Research Status and Clinical Application Progress of Peptide Drugs
-
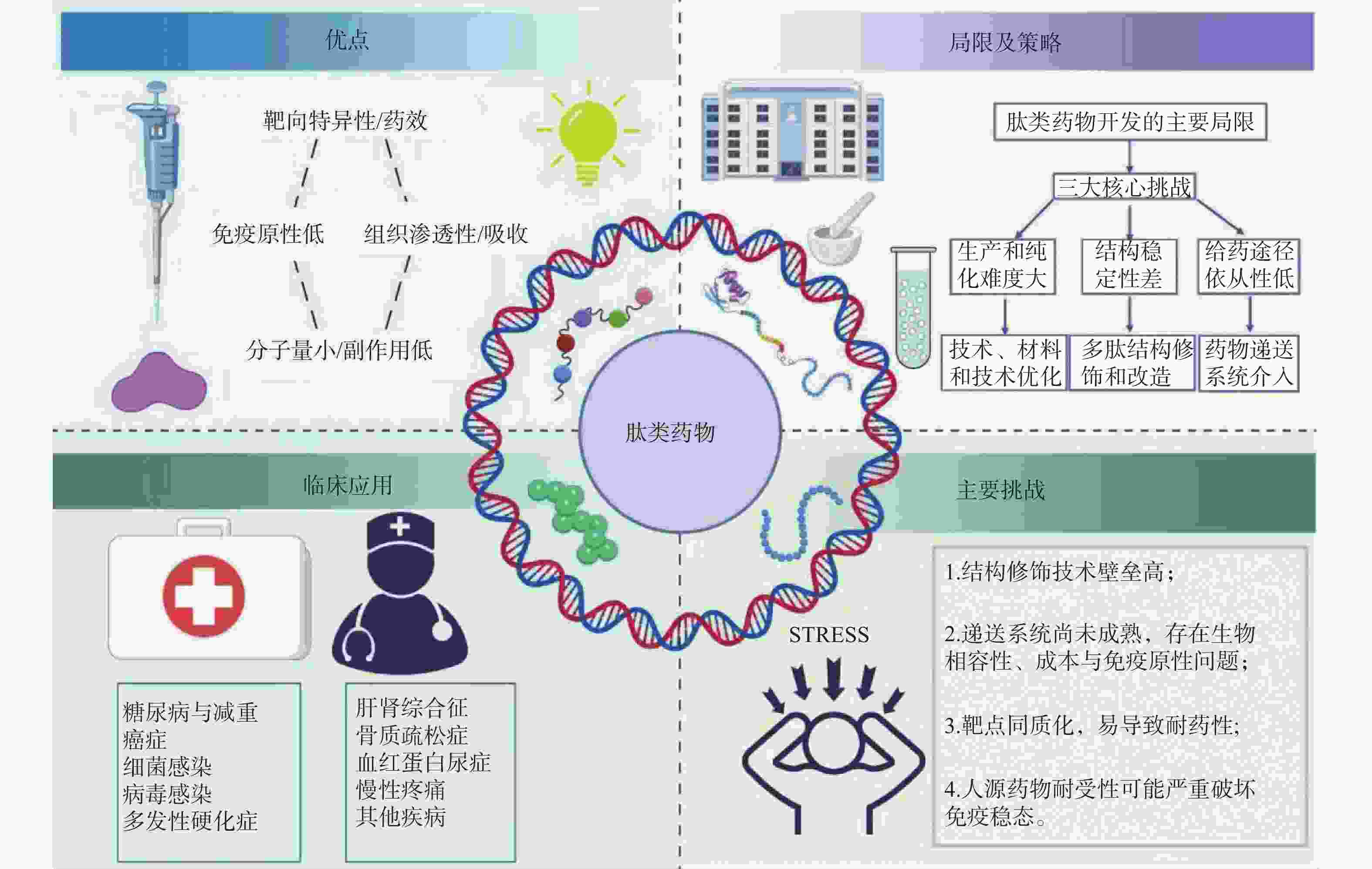
摘要: 随着传统化学药物研发难度的不断增加,肽类药物因具有高特异性、显著疗效、易代谢及低毒性等优势,逐渐成为药物研究与开发的热点。系统阐述了肽类药物的理化特性、主要优势及局限性,并总结了目前常用的结构修饰与递送策略。重点介绍了已获批上市肽类药物在糖尿病、癌症、细菌和病毒感染、多发性硬化症、骨质疏松症等多种疾病中的应用及其作用靶点。同时,对肽类药物在体内稳定性差、生物利用度低、给药途径受限等研发难题进行了分析,并探讨了基于分子修饰、纳米递送及计算机辅助设计等新技术的前景。综上,肽类药物在多领域治疗中展现出独特优势,但仍需突破制备、递送及耐药性等瓶颈,为未来精准治疗提供新的思路与方向。Abstract: With the increasing difficulty of traditional chemical drug research and development, peptide drugs have gradually become a hot spot in drug research and development due to their advantages of high specificity, significant efficacy, easy metabolism and low toxicity. This review systematically expounds the physicochemical properties, main advantages and limitations of peptide drugs, and summarizes the currently common strategies for structural modification and delivery. It focuses on the application and target of approved peptide drugs in various diseases such as diabetes, cancer, bacterial and viral infections, multiple sclerosis, and osteoporosis. Furthermore, the research analyzes the challenges in the research and development of peptide drugs, including poor in vivo stability, low bioavailability, and limited routes of administration. It also discusses the prospects of new technologies based on molecular modification, nanodelivery systems, and computer-aided design. In summary, peptide drugs have shown unique advantages in multi-field therapy, but they still need to break through bottlenecks in preparation, delivery and drug resistance to provide new ideas and directions for future precision therapy.
-
Key words:
- Peptide drugs /
- Research status /
- Clinical application /
- Limitations /
- Therapeutic targets
-
表 1 肽类药物在临床中的应用和作用靶点(1)
Table 1. Clinical application and targets of peptide drugs (1)
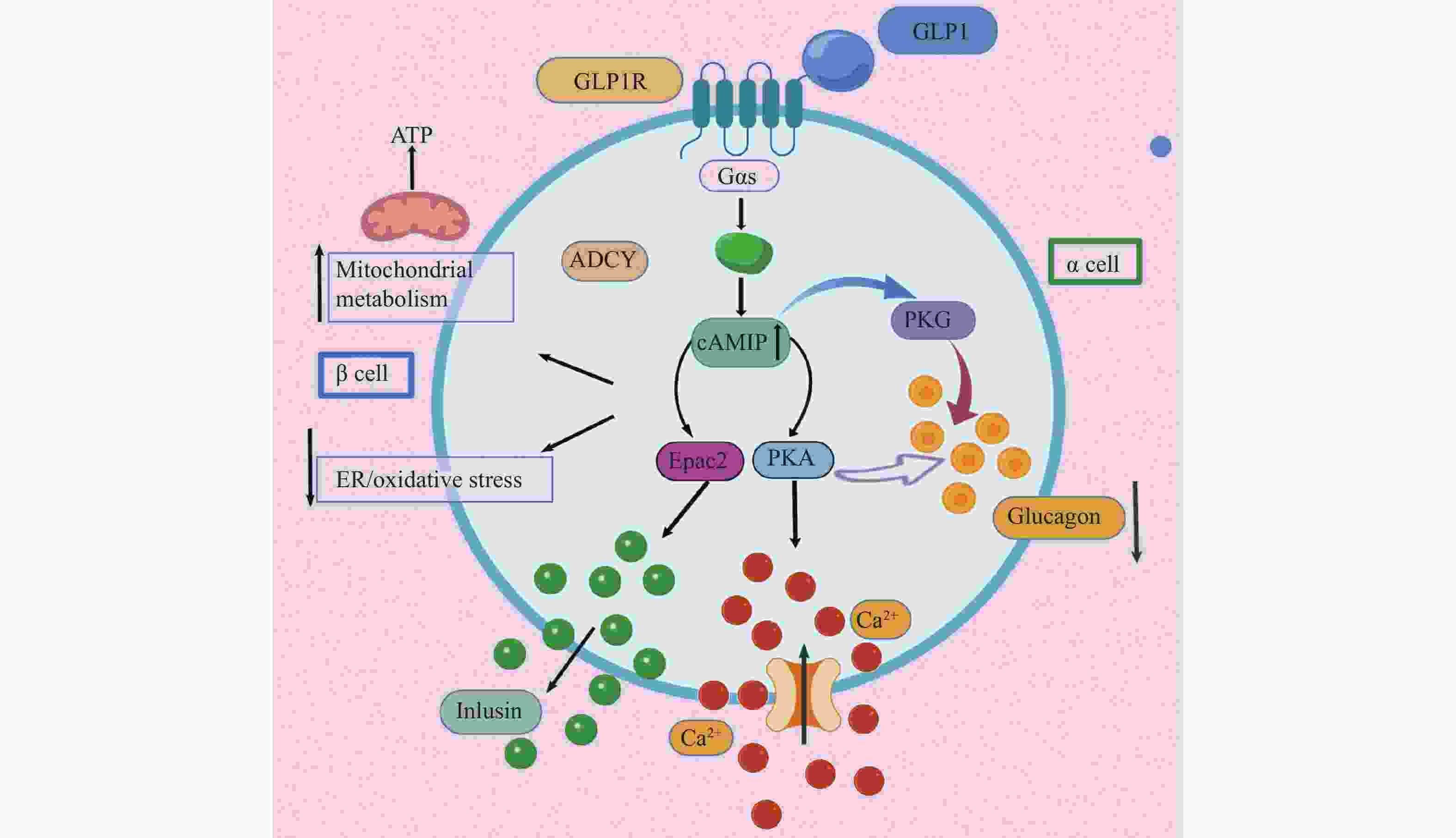
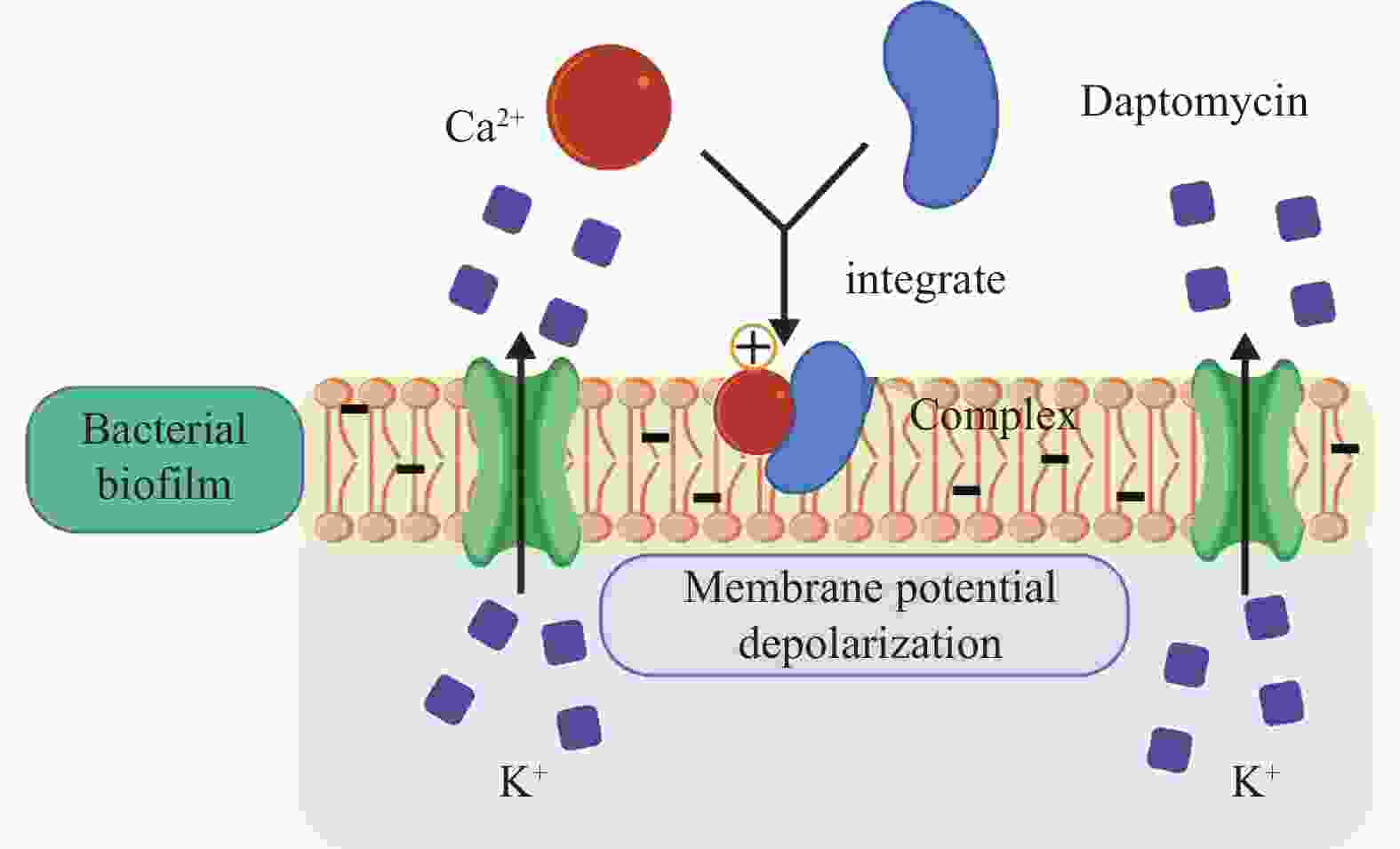
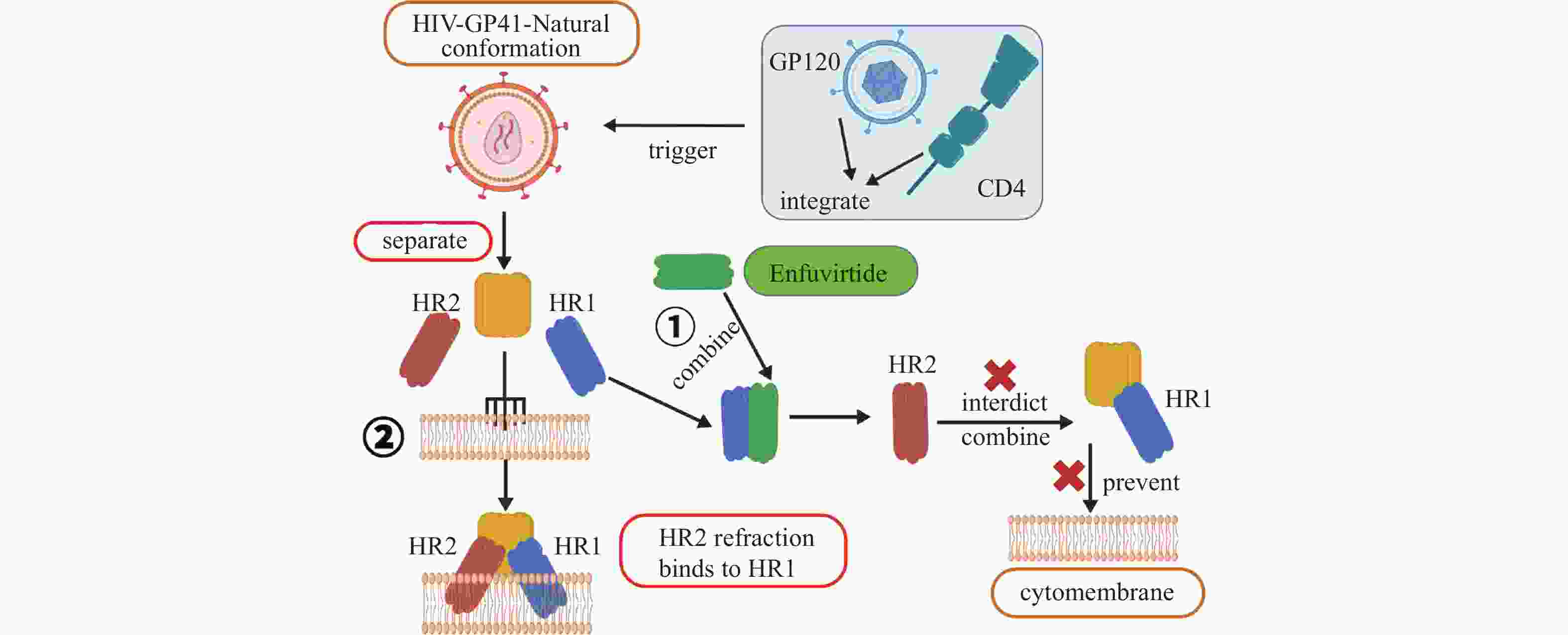
药物名称 主要靶点 适应症 Exenatide GLP-1受体 2型糖尿病 Lixisenatide GLP-1受体 2型糖尿病 Pegylated Liraglutide GLP-1受体 2型糖尿病 Albiglutide GLP-1受体 2型糖尿病 Dulaglutide GLP-1受体 2型糖尿病 Bexagliflozin 钠-葡萄糖共转运蛋白2 2型糖尿病 Sitagliptin DPP-4 2型糖尿病 Linagliptin DPP-4 2型糖尿病 Semaglutide GLP-1受体 2型糖尿病、减重 Liraglutide GLP-1受体 2型糖尿病、减重 Tirzepatide GLP-1受体和GIP受体 2型糖尿病、减重 Pramlintide 降钙素受体 1型和2型糖尿病 Setmelanotide 黑色素皮质激素-4受体 减重 RGD peptide αvβ3整合素受体 抗肿瘤药物递送 Angiopep-2 LRP-1受体 抗胶质瘤药物递送 Sargramostim GM-CSF受体 白血病、乳腺癌 Atezolizumab PD-L1 膀胱癌、非小细胞肺癌 Abarelix GnRH受体 前列腺癌 Degarelix GnRH受体 前列腺癌 Leuprorelin GnRH受体 前列腺癌、子宫肌瘤 Cetrorelix GnRH受体 前列腺癌 Buserelin GnRH受体 前列腺癌 Goserelin GnRH受体 前列腺癌、乳腺癌、子宫内膜易位症 Nafarelin GnRH受体 子宫内膜易位症 Triptorelin GnRH受体 乳腺癌 Ganirelix GnRH受体 女性不孕 Octreotide 生长抑素受体 类癌瘤及血管活性肠肽瘤、肢端肥大症 Lanreotide 生长抑素受体 神经内分泌肿瘤、肢端肥大症 Pasireotide 生长抑素受体 肢端肥大症、库欣综合征 177Lu-dotatate 生长抑素受体 神经内分泌肿瘤 68Ga-dotatate 生长抑素受体 神经内分泌肿瘤 Carfilzomib 20S蛋白酶体活性位点 多发性骨髓瘤、结直肠癌 Vancomycin 抑制细胞壁合成 革兰氏阳性菌感染 Teicoplanin 抑制细胞壁合成 革兰氏阳性菌感染 Dabavancin 抑制细胞壁合成 革兰氏阳性菌感染 Oritavancin 抑制细胞壁合成 革兰氏阳性菌感染 Telavancin 抑制细胞壁合成 革兰氏阳性菌感染 Bacitracin 抑制细胞壁合成 革兰氏阳性菌感染 Daptomycin Ca2+离子依赖型破膜 革兰氏阳性菌感染 Colistin 细菌膜 革兰氏阴性菌感染 Boceprevir NS3/4A蛋白酶 HCV病毒 Daclatasvir NS5A蛋白酶 HCV病毒 Lopinavir 3CL蛋白酶/HIV蛋白酶 SARS冠状病毒、MERS冠状病毒、HIV病毒 表 2 已获批用于糖尿病与减重的GLP-1受体相关肽类药物
Table 2. Approved GLP-1 receptor-related peptide drugs for diabetes and weight loss
药物名称 主要靶点 适应症 特点 艾塞那肽
(Exenatide)GLP-1受体 2型糖尿病 首个获FDA批准的GLP-1RA,源自希拉毒蜥唾液。 利拉鲁肽
(Liraglutide)GLP-1受体 2型糖尿病、减重 脂肪酸链修饰,延长半衰期,每日一次注射。 利司那肽
(Lixisenatide)GLP-1受体 2型糖尿病 作用时间较短,主要控制餐后血糖。 度拉糖肽
(Dulaglutide)GLP-1受体 2型糖尿病、减重 Fc融合蛋白,半衰期长,每周一次注射。 司美格鲁肽
(Semaglutide)GLP-1受体 2型糖尿病、减重 脂肪酸链修饰,长效;首个口服GLP-1RA,代表递送技术突破。 替尔泊肽
(Tirzepatide)GIP受体、GLP-1受体 2型糖尿病、减重 首个双靶点激动剂,在降糖和减重方面展现出卓越疗效。 -
[1] Ganesh A N, Heusser C, Garad S, et al. Patient-centric design for peptide delivery: Trends in routes of administration and advancement in drug delivery technologies[J]. Med Drug Discov, 2021, 9: 100079. doi: 10.1016/j.medidd.2020.100079 [2] Jacobs P G, Levy C J, Brown S A, et al. Research gaps, challenges, and opportunities in automated insulin delivery systems[J]. J Diabetes Sci Technol, 2025, 19(4): 937-949. doi: 10.1177/19322968251338754 [3] Chandarana C, Juwarwala I, Shetty S, et al. Peptide drugs: Current status and it’ s applications in the treatment of various diseases[J]. Curr Drug Res Rev, 2024, 16(3): 381-394. doi: 10.2174/0125899775295960240406073630 [4] 窦树珍, 周治寰, 邹慧, 等. 多肽药物研发和市场格局分析与展望[J]. 中国生物工程杂志, 2024, 44(11): 110-122. doi: 10.13523/j.cb.2404013 [5] Lau J L, Dunn M K. Therapeutic peptides: Historical perspectives, current development trends, and future directions[J]. Bioorg Med Chem, 2018, 26(10): 2700-2707. doi: 10.1016/j.bmc.2017.06.052 [6] Huang W, Wang Y, Wang X, et al. Research progress on bioactive peptides in the treatment of oral diseases[J]. Zhong Nan da Xue Xue Bao Yi Xue Ban, 2025, 50(5): 907-912. [7] Hill T A, Shepherd N E, Diness F, et al. Constraining cyclic peptides to mimic protein structure motifs[J]. Angew Chem Int Ed, 2014, 53(48): 13020-13041. doi: 10.1002/anie.201401058 [8] Jülke E M, Beck-Sickinger A G. Peptide therapeutics: Current status and future opportunity with focus on nose-to-brain delivery[J]. Peptides, 2025, 188: 171404. doi: 10.1016/j.peptides.2025.171404 [9] Wu Z C, Isley N A, Boger D L. N-terminus alkylation of vancomycin: Ligand binding affinity, antimicrobial activity, and site-specific nature of quaternary trimethylammonium salt modification[J]. ACS Infect Dis, 2018, 4(10): 1468-1474. doi: 10.1021/acsinfecdis.8b00152 [10] Sharma K K, Sharma K, Kudwal A, et al. Peptide-heterocycle conjugates as antifungals against cryptococcosis[J]. Asian J Org Chem, 2022, 11(7): e202200196. doi: 10.1002/ajoc.202200196 [11] Sharma K K, Cassell R J, Meqbil Y J, et al. Modulating β-arrestin 2 recruitment at the δ- and μ-opioid receptors using peptidomimetic ligands[J]. RSC Med Chem, 2021, 12(11): 1958-1967. doi: 10.1039/D1MD00025J [12] Lenci E, Trabocchi A. Peptidomimetic toolbox for drug discovery[J]. Chem Soc Rev, 2020, 49(11): 3262-3277. doi: 10.1039/D0CS00102C [13] Yang J, Wang C, Yao C, et al. Site-specific incorporation of multiple thioamide substitutions into a peptide backbone via solid phase peptide synthesis[J]. J Org Chem, 2020, 85(3): 1484-1494. doi: 10.1021/acs.joc.9b02486 [14] Sharma K, Sharma K K, Sharma A, et al. Peptide-based drug discovery: Current status and recent advances[J]. Drug Discov Today, 2023, 28(2): 103464. doi: 10.1016/j.drudis.2022.103464 [15] Jain K K. An overview of drug delivery systems[J]. Methods Mol Biol, 2020, 2059: 1-54. [16] Boyer T L, Chao O, Hakim B, et al. Cartilage targeting cationic peptide carriers display deep cartilage penetration and retention in a rabbit model of post-traumatic osteoarthritis[J]. Osteoarthr Cartil, 2025, 33(6): 721-734. doi: 10.1016/j.joca.2025.04.001 [17] Sánchez-López E, Gómara M J, Haro I. Nanotechnology-based platforms for vaginal delivery of peptide microbicides[J]. Curr Med Chem, 2021, 28(22): 4356-4379. doi: 10.2174/0929867328666201209095753 [18] Jain A, Jain A, Gulbake A, et al. Peptide and protein delivery using new drug delivery systems[J]. Crit Rev Ther Drug Carrier Syst, 2013, 30(4): 293-329. doi: 10.1615/CritRevTherDrugCarrierSyst.2013006955 [19] McLean B A, Wong C K, Campbell J E, et al. Revisiting the complexity of GLP-1 action from sites of synthesis to receptor activation[J]. Endocr Rev, 2021, 42(2): 101-132. doi: 10.1210/endrev/bnaa032 [20] Shahriar S M S, An J M, Hasan M N, et al. Plasmid DNA nanoparticles for nonviral oral gene therapy[J]. Nano Lett, 2021, 21(11): 4666-4675. doi: 10.1021/acs.nanolett.1c00832 [21] Prabhakar S, Veerabhadraswamy P, Gandasi N R. Role of the extracellular matrix in amylin aggregation: Opportunities for improved therapy in type 2 diabetes mellitus[J]. J Biosci, 2025, 50: 68. doi: 10.1007/s12038-025-00554-y [22] Riddle M C, Nahra R, Han J, et al. Control of postprandial hyperglycemia in type 1 diabetes by 24-hour fixed-dose coadministration of pramlintide and regular human insulin: A randomized, two-way crossover study[J]. Diabetes Care, 2018, 41(11): 2346-2352. doi: 10.2337/dc18-1091 [23] Lau J, Bloch P, Schäffer L, et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide[J]. J Med Chem, 2015, 58(18): 7370-7380. doi: 10.1021/acs.jmedchem.5b00726 [24] Fu S, Xu X, Ma Y, et al. RGD peptide-based non-viral gene delivery vectors targeting integrin αvβ3 for cancer therapy[J]. J Drug Target, 2019, 27(1): 1-11. doi: 10.1080/1061186X.2018.1455841 [25] di Polidoro A C, Cafarchio A, Vecchione D, et al. Revealing angiopep-2/LRP1 molecular interaction for optimal delivery to glioblastoma (GBM)[J]. Molecules, 2022, 27(19): 6696. doi: 10.3390/molecules27196696 [26] Rahbar A, Hossain M S, Giver C R, et al. Intermittent sargramostim administration expands proliferating Naïve T cells, tregs, HLA-DR+ PD-L1+ monocytes and myeloid-derived suppressor cells: Results from a randomized placebo-controlled clinical trial of GM-CSF in patients with peripheral artery disease[J]. Blood, 2023, 142(Supplement 1): 5355. [27] Meyer C, Sims A H, Morgan K, et al. Transcript and protein profiling identifies signaling, growth arrest, apoptosis, and NF-κB survival signatures following GNRH receptor activation[J]. Endocr Relat Cancer, 2013, 20(1): 123-136. [28] Al Musaimi O. Peptide therapeutics: Unveiling the potential against cancer-a journey through 1989[J]. Cancers (Basel), 2024, 16(5): 1032. doi: 10.3390/cancers16051032 [29] Bandow J E, Metzler-Nolte N. New ways of killing the beast: Prospects for inorganic-organic hybrid nanomaterials as antibacterial agents[J]. Chembiochem, 2009, 10(18): 2847-2850. doi: 10.1002/cbic.200900628 [30] Flint A J, Davis A P. Vancomycin mimicry: Towards new supramolecular antibiotics[J]. Org Biomol Chem, 2022, 20(39): 7694-7712. doi: 10.1039/D2OB01381A [31] Grein F, Müller A, Scherer K M, et al. Ca2+-Daptomycin targets cell wall biosynthesis by forming a tripartite complex with undecaprenyl-coupled intermediates and membrane lipids[J]. Nat Commun, 2020, 11: 1455. doi: 10.1038/s41467-020-15257-1 [32] Heidary M, Khosravi A D, Khoshnood S, et al. Daptomycin[J]. J Antimicrob Chemother, 2018, 73(1): 1-11. doi: 10.1093/jac/dkx349 [33] Bialvaei A Z, Samadi Kafil H. Colistin, mechanisms and prevalence of resistance[J]. Curr Med Res Opin, 2015, 31(4): 707-721. doi: 10.1185/03007995.2015.1018989 [34] Xu W, Pu J, Su S, et al. Revisiting the mechanism of enfuvirtide and designing an analog with improved fusion inhibitory activity by targeting triple sites in gp41[J]. AIDS, 2019, 33(10): 1545-1555. doi: 10.1097/QAD.0000000000002208 [35] Figueira T N, Domingues M M, Illien F, et al. Enfuvirtide-protoporphyrin IX dual-loaded liposomes: in vitro evidence of synergy against HIV-1 entry into cells[J]. ACS Infect Dis, 2020, 6(2): 224-236. doi: 10.1021/acsinfecdis.9b00285 [36] Yuan R, Wang B, Lu W, et al. A distinct region in erythropoietin that induces immuno/inflammatory modulation and tissue protection[J]. Neurotherapeutics, 2015, 12(4): 850-861. doi: 10.1007/s13311-015-0379-1 [37] Soares de Souza A, Rudin S, Yu D, et al. Treatment with ATX-MS-1467 persistently triggers IL-10, but not pro-inflammatory cytokine release and induces a population of lag3+CD4+ cells in a humanized HLA/TCR mouse model (P4.002)[J]. Neurology, 2015, 84(14_supplement): P4.002. doi: 10.1212/WNL.84.14_supplement.P4.002 [38] Gaindh D, Choi Y B, Marchese M, et al. Prolonged beneficial effect of brief erythropoietin peptide JM4 therapy on chronic relapsing EAE[J]. Neurotherapeutics, 2021, 18(1): 401-411. doi: 10.1007/s13311-020-00923-5 [39] Charles T, Moss D L, Bhat P, et al. CD4+ T-cell epitope prediction by combined analysis of antigen conformational flexibility and peptide-MHCII binding affinity[J]. Biochemistry, 2022, 61(15): 1585-1599. doi: 10.1021/acs.biochem.2c00237 [40] Ng S L, Leno-Duran E, Samanta D, et al. Genetically modified hematopoietic stem/progenitor cells that produce IL-10-secreting regulatory T cells[J]. Proc Natl Acad Sci USA, 2019, 116(7): 2634-2639. doi: 10.1073/pnas.1811984116 [41] Papaluca T, Gow P. Terlipressin: Current and emerging indications in chronic liver disease[J]. J Gastroenterol Hepatol, 2018, 33(3): 591-598. doi: 10.1111/jgh.14009 [42] Terbah R, Gow P, Sinclair M, et al. Terlipressin for type 1 hepatorenal syndrome[J]. Dig Dis Sci, 2020, 65(8): 2454-2455. doi: 10.1007/s10620-020-06370-8 [43] Jamil K, Pappas S C, Devarakonda K R. In vitro binding and receptor-mediated activity of terlipressin at vasopressin receptors V1 and V2[J]. J Exp Pharmacol, 2018, 10: 1-7. [44] Felsenfeld A J, Levine B S. Calcitonin, the forgotten hormone: Does it deserve to be forgotten?[J]. Clin Kidney J, 2015, 8(2): 180-187. doi: 10.1093/ckj/sfv011 [45] Xu J, Wang J, Chen X, et al. The effects of calcitonin gene-related peptide on bone homeostasis and regeneration[J]. Curr Osteoporos Rep, 2020, 18(6): 621-632. doi: 10.1007/s11914-020-00624-0 [46] Casado E, Martínez-Díaz-Guerra G, Caeiro J R. PTH/PTHrP analogues as osteoanabolic treatment in patients with osteoporosis[J]. Med Clin (Barc), 2025, 165(4): 107076. doi: 10.1016/j.medcli.2025.107076 [47] Gurnari C, Awada H, Pagliuca S, et al. Paroxysmal nocturnal hemoglobinuria–related thrombosis in the era of novel therapies: A 2043-patient-year analysis[J]. Blood, 2024, 144(2): 145-155. doi: 10.1182/blood.2024023988 [48] Hillmen P, Szer J, Weitz I, et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria[J]. N Engl J Med, 2021, 384(11): 1028-1037. doi: 10.1056/NEJMoa2029073 [49] Sukhanova V A, Uspenskaya E V, Ainaz S, et al. Development of a comprehensive approach to quality control of dermorphin derivative—Representative of synthetic opioid peptides with non-narcotic type of analgesia[J]. Sci Pharm, 2025, 93(1): 3. [50] Miljanich G P. Ziconotide: Neuronal calcium channel blocker for treating severe chronic pain[J]. Curr Med Chem, 2004, 11(23): 3029-3040. doi: 10.2174/0929867043363884 [51] Lin J, Chen S, Butt U D, et al. A comprehensive review on ziconotide[J]. Heliyon, 2024, 10(10): e31105. doi: 10.1016/j.heliyon.2024.e31105 [52] Massironi S, Cavalcoli F, Rausa E, et al. Understanding short bowel syndrome: Current status and future perspectives[J]. Dig Liver Dis, 2020, 52(3): 253-261. doi: 10.1016/j.dld.2019.11.013 [53] Jamshidi Kandjani O, Alizadeh A A, Moosavi-Movahedi A A, et al. Expression, purification and molecular dynamics simulation of extracellular domain of glucagon-like peptide-2 receptor linked to teduglutide[J]. Int J Biol Macromol, 2021, 184: 812-820. doi: 10.1016/j.ijbiomac.2021.06.141 [54] Schmid H. Peginesatide for the treatment of renal disease-induced Anemia[J]. Expert Opin Pharmacother, 2013, 14(7): 937-948. doi: 10.1517/14656566.2013.780695 [55] Piehl E, Fernandez-Bustamante A. Lucinactant for the treatment of respiratory distress syndrome in neonates[J]. Drugs Today (Barc), 2012, 48(9): 587-593. doi: 10.1358/dot.2012.48.9.1835160 [56] Zhu K, Meng L, Luo J, et al. Taltirelin induces TH expression by regulating TRHR and RARα in medium spiny neurons[J]. J Transl Med, 2024, 22(1): 1158. doi: 10.1186/s12967-024-06020-x [57] Shirley M. Zilucoplan: First approval[J]. Drugs, 2024, 84(1): 99-104. doi: 10.1007/s40265-023-01977-3 [58] Wensink D, Wagenmakers M A E M, Langendonk J G. Afamelanotide for prevention of phototoxicity in erythropoietic protoporphyria[J]. Expert Rev Clin Pharmacol, 2021, 14(2): 151-160. doi: 10.1080/17512433.2021.1879638 [59] Cho Y M. Glucagon-like peptide-1 therapy for youth with type 2 diabetes[J]. J Diabetes Investig, 2023, 14(3): 362-363. doi: 10.1111/jdi.13953 [60] Tran K L, Park Y I, Pandya S, et al. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes[J]. Am Health Drug Benefits, 2017, 10(4): 178-188. [61] Mookherjee N, Anderson M A, Haagsman H P, et al. Antimicrobial host defence peptides: Functions and clinical potential[J]. Nat Rev Drug Discov, 2020, 19(5): 311-332. doi: 10.1038/s41573-019-0058-8 [62] Basith S, Manavalan B, Hwan Shin T, et al. Machine intelligence in peptide therapeutics: A next-generation tool for rapid disease screening[J]. Med Res Rev, 2020, 40(4): 1276-1314. doi: 10.1002/med.21658 -





 下载:
下载: