Clinical Value of Matrix Metalloproteinase 7,Glutamyl Transferase,and Total Bile Acids in the Joint Diagnosis of Biliary Atresia
-
摘要:
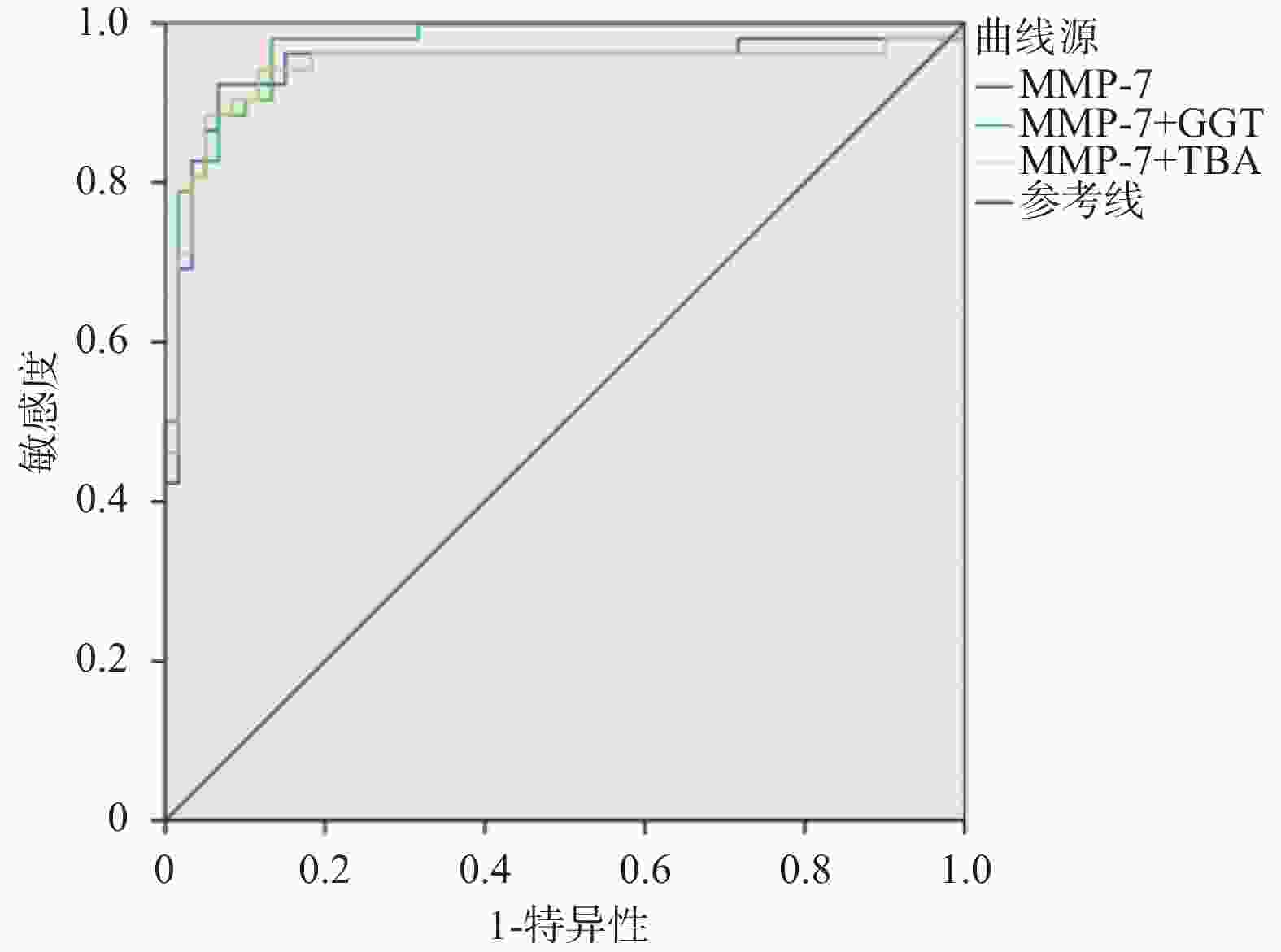
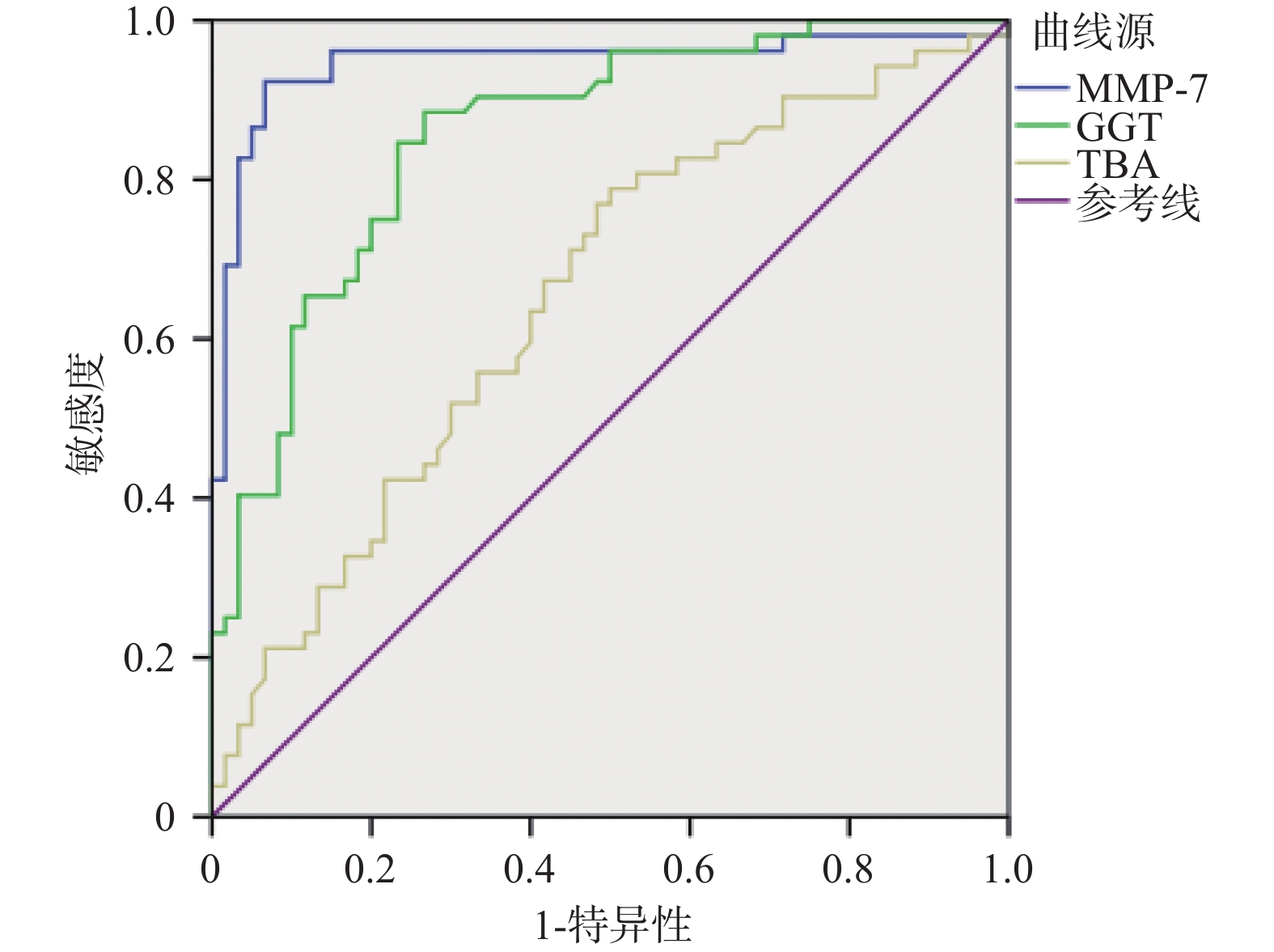
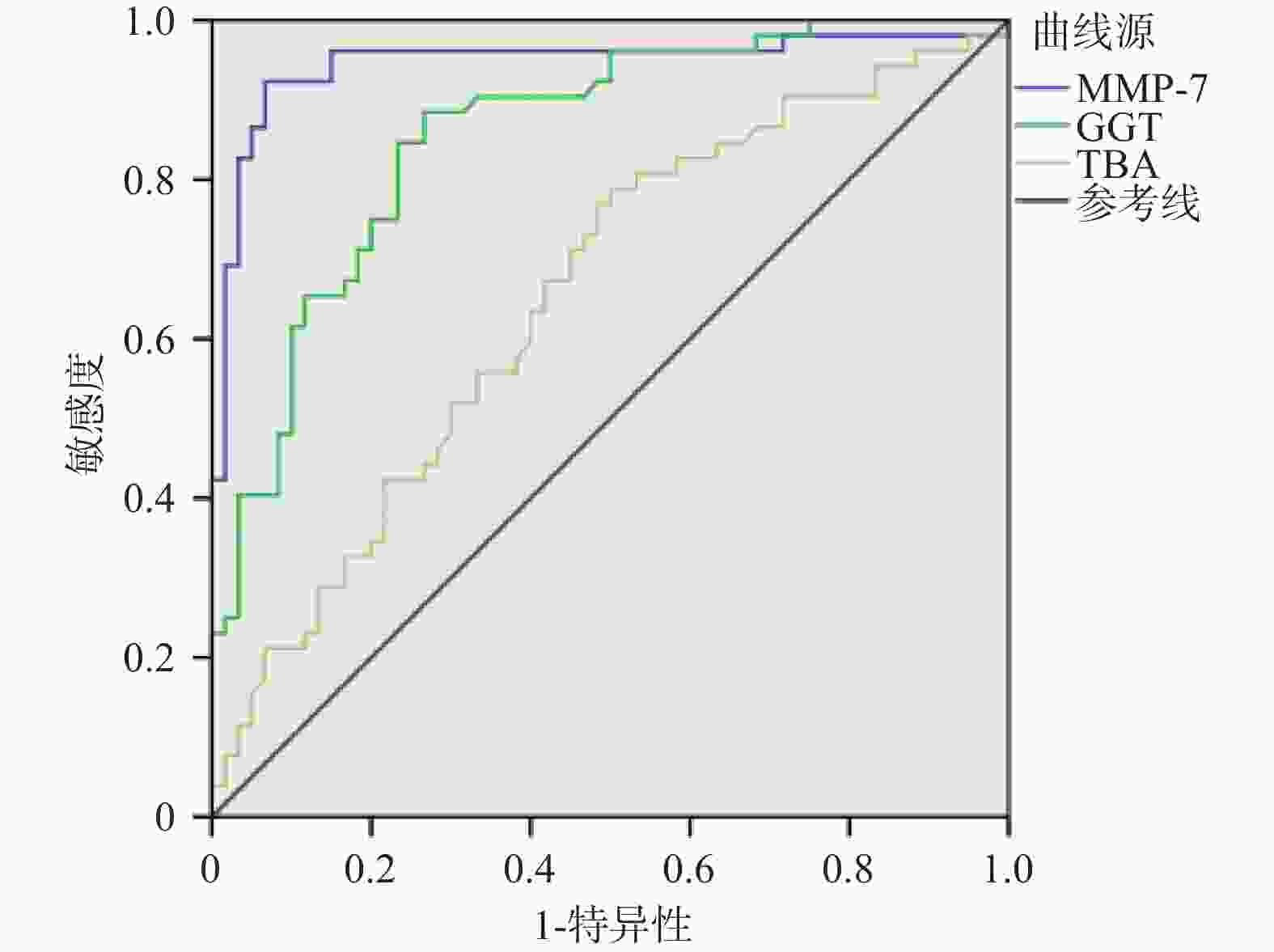
目的 探讨血清基质金属蛋白酶7联合谷氨酰转移酶、总胆汁酸诊断胆道闭锁的价值。 方法 选取昆明市儿童医院2023年7月至2024年9月住院胆汁淤积性黄疸患儿112例为研究对象。根据手术探查、术中胆道造影、肝活检及随访情况,将患儿分为胆道闭锁组52例(BA)和非胆道闭锁组60例(Non-BA)。比较两组患儿的日龄、性别、血清基质金属蛋白酶7(MMP-7)、谷氨酰转移酶(GGT)、丙氨酸氨基转氨酶(ALT)、天冬氨酸转氨酶(AST)、总胆红素(TB)、直接胆红素(DB)、总胆汁酸(TBA)、天冬氨酸转氨酶/血小板指数(APRI)。将有统计学意义的指标纳入受试者工作特征曲线(ROC)分析,计算ROC曲线下面积(AUC)和最佳诊断界值(约登指数)。 结果 两组患儿在日龄、ALT、AST、DB、TB、APRI水平比较差异无统计学意义(P > 0.05);两组性别构成比较差异有统计学意义(P = 0.006);BA组MMP-7、GGT、TBA水平显著高于Non-BA组,比较差异有统计学意义(P < 0.05);MMP-7、GGT、TBA诊断BA的AUC分别为0.946(95%CI 0.897~0.996),0.857(95%CI 0.789~0.926),0.654(95%CI 0.552~0.755);当MMP-7截断值为22.37 ng/mL,诊断BA的敏感度和特异度分别为0.923和0.933;当GGT的截断值为151.5 U/L,诊断BA的敏感度和特异度分别为0.885和0.733;当TBA的截断值为119.5 μmol/L,诊断BA的敏感度和特异度分别为0.788和0.500。MMP-7 + GGT、MMP-7 + TBA联合诊断BA的AUC分别为0.971(95%CI 0.946~0.997),0.943(95%CI 0.889~0.996)。 结论 血清MMP-7作为单独诊断BA的指标,具有较好的诊断价值;MMP-7联合GGT诊断BA优于单一指标;MMP-7联合TBA并不能提高诊断效能。 Abstract:Objective To explore the value of serum matrix metalloproteinase 7 combined with glutamyl transferase and total bile acids in the diagnosis of biliary atresia. Methods 112 children hospitalized in Kunming Children's Hospital with the cholestatic jaundice from July 2023 to September 2024 were selected as the research subjects. According to the surgical exploration, intraoperative cholangiography, liver biopsy and follow-up, the children were divided into the biliary atresia group (BA) (n = 52) and non-biliary atresia group (Non-BA) (n = 60) respecvely. The age, gender, serum matrix metalloproteinase 7 (MMP-7), glutamyl transferase (GGT), alanine aminative aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), direct bilirubin (DB), total bile acid (TBA) and aspartate aminotransferase/platelet index (APRI) were compared between the two groups. Statistically significant indicators were included in receiver operating characteristic curve (ROC) analysis, and the area under the ROC curve (AUC) and optimal diagnostic margin (Youden index) were calculated. Results There was no significant difference in age, ALT, AST, DB, TB and APRI levels between the two groups of patients (P > 0.05); There was a difference in gender composition ratio between the two groups (P = 0.006); MMP-7 , GGT, TBA levels in the BA group were significantly higher than those in the Non-BA group, and the difference was statistically significant (P < 0.05); the AUC of MMP-7, GGT, and TBA in diagnosing BA were 0.946 (95%CI 0.897~0.996) , 0.857 (95%CI 0.789~0.926), 0.654 (95%CI 0.552~0.755) respectively; When the cut-off value of MMP-7 was 22.37 ng/ml, the sensitivity and specificity for diagnosing BA were 0.923 and 0.933 respectively; When the cut-off value of GGT was 151.5 U/L, the sensitivity and specificity of diagnosing BA were 0.885 and 0.733 respectively; When the cut-off value of TBA was 119.5 μmol/L, the sensitivity and specificity of diagnosing BA were 0.788 and 0.500, respectively. The AUC of MMP-7 + GGT and MMP-7 + TBA combined to diagnose BA were 0.971 (95%CI 0.946~0.997) and 0.943 (95%CI 0.889~0.996) respectively. Conclusion Serum MMP-7 has the good diagnostic value as a single indicator for diagnosing BA; MMP-7 combined with GGT is better than a single indicator for diagnosing BA; MMP-7 combined with TBA cannot improve the diagnostic efficiency. -
Key words:
- Biliary atresia /
- Matrix metalloproteinase-7 /
- Glutamyl transferase /
- Bile acids /
- Diagnosis
-
表 1 BA组和Non-BA组临床资料比较[M(P25,P75)]
Table 1. Comparison of clinical data between BA and non-BA groups [M(P25,P75)]
指标 组别 χ2/Z P BA组(n=52) Non-BA组(n=60) 例数(n) 52 60 − − 性别(男/女) 23/29 42/18 7.596 0.006* 日龄(d) 55(30,70) 53(35,66) −0.163 0.87 MMP-7(ng/mL) 57.02(34.65,85.96) 12.39(9.27,15.39) −8.127 < 0.001* GGT(U/L) 383.50(226.50,711.25) 100.00(64.50,182.50) −5.505 < 0.001* DB(μmol/L) 105.10(84.00,129.73) 103.50(72.63,132.38) −0.607 0.544 TBA(μmol/L) 163.00(120.75,196.48) 119.85(92.43,167.63) −2.795 0.005* TB(μmol/L) 154.30(119.60,184.25) 145.55(101.45,181.88) −1.292 0.196 ALT(U/L) 101.50(59.00,207.25) 129.50(56.00,203.75) −0.723 0.469 AST(U/L) 182.00(101.00,310.50) 179.00(104.00,376.50) −0.505 0.614 APRI 0.49(0.31,1.10) 0.54(0.28,1.44) −0.896 0.37 *P < 0.05。 表 2 MMP-7、GGT、TBA对BA的诊断效能
Table 2. Diagnostic efficacy of MMP-7,GGT,and TBA for BA
指标 AUC 截断值 95%CI 敏感度 特异度 约登指数 MMP-7 0.946 22.37 0.897~0.996 0.923 0.933 0.856 GGT 0.857 151.5 0.789~0.926 0.885 0.733 0.618 TBA 0.654 119.5 0.552~0.755 0.788 0.500 0.288 表 3 MMP-7联合GGT、TBA对BA的诊断效能
Table 3. Diagnostic efficacy of MMP-7 combined with GGT and TBA for BA
指标 AUC P 95%CI 敏感度 特异度 约登指数 MMP-7 0.946 < 0.001* 0.897~0.996 0.923 0.933 0.856 MMP-7+GGT 0.971 < 0.001* 0.946~0.997 0.981 0.867 0.848 MMP-7+TBA 0.943 < 0.001* 0.889~0.996 0.885 0.950 0.835 *P < 0.05。 -
[1] Vij M,Rela M. Biliary atresia: Pathology,etiology and pathogenesis[J]. Future Sci OA,2020,6(5):FSO466. doi: 10.2144/fsoa-2019-0153 [2] Nio M. Japanese Biliary atresia registry[J]. Pediatr Surg Int,2017,33(12):1319-1325. doi: 10.1007/s00383-017-4160-x [3] Lertudomphonwanit C,Mourya R,Fei L,et al. Large-scale proteomics identifies MMP-7 as a sentinel of epithelial injury and of biliary atresia[J]. Science Translational Medicine,2017,9(417):1-22. [4] Fawaz R,Baumann U,Ekong U,et al. Guideline for the evaluation of cholestatic jaundice in infants: Joint recommendations of the North American Society for pediatric gastroenterology,hepatology,and nutrition and the european society for pediatric gastroenterology,hepatology,and nutrition[J]. J Pediatr Gastroenterol Nutr,2017,64(1):154-168. doi: 10.1097/MPG.0000000000001334 [5] Davenport M,Muntean A,Hadzic N. Biliary atresia: Clinical phenotypes and aetiological heterogeneity[J]. Journal of Clinical Medicine,2021,10(23):5675. doi: 10.3390/jcm10235675 [6] Davenport M,Kronfli R,Makin E. Advances in understanding of biliary atresia pathogenesis and progression - a riddle wrapped in a mystery inside an enigma[J]. Expert Review of Gastroenterology & Hepatology,2023,17(4):343-352. [7] 陈功,姜璟㼆,汤悦,等. 胆道闭锁诊断与治疗循证实践指南[J]. 中国循证儿科杂志,2022,17(4):245-259. doi: 10.3969/j.issn.1673-5501.2022.04.001 [8] 汤悦,朱叶,姜璟㼆,等. 新生儿胆道闭锁筛查和诊断系统评价和Meta分析[J]. 中国循证儿科杂志,2020,15(6):411-418. doi: 10.3969/j.issn.1673-5501.2020.06.003 [9] Kaczmarek L. Mmp-9 inhibitors in the brain: Can old bullets shoot new targets?[J]. Curr Pharm Des,2013,19(6):1085-1089. [10] Garalla H M,Lertkowit N,Tiszlavicz L,et al. Matrix metalloproteinase (MMP)-7 in Barrett's esophagus and esophageal adenocarcinoma: Expression,metabolism,and functional significance[J]. Physiol Rep,2018,6(10):e13683. doi: 10.14814/phy2.13683 [11] Leelawat K,Narong S,Wannaprasert J,et al. Prospective study of MMP7 serum levels in the diagnosis of cholangiocarcinoma[J]. World Journal of Gastroenterology,2010,16(37):4697-4703. doi: 10.3748/wjg.v16.i37.4697 [12] Kuhlmann K F,van Till J W,Boermeester M A,et al. Evaluation of matrix metalloproteinase 7 in plasma and pancreatic juice as a biomarker for pancreatic cancer[J]. Cancer Epidemiology Biomarkers & Prevention,2007,16(5):886-891. [13] Morais A,Beltrao M,Sokhatska O,et al. Serum metalloproteinases 1 and 7 in the diagnosis of idiopathic pulmonary fibrosis and other interstitial pneumonias[J]. Respiratory Medicine,2015,109(8):1063-1068. doi: 10.1016/j.rmed.2015.06.003 [14] Hung T M,Chang S C,Yu W H,et al. A novel nonsynonymous variant of matrix metalloproteinase-7 confers risk of liver cirrhosis[J]. Hepatology,2009,50(4):1184-1193. doi: 10.1002/hep.23137 [15] Theocharis A D,Skandalis S S,Gialeli C,et al. Extracellular matrix structure[J]. Advanced Drug Delivery Reviews,2016,97(2):4-27. [16] Cui N,Hu M,Khalil R A. Biochemical and biological attributes of matrix metalloproteinases[J]. Progress in Molecular Biology and Translational Science,2017,147(1):1-73. [17] Mao X,Duan X,Jiang B. Fascin induces epithelial-mesenchymal transition of cholangiocarcinoma cells by regulating Wnt/β-catenin signaling[J]. Med Sci Monit,2016,22(9):3479-3485. [18] Asai A,Miethke A,Bezerra JA. Pathogenesis of biliary atresia: Defining biology to understand clinical phenotypes[J]. Nature Reviews Gastroenterology & Hepatology,2015,12(6):342-352. [19] Jiang J Y,Liu S Y,Du M,et al. Measurement of MMP-7 in micro-volume peripheral blood: Development of dried blood spot approach[J]. Front Pediatr,2023,1293329(11):1293329. doi: 10.3389/fped.2023.1293329 [20] Pandurangi S,Mourya R,Nalluri S,et al. Childhood liver disease research network. Diagnostic accuracy of serum matrix metalloproteinase-7 as a biomarker of biliary atresia in a large North American cohort[J]. Hepatology,2024,80(1):152-162. doi: 10.1097/HEP.0000000000000827 [21] Muraoka M,Yoshida S,Ohno M,et al. Reactivity of γ-glutamyl-cysteine with intracellular and extracellular glutathione metabolic enzymes[J]. FEBS Lett,2022,596(2):180-188. doi: 10.1002/1873-3468.14261 [22] Deneau M,Perito E,Ricciuto A,et al. Ursodeoxycholic acid therapy in pediatric primary sclerosing cholangitis: Predictors of gamma glutamyltransferase normalization and favorable clinical course[J]. J Pediatr,2019,209(6):92-96. [23] 纳钊,白强,陈莉,等. 谷氨酰转移酶直接胆红素及天冬氨酸转氨酶诊断胆汁淤积性黄疸患儿胆道闭锁的临床意义[J]. 中国妇幼保健,2024,39(20):3975-3978. [24] 姜璟 㼆,汤悦,朱叶,等. 基于超声、肝胆核素显像和磁共振胆胰管成像影像学检查诊断胆道闭锁准确性研究的系统评价和Meta分析[J]. 中国循证儿科杂志,2020,15(3):166-176. [25] Bathena S P,Thakare R,Gautam N,et al. Urinary bile acids as biomarkers for liver diseases II. Signature profiles in patients[J]. Toxicol Sci,2015,143(2):308-318. doi: 10.1093/toxsci/kfu228 [26] Liu J,Lu H,Lu Y F,et al. Potency of individual bile acids to regulate bile acid synthesis and transport genes in primary human hepatocyte cultures[J]. Toxicol Sci,2014,141(2):538-546. doi: 10.1093/toxsci/kfu151 [27] Han Y J,Hu S Q,Zhu J H,et al. Accurate prediction of biliary atresia with an integrated model using MMP-7 levels and bile acids[J]. World Journal of Pediatrics,2024,20(8):822-833. doi: 10.1007/s12519-023-00779-7 -





 下载:
下载: