cancer Res Commun, 2023, 3(4): 640-658. doi: 10.1158/2767-9764.CRC-22-0310 [15] Gao Y, Yuan L, Ke C, et al. Caprin-1 plays a role in cell proliferation and Warburg metabolism of esophageal carcinoma by regulating METTL3 and WTAP[J]. J Transl Med, 2023, 21(1): 159. doi: 10.1186/s12967-023-04001-0 [16] Luo H L, Luo T, Liu J J, et al. Macrophage polarization-associated lnc-Ma301 interacts with caprin-1 to inhibit hepatocellular carcinoma metastasis through the Akt/Erk1 pathway[J]. Cancer Cell Int, 2021, 21(1): 422. doi: 10.1186/s12935-021-02133-1 [17] Zhang T, Han Y, Zou Y, et al. Dual roles of LMO2 in promoting tumor angiogenesis and progression[J]. Cell Investig, 2025, 1(3): 100024. doi: 10.1016/j.clnves.2025.100024 [18] Liu J, Niu L, Hao J, et al. circIPO7 dissociates caprin-1 from ribosomes and inhibits gastric cancer cell proliferation by suppressing EGFR and mTOR[J]. Oncogene, 2023, 42(13): 980-993. doi: 10.1038/s41388-023-02610-z [19] Yang J, Xu R, Wang C, et al. Early screening and diagnosis strategies of pancreatic cancer: A comprehensive review[J]. Cancer Commun, 2021, 41(12): 1257-1274. -





 下载:
下载: