Investigating the Effect of Dihydroartemisinin on Proliferation and Metastasis of Prostate Cancer Cells based on the SPOP/HnRNPK Pathway
-
摘要:
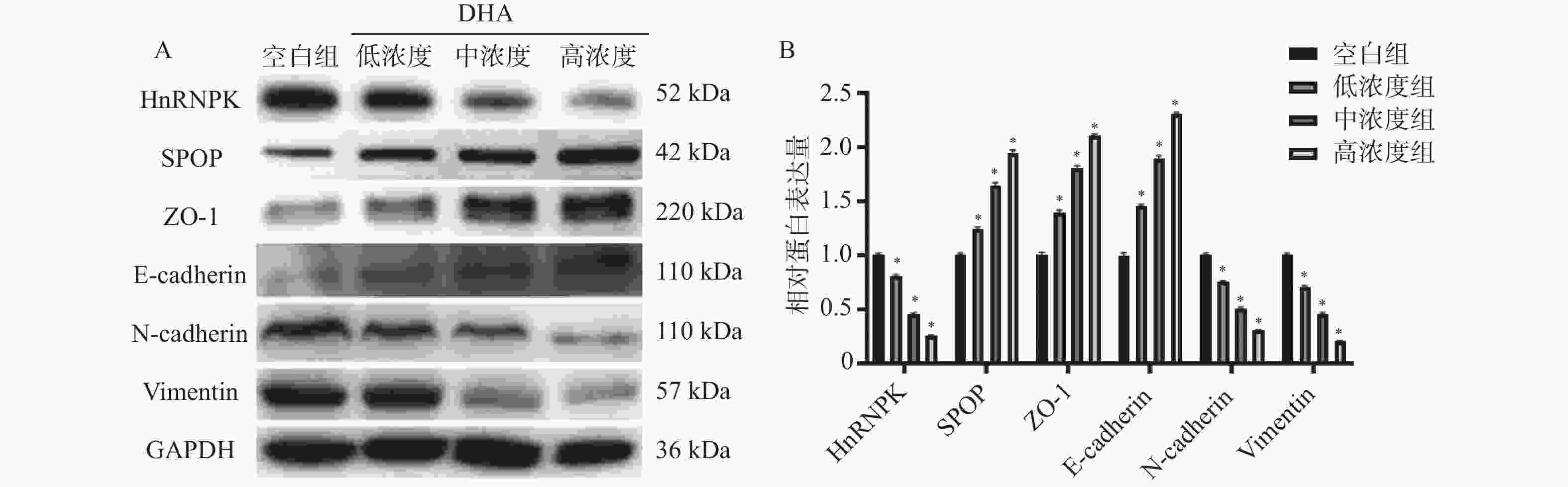
目的 基于斑点型POZ蛋白(speckle-type POZ protein,SPOP)/核内不均一核糖核蛋白K(HnRNPK)通路探讨双氢青蒿素(DHA)对前列腺癌细胞增殖和转移的影响。 方法 体外培养前列腺癌细胞系DU145并采用不同浓度DHA处理,根据DHA浓度将细胞分组为空白组(0 μmol/L)、低浓度组(10 μmol/L)、中浓度组(50 μmol/L)、高浓度组(100 μmol/L)。通过克隆形成实验、划痕实验、Transwell实验分别观察不同浓度DHA作用下DU145细胞增殖、迁移、侵袭活性变化,Western blot检测SPOP、HnRNPK、波形蛋白(Vimentin)、神经型钙黏蛋白(N-cadherin)、上皮型钙黏蛋白(E-cadherin)、紧密连接蛋白1(ZO-1)表达水平。对DU145细胞进行SPOP表达敲低处理(SPOP-shNC-DU145,SPOP-sh-DU145),分别给予前述低浓度组、中浓度组、高浓度组干预条件进行回复实验。将敲低SPOP细胞注射到小鼠体内建立异种移植小鼠模型,同时灌胃给药DHA低浓度(2.5 mg/kg)、中浓度(5 mg/kg)、高浓度(10 mg/kg)。 结果 与空白组相比,DHA低浓度组、DHA中浓度组和DHA高浓度组DU145细胞增殖、迁移和侵袭活性均受到抑制(P < 0.05),且细胞内HnRNPK、Vimentin、N-cadherin表达水平降低(P < 0.05),SPOP、E-cadherin、ZO-1表达水平升高(P < 0.05)。与SPOP-shNC-DU145细胞比较,SPOP-sh-DU145受到的DHA抑制作用减弱,细胞内HnRNPK、Vimentin、N-cadherin表达水平升高(P < 0.05),E-cadherin、ZO-1表达水平降低(P < 0.05)。 结论 DHA可以靶向诱导SPOP表达升高,抑制DU145细胞增殖和转移。 -
关键词:
- 斑点型POZ蛋白 /
- 核内不均一核糖核蛋白K /
- 双氢青蒿素 /
- 前列腺癌
Abstract:Objective To investigate the effects of dihydroartemisinin (DHA) on the proliferation and metastasis of prostate cancer cells via the speckle-type POZ protein (SPOP)/heterogeneous nuclear ribonucleoprotein K (HnRNPK) pathway. Methods The prostate cancer cell line DU145 was cultured in vitro and treated with different concentrations of DHA. Cells were divided into groups based on DHA concentration: blank group (0 μmol/L), low-concentration group (10 μmol/L), medium-concentration group (50 μmol/L), and high-concentration group (100 μmol/L). Colony formation assay, scratch assay, and Transwell assay were performed to observe changes in proliferation, migration, and invasion of DU145 cells under different DHA treatments. Western blotting was used to detect the expression levels of SPOP, HnRNPK, Vimentin, Neural-cadherin (N-cadherin), Epithelial-cadherin (E-cadherin), and Zonula occludens-1 (ZO-1). SPOP expression was knocked down in DU145 cells (SPOP-shNC-DU145, SPOP-sh-DU145), which were then subjected to the aforementioned low, medium, and high DHA concentration interventions for rescue experiments. A xenograft mouse model was established by injecting SPOP-knockdown cells into mice, followed by oral administration of low (2.5 mg/kg), medium (5 mg/kg), and high (10 mg/kg) concentrations of DHA. Results Compared with the blank group, the proliferation, migration, and invasion of DU145 cells in the low-, medium-, and high-concentration groups were significantly inhibited (P < 0.05), Intracellular expression levels of HnRNPK, Vimentin, and N-cadherin were decreased (P < 0.05), while the expression levels of SPOP, E-cadherin, and ZO-1 were increased (P < 0.05). Compared with SPOP-shNC DU145 cells, the inhibitory effects of DHA on SPOP-sh DU145 cells were attenuated, with increased expression levels of HnRNPK, Vimentin, and N-cadherin (P < 0.05), and decreased expression levels of E-cadherin and ZO-1 (P < 0.05). Conclusion DHA may target and induce increased SPOP expression, thereby inhibiting the proliferation and metastasis of DU145 cells. -
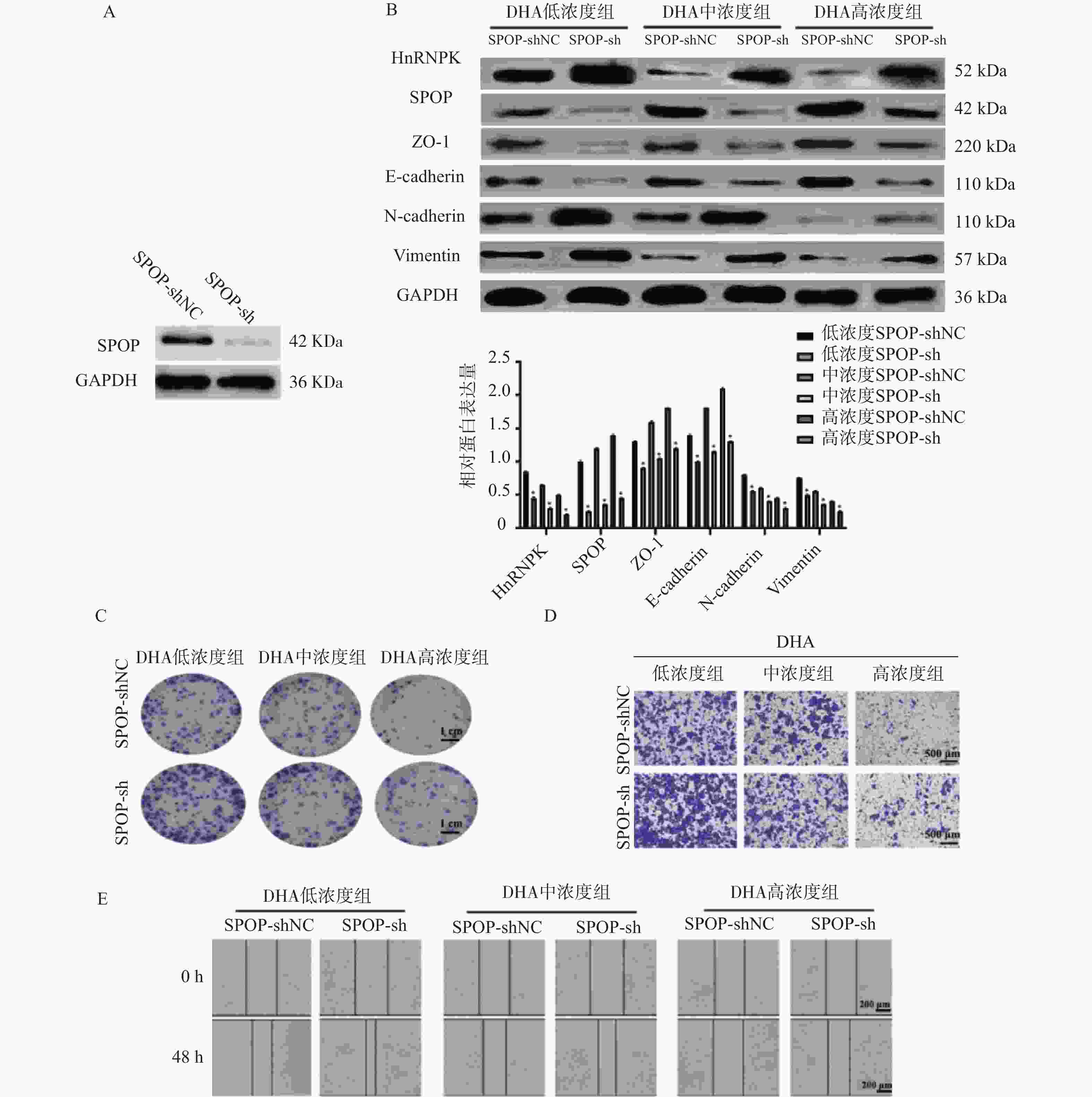
图 4 SPOP下调对DHA作用下DU145细胞EMT、迁移及侵袭的影响($\bar x \pm s $,n = 3)
A:Western blot检测SPOP干扰效率;B:Western blot及灰度定量分析SPOP干扰在不同DHA浓度处理下对DU145细胞EMT相关蛋白(HnRNPK、SPOP、ZO-1、E-cadherin、N-cadherin、Vimentin)表达的影响;与空白组比较,*P < 0.05;C:克隆形成实验显示不同DHA浓度下SPOP干扰对DU145细胞增殖能力的影响(×100,标尺:1 cm);D:Transwell实验检测不同DHA浓度下SPOP干扰对DU145细胞侵袭能力的影响(×200,标尺:500 μm);E:划痕愈合实验检测SPOP干扰对DU145细胞迁移能力的影响(×100,标尺:200 μm)。
Figure 4. Effects of SPOP knockdown on EMT,migration,and invasion of DU145 cells treated with DHA ($\bar x \pm s $,n = 3)
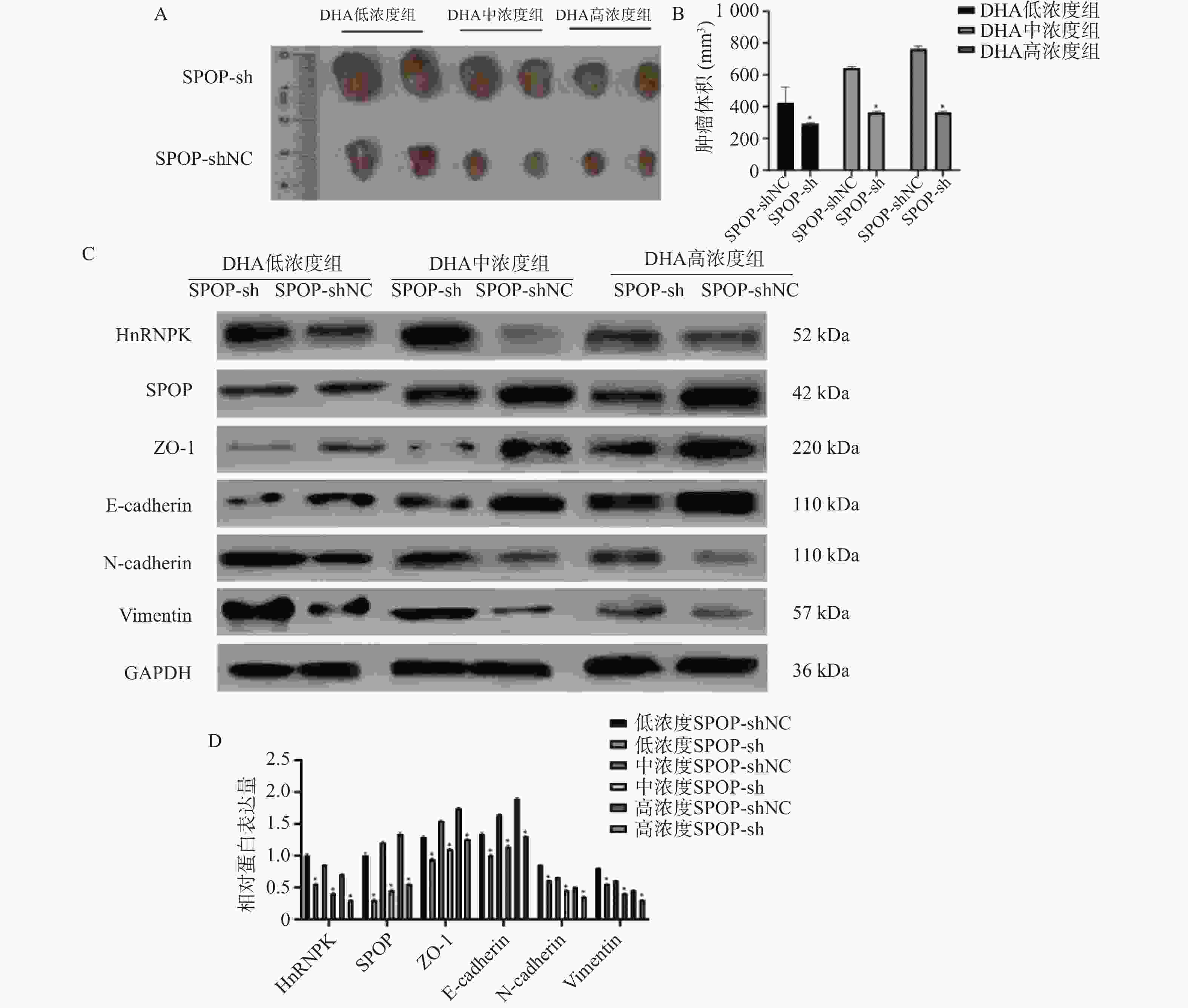
图 5 SPOP下调部分逆转DHA对DU145细胞异种移植瘤生长的抑制作用($\bar x \pm s $,n = 5)
A:SPOP敲低后DHA不同浓度处理下小鼠移植瘤生长变化;B:肿瘤体积定量分析;与SPOP-shNC组相比,*P < 0.05;C:Western blot检测不同DHA浓度下SPOP敲低后DU145异种移植瘤组织中EMT相关蛋白(HnRNPK、SPOP、ZO-1、E-cadherin、N-cadherin、Vimentin)的表达变化;D:相应蛋白灰度值定量分析;*P < 0.05。
Figure 5. Knockdown of SPOP partially reverses the inhibitory effects of DHA on DU145 xenograft tumor growth ($\bar x \pm s $,n = 5)
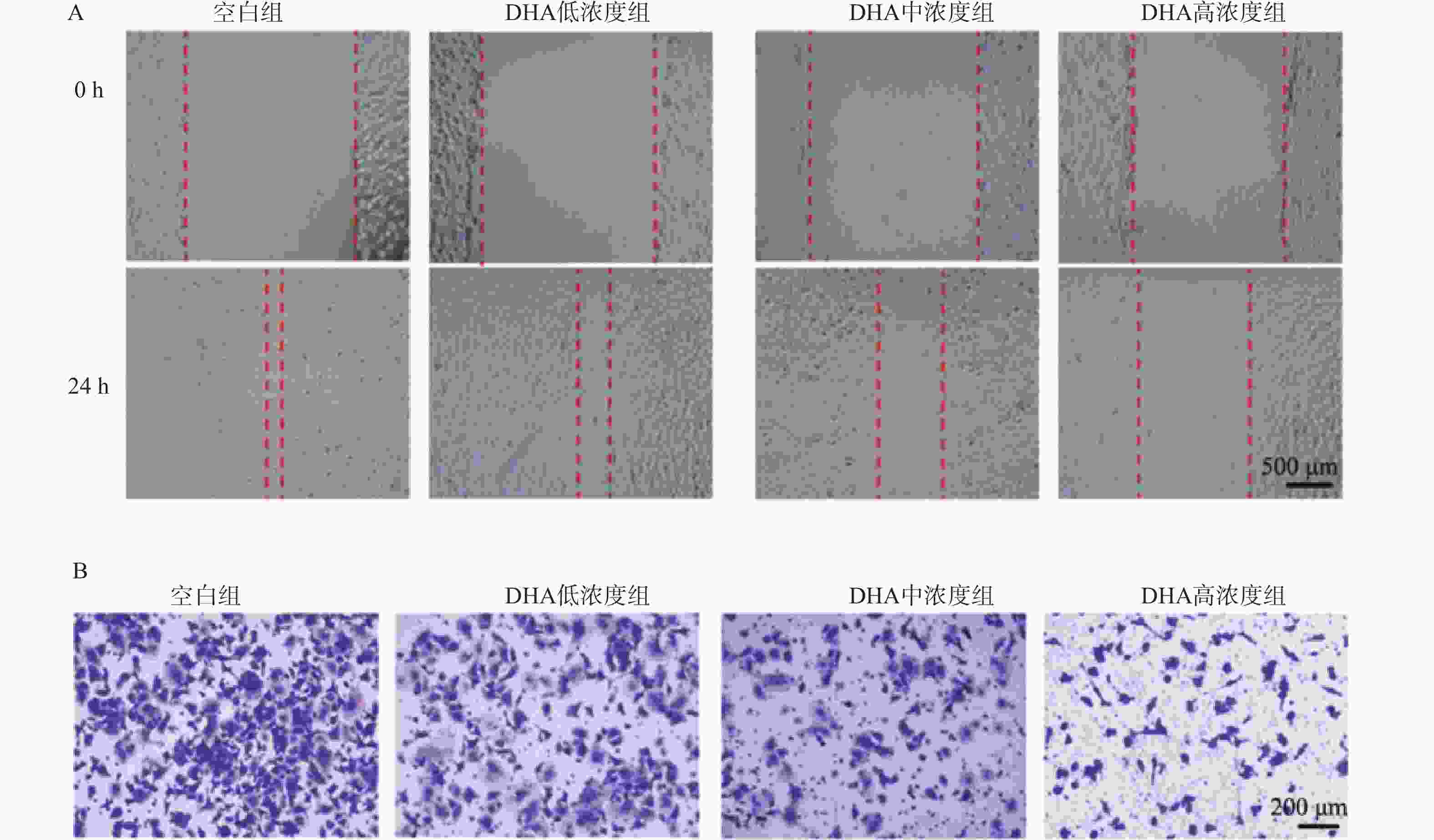
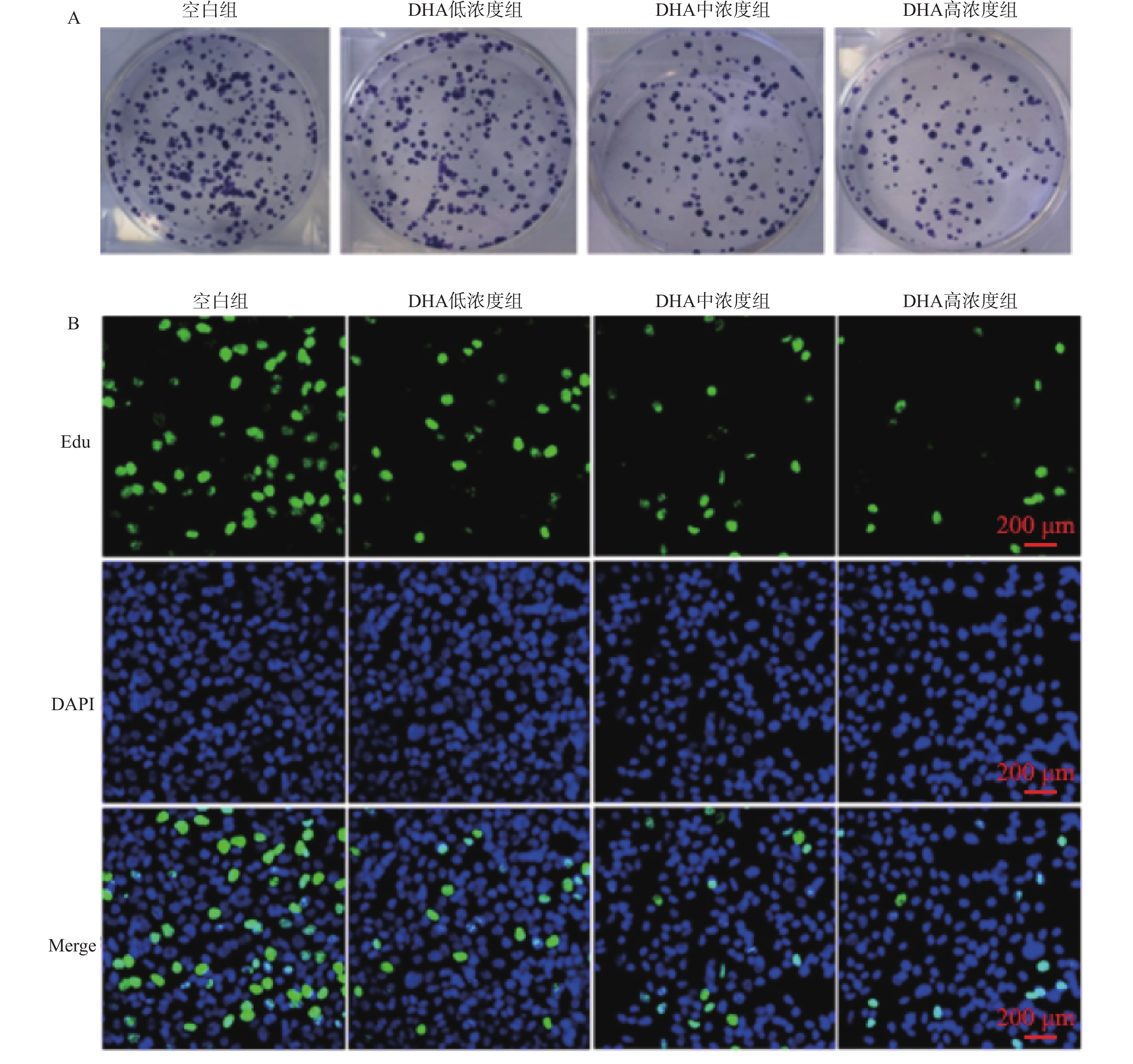
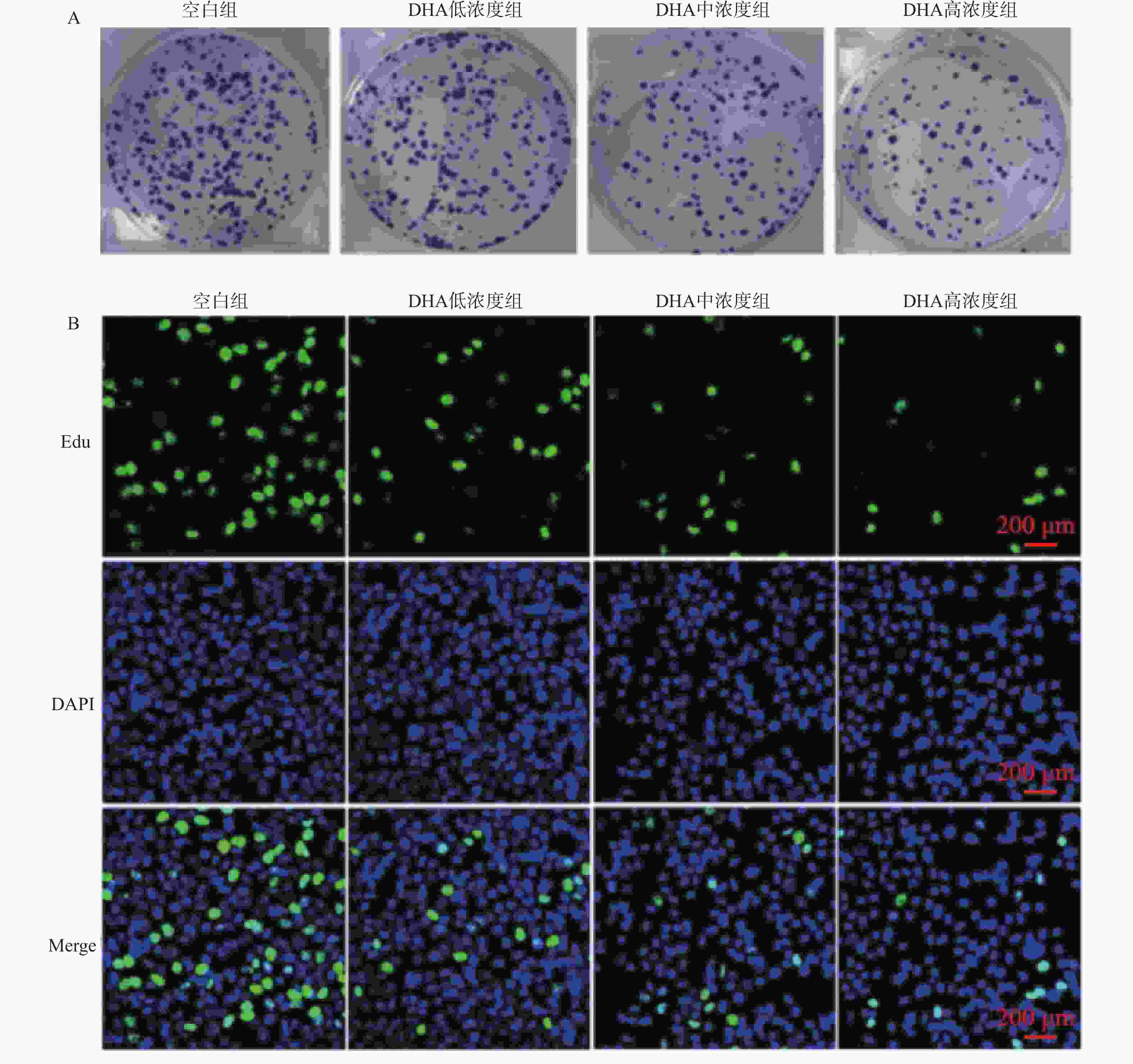
表 1 不同浓度DHA处理后DU145细胞增殖、迁移、侵袭、EMT及SPOP/HnRNPK蛋白表达水平比较($\bar x \pm s $,n = 3)
Table 1. Comparison of proliferation,migration,invasion,EMT markers,and SPOP/HnRNPK protein expression levels in DU145 cells after treatment with different concentrations of DHA ($\bar x \pm s $,n = 3)
指标 空白组 DHA低浓度组 DHA中浓度组 DHA高浓度组 F P 克隆形成数量(个) 3.67 ± 6.51 111.67 ± 5.69* 90.33 ± 4.51* 63.33 ± 3.51* 177.86 <0.001* 48 h划痕愈合面积比例(%) 86.15 ± 1.53 76.40 ± 2.08* 52.76± 35.33* 35.28 ± 1.53* 165.31 <0.001* 48 h侵袭细胞数量(个) 363.00 ± 14.11 284.33 ± 12.10* 179.00 ± 9.02* 102.67 ± 11.68* 89.23 <0.001* *P < 0.05。 表 2 SPOP敲低后各组DU145细胞增殖、迁移、侵袭、EMT及HnRNPK水平比较($\bar x \pm s $,n = 3)
Table 2. Comparison of proliferation,migration,invasion,EMT markers,and HnRNPK levels in SPOP-knockdown DU145 cells across treatment groups ($\bar x \pm s $,n = 3)
指标 DHA低浓度组 DHA中浓度组 DHA高浓度组 SPOP-shNC SPOP-sh t P SPOP-shNC SPOP-sh t P SPOP-shNC SPOP-sh t P 克隆形成
数量(个)106.33 ± 4.04 131.00 ± 3.61* 76.78 <0.001* 90.33 ± 4.51 109.67 ± 3.51* 15.72 <0.001* 63.33 ± 3.51 76.00 ± 3.61* 9.84 <0.001* 48 h划痕愈
合面积比例(%)80.50 ± 1.47 90.12 ± 1.86* 19.21 <0.001* 53.40 ± 1.08 66.05 ± 1.42* 21.88 <0.001* 35.33 ± 1.53 43.86 ± 1.46* 6.74 <0.01* 48 h侵袭
细胞数量
(个)303.00 ± 8.19 365.67 ± 8.51* 66.05 <0.001* 179.33 ± 9.02 259.67 ± 11.93* 81.21 <0.001* 102.67 ± 11.68 157.00 ± 9.00* 19.21 <0.001* *P < 0.05。 表 3 SPOP敲低后各组DU145细胞异种移植瘤小鼠肿瘤生长及相关蛋白表达的比较($\bar x \pm s $,n = 5)
Table 3. Comparison of tumor growth and related protein expression in xenograft mouse models with SPOP-knockdown DU145 cells across different groups ($\bar x \pm s $,n = 5)
指标 DHA低浓度组 DHA中浓度组 DHA高浓度组 shSPOP-NC shSPOP t P shSPOP-NC shSPOP t P shSPOP-NC shSPOP t P 肿瘤体积 (mm3) 105.0 ± 10.5 150.0 ± 15.0* 9.12 <0.001* 95.0 ± 9.5 110.0 ± 11.0* 6.71 <0.001* 80.0 ± 3.0 95.0 ± 3.5* 3.11 0.014* 肿瘤重量 (g) 0.52 ± 0.05 0.75 ± 0.08* 3.52 0.008* 0.47 ± 0.05 0.55 ± 0.06* 4.54 0.002* 0.40 ± 0.04 0.52 ± 0.04* 3.68 0.007* *P < 0.05。 -
[1] Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2024, 74(3): 229-263. [2] Desai K, McManus J M, Sharifi N. Hormonal therapy for prostate cancer[J]. Endocr Rev, 2021, 42(3): 354-373. doi: 10.1210/endrev/bnab002 [3] Geng C, Zhang M C, Manyam G C, et al. SPOP mutations target STING1 signaling in prostate cancer and create therapeutic vulnerabilities to PARP inhibitor-induced growth suppression[J]. Clin Cancer Res, 2023, 29(21): 4464-4478. doi: 10.1158/1078-0432.CCR-23-1439 [4] Wang Z, Song Y, Ye M, et al. The diverse roles of SPOP in prostate cancer and kidney cancer[J]. Nat Rev Urol, 2020, 17(6): 339-350. doi: 10.1038/s41585-020-0314-z [5] Xie W, Zhu H, Zhao M, et al. Crucial roles of different RNA-binding hnRNP proteins in Stem Cells[J]. Int J Biol Sci, 2021, 17(3): 807-817. doi: 10.7150/ijbs.55120 [6] Puvvula P K, Buczkowski S, Moon A M. hnRNPK-derived cell-penetratingpeptide inhibits cancer cell survival[J]. Mol Ther Oncolytics, 2021, 23: 342-354. doi: 10.1016/j.omto.2021.10.004 [7] Xi Z, Huang H, Hu J, et al. LINC00571 drives tricarboxylic acid cycle metabolism in triple-negative breast cancer through HNRNPK/ILF2/IDH2 axis[J]. J Exp Clin Cancer Res, 2024, 43(1): 22. doi: 10.1186/s13046-024-02950-y [8] 李先缪. SPOP介导癌蛋白HnRNPK的泛素化降解并抑制前列腺癌细胞增殖的机制研究[D]. 武汉: 华中科技大学, 2023. [9] 徐嘉若, 贾丰菁, 陈佳靓, 等. 双氢青蒿素通过调控MAPK/PI3K/Akt信号通路抗结直肠癌作用研究[J]. 上海中医药大学学报, 2024, 38(2): 83-92. [10] 杨韬, 封龙飞, 郭欢, 等. 双氢青蒿素通过自噬作用抗肿瘤的研究现状[J]. 中国临床药理学杂志, 2023, 39(15): 2271-2275. [11] Papanikolaou S, Vourda A, Syggelos S, et al. Cell plasticity and prostate cancer: The role of epithelial-mesenchymal transition in tumor progression, invasion, metastasis and cancer therapy resistance[J]. Cancers (Basel), 2021, 13(11): 2795. doi: 10.3390/cancers13112795 [12] Akrida I, Papadaki H. Adipokines and epithelial-mesenchymal transition (EMT) in cancer[J]. Mol Cell Biochem, 2023, 478(11): 2419-2433. doi: 10.1007/s11010-023-04670-x [13] Rao Q, Yu H, Li R, et al. Dihydroartemisinin inhibits angiogenesis in breast cancer via regulating VEGF and MMP-2 /-9[J]. Fundam Clin Pharmacol, 2024, 38(1): 113-125. doi: 10.1111/fcp.12941 [14] Ma Y, Zhang P, Zhang Q, et al. Dihydroartemisinin suppresses proliferation, migration, the Wnt/β-catenin pathway and EMT via TNKS in gastric cancer[J]. Oncol Lett, 2021, 22(4): 688. doi: 10.3892/ol.2021.12949 [15] Yu R, Jin G, Fujimoto M. Dihydroartemisinin: A potential drug for the treatment of malignancies and inflammatory diseases[J]. Front Oncol, 2021, 11: 722331. doi: 10.3389/fonc.2021.722331 [16] Chen J, Yao G. Herbal medicine dihydroartemisinin inhibits colorectal cancer by regulating PI3K/AKT signaling pathway[J]. J Oncology, 2023, 3(1): 1080. [17] Zhang H, Jin X, Huang H. Deregulation of SPOP in cancer[J]. Cancer Res, 2023, 83(4): 489-499. doi: 10.1158/0008-5472.CAN-22-2801 [18] Nakazawa M, Fang M, H Marshall C, et al. Clinical and genomic features of SPOP-mutant prostate cancer[J]. Prostate, 2022, 82(2): 260-268. doi: 10.1002/pros.24269 [19] Pedrani M, Salfi G, Merler S, et al. Prognostic and predictive role of SPOP mutations in prostate cancer: A systematic review and meta-analysis[J]. Eur Urol Oncol, 2024, 7(6): 1199-1215. doi: 10.1016/j.euo.2024.04.011 [20] Chang W, Feng K, Zhou P, et al. SPOP suppresses hepatocellular carcinoma growth and metastasis by ubiquitination and proteasomal degradation of TRAF6[J]. Cancer Sci, 2025, 116(5): 1295-1307. doi: 10.1111/cas.70025 [21] Li Y, Wang H, Wan J, et al. The hnRNPK/A1/R/U complex regulates gene transcription and translation and is a favorable prognostic biomarker for human colorectal adenocarcinoma[J]. Front Oncol, 2022, 12: 845931. doi: 10.3389/fonc.2022.845931 [22] Wu H L, Li S M, Huang Y C, et al. Transcriptional regulation and ubiquitination-dependent regulation of HnRNPK oncogenic function in prostate tumorigenesis[J]. Cancer Cell Int, 2021, 21(1): 641. doi: 10.1186/s12935-021-02331-x [23] Jiang Q, Zheng N, Bu L, et al. SPOP-mediated ubiquitination and degradation of PDK1 suppresses AKT kinase activity and oncogenic functions[J]. Mol Cancer, 2021, 20(1): 100. doi: 10.1186/s12943-021-01397-5 [24] Shi H, Xiong L, Yan G, et al. Susceptibility of cervical cancer to dihydroartemisinin-induced ferritinophagy-dependent ferroptosis[J]. Front Mol Biosci, 2023, 10: 1156062. doi: 10.3389/fmolb.2023.1156062 [25] Wang X, Meng F, Mao J. Progress of natural sesquiterpenoids in the treatment of hepatocellular carcinoma[J]. Front Oncol, 2024, 14: 1445222. doi: 10.3389/fonc.2024.1445222 [26] Bernasocchi T, El Tekle G, Bolis M, et al. Dual functions of SPOP and ERG dictate androgen therapy responses in prostate cancer[J]. Nat Commun, 2021, 12(1): 734. doi: 10.1038/s41467-020-20820-x [27] Sun X X, Li Y, Sears R C, et al. Targeting the MYC ubiquitination-proteasome degradation pathway for cancer therapy[J]. Front Oncol, 2021, 11: 679445. doi: 10.3389/fonc.2021.679445 -





 下载:
下载: