Mechanisms of Collagen-associated Integrin α1,α2 and β1 in the Caveolin-1/AQP1 Pathway During Scleral Remodeling in the Recovery Phase of Form-Deprivation Myopia
-
摘要:
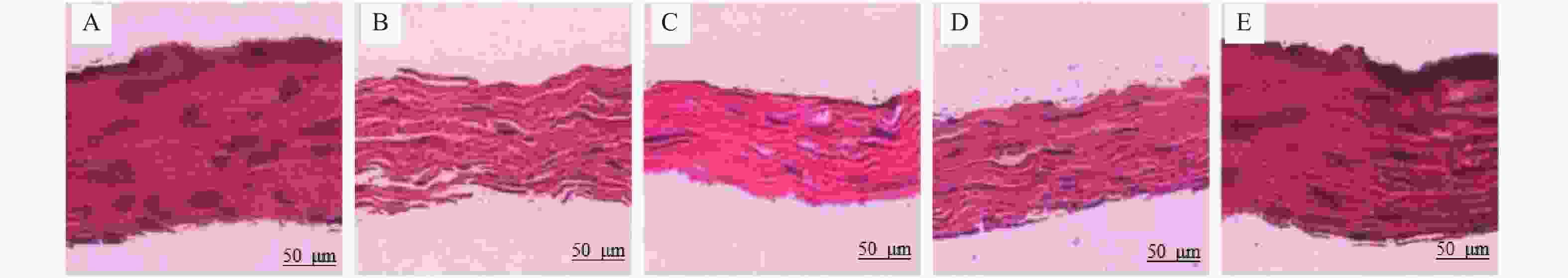
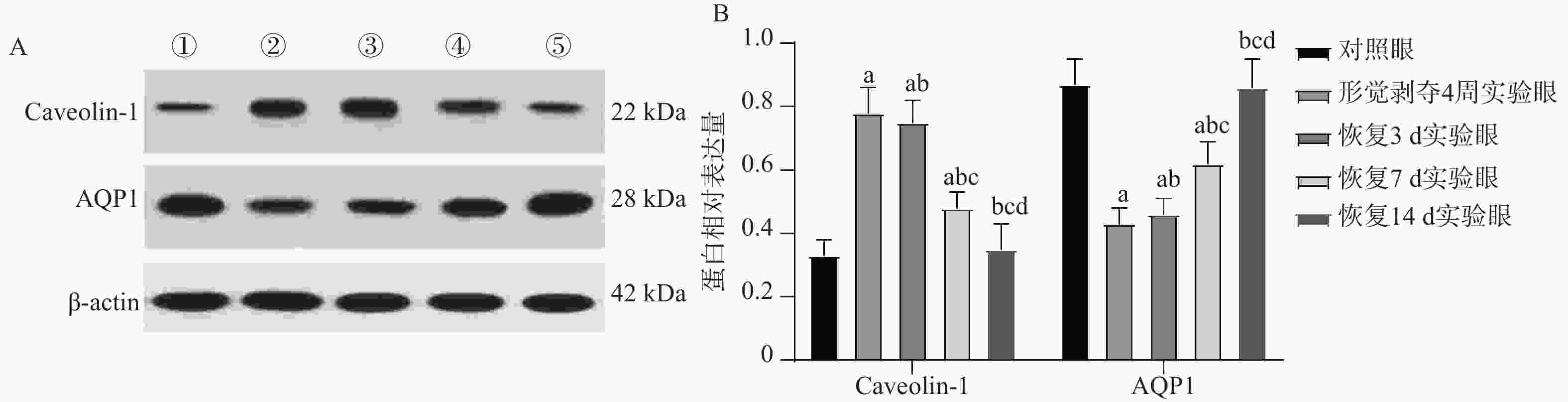
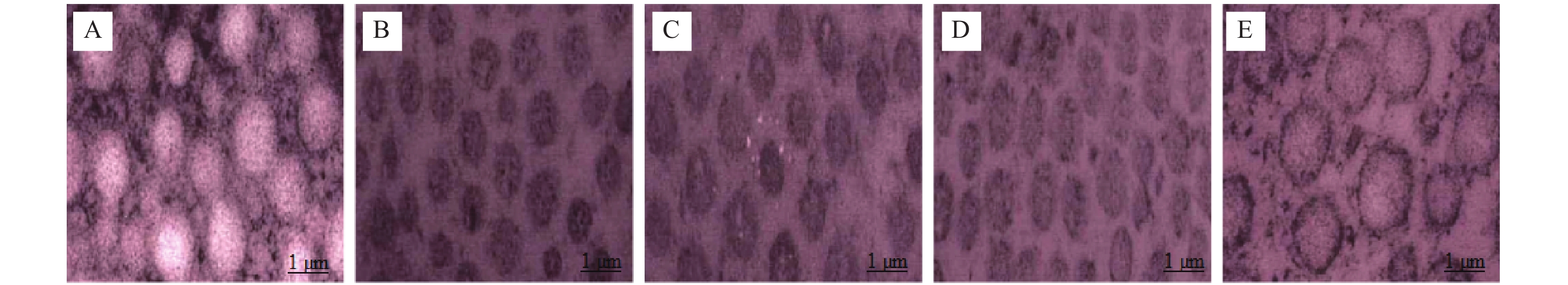
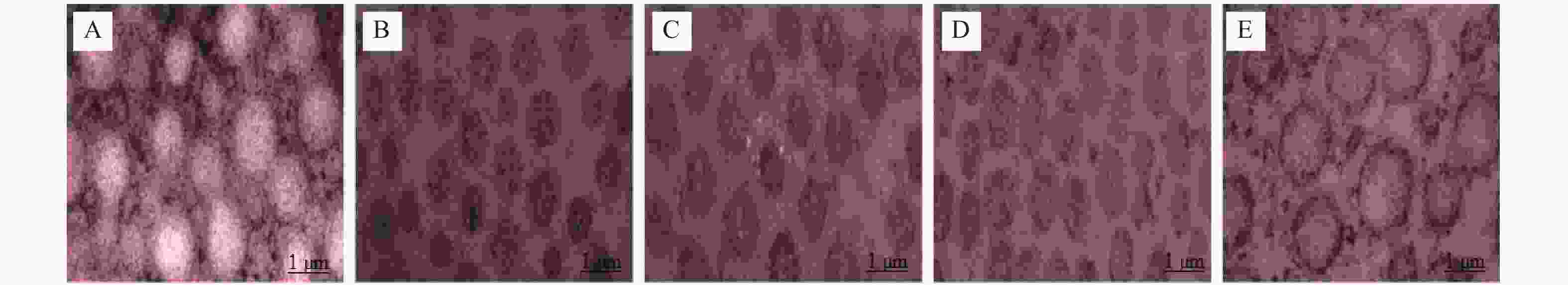
目的 研究胶原相关整合素(Integrin)α1、α2、β1对形觉剥夺近视(form-deprivationmyopia,FDM)恢复期巩膜重塑中小窝蛋白-1(Caveolin-1,Cav-1)/水通道蛋白1(aquaporin1,AQP1)通路的作用机制。 方法 50只普通级豚鼠随机分为研究组(n = 40)和对照组(n = 10)。研究组建立FDM诱导模型,遮盖豚鼠单侧眼作为实验眼,持续遮盖4周后去除豚鼠头套,根据去除时间建立遮盖4周、3 d、7 d、14 d不同时间点恢复期FDM豚鼠模型(各10只)。研究组豚鼠对侧眼作为自身对照眼,对照组的10只豚鼠双眼作为正常对照眼。分别检测对照眼、形觉剥夺4周、恢复3 d、恢复7 d、恢复14 d实验眼屈光度、眼轴长度;RT-PCR检测巩膜组织胶原相关Integrin α1、α2、β1 mRNA表达;电镜观察巩膜超微结构;HE染色观察巩膜形态;免疫印记检测巩膜组织中Caveolin-1、AQP1蛋白表达。 结果 与对照眼比较,形觉剥夺4周实验眼、恢复3 d、恢复7 d实验眼屈光度、AQP1蛋白降低,眼轴长度、Caveolin-1升高(P < 0.0083 ),恢复14 d实验眼屈光度、眼轴长度无显著变化(P >0.0083 )。与形觉剥夺4周、恢复3 d实验眼比较,恢复7 d实验眼屈光度、AQP1蛋白表达提升,眼轴长度、Caveolin-1降低(P <0.0083 )。形觉剥夺4周实验眼与恢复3 d实验眼屈光度、眼轴长度、AQP1、Caveolin-1比较无显著差异(P >0.0083 )。与对照眼比较,形觉剥夺4周实验眼、恢复3 d、恢复7 d实验眼Integrin α1、α2、β1 mRNA水平降低(P <0.0083 ),恢复14 d实验眼无显著变化(P >0.0083 )。与形觉剥夺4周实验眼比较,恢复3 d、7 d实验眼,形觉剥夺4周实验眼、恢复3 d、恢复7 d实验眼Integrin α1、α2、β1 mRNA升高,且恢复7 d实验眼最高(P <0.0083 )。电镜显示恢复7 d、恢复14 d实验眼巩膜胶原纤维分布情况改善,且恢复14 d实验眼巩膜胶原纤维分布接近正常。HE染色结果显示恢复14 d实验眼巩膜形态接近正常。结论 在形觉剥夺近视恢复期巩膜重塑过程中,胶原相关Integrin α1、α2、β1可能通过直接或间接调控对Caveolin-1/AQP1通路产生影响,影响巩膜细胞的水转运及信号转导,进而参与巩膜重塑。 Abstract:Objective To investigate the mechanism of collagen-related integrins α1, α2 and β1 on the Caveolin-1 (Cav-1)/aquaporin 1 (AQP1) signaling pathway during scleral remodeling in the recovery phase of form-deprivation myopia (FDM). Methods 50 guinea pigs were randomly divided into a study group (n = 40) and a control group (n = 10). The study group underwent FDM induction with monocular occlusion for 4 consecutive weeks. After removing the eye cover, FDM guinea pig models at different recovery time points were established: 4-week deprivation, 3-day recovery, 7-day recovery, and 14-day recovery (n = 10 each). The contralateral eye of study group animals served as internal control, and both eyes of the 10 control group animals served as normal control. Refraction and axial length of control eyes, 4-week deprivation eyes, 3-day recovery eyes, 7-day recovery eyes, and 14-day recovery eyes were measured. RT-PCR was used to detect the mRNA expression of collagen-related integrin α1, α2, and β1 in the scleral tissue; electron microscopy was used to observe the ultrastructure of the sclera; HE staining was used to observe the morphology of the sclera; and immunoblotting was used to detect the expression of Caveolin-1 and AQP1 proteins in the scleral tissue. Results Compared with the control eyes, 4-week deprivation eyes, 3-day recovery eyes, and 7-day recovery eyes showed decreased refraction and AQP1 protein, with increased axial length and Caveolin-1 (P < 0.0083 ). At 14-day recovery, no significant changes were observed (P >0.0083 ). Compared with 4-week deprivation and 3-day recovery eyes, 7-day recovery eyes showed elevated refraction and AQP1 protein expression, with decreased axial length and Caveolin-1 (P <0.0083 ). No significant differences were found between 4-week deprivation and 3-day recovery eyes (P >0.0083 ). Compared with control eyes, Integrinα1, α2, and β1 mRNA levels were decreased in 4-week deprivation, 3-day recovery, and 7-day recovery eyes(P <0.0083 ), with no significant changes at 14-day recovery(P >0.0083 ). Compared with 4-week deprivation eyes, Integrinα1, α2 and β1 mRNA levels increased in 3-day and 7-day recovery eyes, with the highest levels at 7-day recovery (P <0.0083 ). Electron microscopy showed improved collagen fibril distribution in 7-day and 14-day recovery eyes, with 14-day recovery eyes showing near-normal distribution. HE staining revealed that 14-day recovery eyes had near-normal scleral morphology.Conclusion During scleral remodeling in the recovery period of form-deprivation myopia, collagen-related Integrins α1, α2 and β1 may directly or indirectly regulate the Caveolin-1/AQP1 pathway, affecting water transport and signal transduction in scleral cells, thereby participating in scleral remodeling. -
Key words:
- Form deprivation myopia /
- Scleral remodeling /
- Caveolin-1 /
- Aquaporin-1 /
- Integrin α1 /
- Integrin α2 /
- Integrin β1
-
表 1 RT-PCR引物序列
Table 1. RT-PCR primer sequences
基因 引物序列(5'-3') 产物长度(bp) Integrinα1 上游:GGGAACAGAGAAAGAAGAACAAGG 169 下游:TCCAAAACGAGCCCCACA Integrinα2 上游:ACGATGTGGGTGAGTGTAGAGG 99 下游:CTGGCTGTTTGGGTTCTGG Integrinβ1 上游:ACTCCCTTTCTTCAGAGGTCATTTT 97 下游:GTCCCGTTTACCCCGTTCTT β-actin 上游:GGCACCAGGGAGTCATGGTA 96 下游:TGGGGTATTTCAGGGTCAGG 表 2 形觉剥夺恢复期屈光度、眼轴长度比较[$ \bar x \pm s $,n = 10]
Table 2. Comparison of diopter and axial length in the recovery period of form deprivation [$ \bar x \pm s $,n = 10]
组别 屈光度(D) 眼轴长度(mm) 对照眼 2.29 ± 0.47 8.38 ± 0.06 形觉剥夺4周实验眼 −2.50 ± 0.35△ 8.53 ± 0.08△ 恢复3 d实验眼 −2.00 ± 0.37△ 8.51 ± 0.07△ 恢复7 d实验眼 1.00 ± 0.31△#+ 8.45 ± 0.05△#+ 恢复14 d实验眼 2.00 ± 0.39#+≠ 8.40 ± 0.05#+≠ F 349.500 10.880 P <0.001*** <0.001*** ***P < 0.001;与对照眼比较,△P < 0.0083 ;与形觉剥夺4周实验眼比较,#P <0.0083 ;与恢复3 d实验眼比较,+P <0.0083 ;与恢复7 d实验眼比较,≠P <0.0083 ;表 3 形觉剥夺恢复期Integrinα1、α2、β1mRNA比较[($ \bar x \pm s $),n = 10]
Table 3. Comparison of Integrinα1,α2,β1 mRNA in the recovery period of form deprivation[($ \bar x \pm s $),n = 10]
组别 Integrinα1 Integrinα2 Integrinβ1 对照眼 0.63 ± 0.03 0.58 ± 0.04 0.74 ± 0.03 形觉剥夺4周实验眼 0.36 ± 0.04△ 0.34 ± 0.03△ 0.48 ± 0.02△ 恢复3 d实验眼 0.48 ± 0.03△# 0.44 ± 0.04△# 0.54 ± 0.04△# 恢复7 d实验眼 0.57 ± 0.02△#+ 0.54 ± 0.02△#+ 0.63 ± 0.03△#+ 恢复14 d实验眼 0.61 ± 0.04#+≠ 0.59 ± 0.03#+≠ 0.72 ± 0.02#+≠ F 114.400 104.800 150.200 P <0.001*** <0.001*** <0.001*** ***P < 0.001;与对照眼比较,△P < 0.0083 ;与形觉剥夺4周实验眼比较,#P <0.0083 ;与恢复3 d实验眼比较,+P <0.0083 ;与恢复7 d实验眼比较,≠P <0.0083 。表 4 Caveolin-1、AQP1蛋白表达对比[($ \bar x \pm s $),n = 10]
Table 4. Comparison of Caveolin-1 and AQP1 protein expression[($ \bar x \pm s $),n = 10]
组别 Caveolin-1 AQP1 对照眼 0.33 ± 0.06 0.87 ± 0.08 形觉剥夺4周实验眼 0.78 ± 0.08△ 0.43 ± 0.05△ 恢复3 d实验眼 0.75 ± 0.07△ 0.46 ± 0.05△ 恢复7 d实验眼 0.48 ± 0.05△#+ 0.62 ± 0.07△#+ 恢复14 d实验眼 0.35 ± 0.08#+≠ 0.86 ± 0.09#+≠ F 97.420 91.130 P <0.001*** <0.001*** ***P < 0.001;与对照眼比较,△P < 0.0083 ;与形觉剥夺4周实验眼比较,#P <0.0083 ;与恢复3 d实验眼比较,+P <0.0083 ;与恢复7 d实验眼比较,≠P <0.0083 。 -
[1] 李聪颖, 甘嘉禾, 王美君, 等. 不同强度光照对豚鼠屈光发育和形觉剥夺性近视的影响[J]. 中华实验眼科杂志, 2022, 40(6): 491-497. doi: 10.3760/cma.j.cn115989-20220228-00079 [2] 杨心怡, 黄洪鹏, 郭晓萱, 等. 新托品对豚鼠形觉剥夺性近视的抑制作用及机制[J]. 中国药理学与毒理学杂志, 2024, 38(5): 360-368. doi: 10.3867/j.issn.1000-3002.2024.05.005 [3] Qian K W, Li Y Y, Wu X H, et al. Altered retinal dopamine levels in a melatonin-proficient mouse model of form-deprivation myopia[J]. Neurosci Bull, 2022, 38(9): 992-1006. doi: 10.1007/s12264-022-00842-9 [4] Feng J, Zhang X, Li R, et al. Widespread involvement of acetylation in the retinal metabolism of form-deprivation myopia in guinea pigs[J]. ACS Omega, 2023, 8(26): 23825-23839. doi: 10.1021/acsomega.3c02219 [5] Li J, Jo M H, Yan J, et al. Ligand binding initiates single-molecule integrin conformational activation[J]. Cell, 2024, 187(12): 2990-3005. e17. [6] Johnson R T, Solanki R, Wostear F, et al. Piezo1-mediated regulation of smooth muscle cell volume in response to enhanced extracellular matrix rigidity[J]. Br J Pharmacol, 2024, 181(11): 1576-1595. doi: 10.1111/bph.16294 [7] 张凯雪, 方文卿, 陈娟, 等. 约束视距下照度诱导近视动物模型的研究[J]. 眼科, 2023, 32(4): 294-298. doi: 10.13281/j.cnki.issn.1004-4469.2023.04.005 [8] 姚丽. 当归对形觉剥夺性气血不足型近视豚鼠脉络膜厚度的影响研究[D]. 沈阳: 辽宁中医药大学, 2023. [9] Cui Z, Huang Y, Chen X, et al. Identification of miR-671-5p and its related pathways as general mechanisms of both form-deprivation and lens-induced myopia in mice[J]. Curr Issues Mol Biol, 2023, 45(3): 2060-2072. doi: 10.3390/cimb45030132 [10] Kanchanawong P, Calderwood D A. Organization, dynamics and mechanoregulation of integrin-mediated cell-ECM adhesions[J]. Nat Rev Mol Cell Biol, 2023, 24(2): 142-161. [11] Sawant M, Wang F, Koester J, et al. Ablation of integrin-mediated cell-collagen communication alleviates fibrosis[J]. Ann Rheum Dis, 2023, 82(11): 1474-1486. doi: 10.1136/ard-2023-224129 [12] Metlapally R, Jobling A I, Gentle A, et al. Characterization of the integrin receptor subunit profile in the mammalian sclera[J]. Mol Vis, 2006, 12: 725-734. [13] 李兴钰, 汤锦菲, 李菲菲. 藏红花酸调控EGR-1信号轴抑制近视模型小鼠巩膜重塑的作用机制研究[J]. 浙江中西医结合杂志, 2025, 35(5): 396-401+406. doi: 10.3969/j.issn.1005-4561.2025.05.002 [14] 张新, 巨朝娟, 金鑫, 等. 外源性神经生长因子对形觉剥夺性近视豚鼠巩膜组织的保护作用及其机制[J]. 吉林大学学报(医学版), 2021, 47(6): 1455-1461. doi: 10.13481/j.1671-587X.20210615 [15] Shu Z, Chen K, Wang Q, et al. The role of retinal dopamine D1 receptors in ocular growth and myopia development in mice[J]. J Neurosci, 2023, 43(48): 8231-8242. doi: 10.1523/JNEUROSCI.1196-23.2023 [16] Dong J, Hu L, Li C, et al. Expression of osteopontin and integrin αvβ3 receptor in retina of diabetic guinea pigs with high myopia[J]. Ophthalmic Res, 2023, 66(1): 144-150. doi: 10.1159/000526586 [17] 王园. 胶原相关整合素α1、α2和β1在形觉剥夺性近视诱导及恢复期中调节巩膜重塑作用的研究[D]. 青岛: 青岛大学, 2015. [18] Liu X, Yuan Y, Wu Y, et al. Extracellular matrix stiffness modulates myopia scleral remodeling through integrin/F-actin/YAP axis[J]. Invest Ophthalmol Vis Sci, 2025, 66(2): 22. doi: 10.1167/iovs.66.2.22 [19] 张悦之, 邓燕, 殷小龙, 等. 人巩膜成纤维细胞中miR-184表达与近视发生、发展的关系及其对PI3K-Akt信号通路的调控作用[J]. 眼科新进展, 2023, 43(6): 444-448. doi: 10.13389/j.cnki.rao.2023.0089 [20] Stone R A, Tobias J W, Wei W, et al. Diurnal gene expression patterns in retina and choroid distinguish myopia progression from myopia onset[J]. PLoS One, 2024, 19(7): e0307091. doi: 10.1371/journal.pone.0307091 [21] Chen W, Li Z, Wang Q, et al. The role of C-Jun N-terminal kinase-1 in controlling aquaporin-1 and choroidal thickness during recovery from form-deprivation myopia in guinea pigs[J]. Curr Eye Res, 2021, 46(6): 885-894. doi: 10.1080/02713683.2020.1839107 [22] Li Z, Chen W, Zhang H, et al. The aquaporin-1 depletion downregulates the sclera biomechanical strength[J]. Curr Eye Res, 2020, 45(10): 1240-1244. doi: 10.1080/02713683.2020.1730404 [23] 王绍娟, 杨菁. 整合素调节Fak/SFKs与生长因子受体信号传导的研究进展[J]. 国际免疫学杂志, 2014, 37(4): 307-311. doi: 10.3760/cma.j.issn.1673-4394.2014.04.011 [24] Petpiroon N, Bhummaphan N, Tungsukruthai S, et al. Chrysotobibenzyl inhibition of lung cancer cell migration through Caveolin-1-dependent mediation of the integrin switch and the sensitization of lung cancer cells to cisplatin-mediated apoptosis[J]. Phytomedicine, 2019, 58: 152888. doi: 10.1016/j.phymed.2019.152888 -





 下载:
下载: