Feasibility of Optic Nerve Sheath Diameter to Guide the Treatment of Dehydration in Patients Undergoing Glioma Surgery
-
摘要:
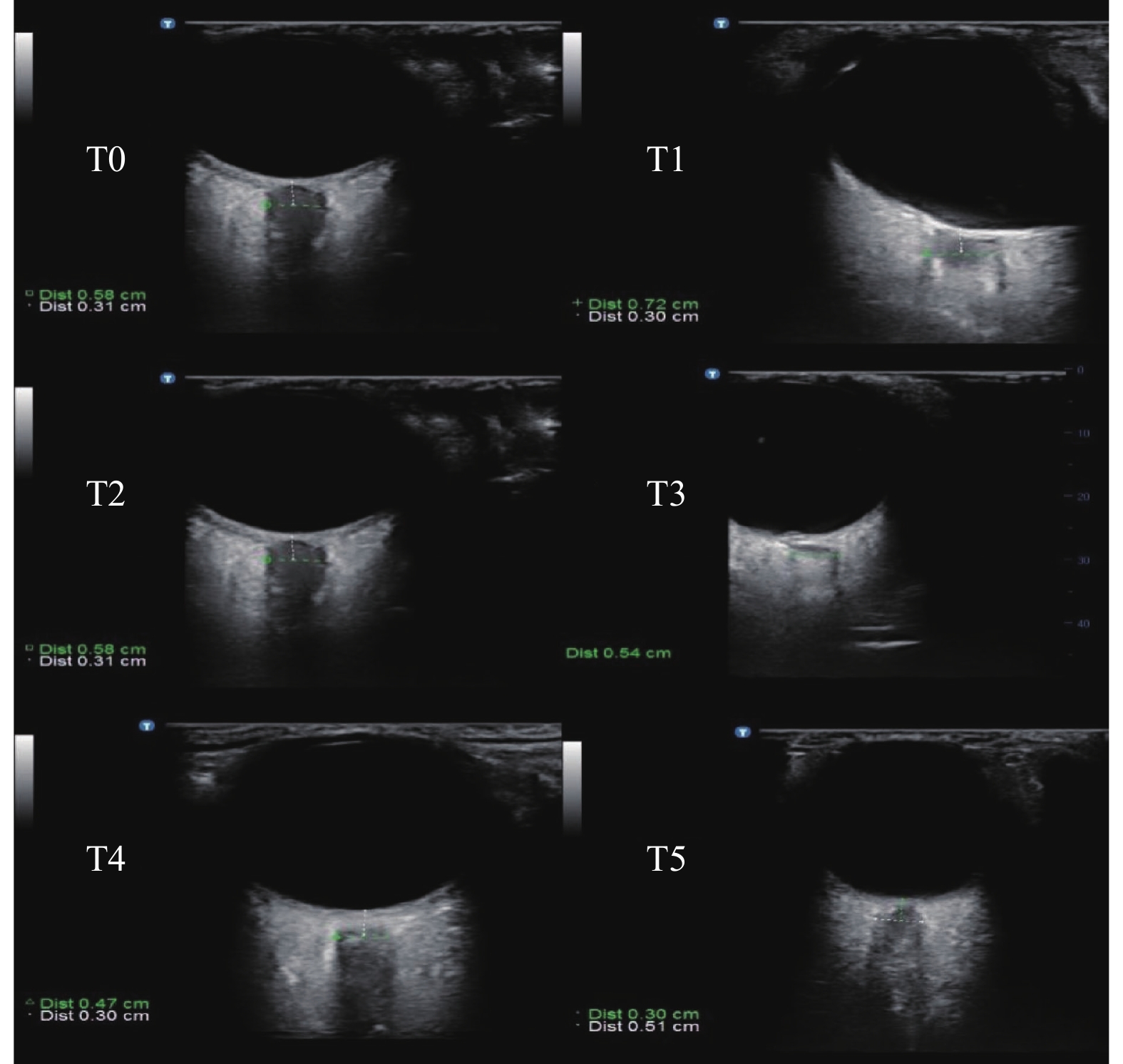
目的 探讨超声测量视神经鞘直径(ONSD)对神经胶质瘤手术患者颅高压(ICH)脱水治疗效果的可行性。 方法 选取择期行额部神经胶质瘤切除术的颅高压患者共40例,随机分为2组(n = 20)。在气管插管10 min后,甘露醇组(M组)予20%甘露醇0.5 g/kg在15 min内静脉滴注完毕,对照组(C组)予等量生理盐水在15 min内静脉滴注完毕。测量麻醉诱导前(T0)、气管插管后即刻(T1)、药物开始滴注时(T2)、药物滴注完毕后30 min(T3)、手术结束即刻(T4)、术后24 h(T5)双眼的ONSD值。检测各时刻血清中VEGF-A、MMP-9和ET-1水平。记录打开硬脑膜时的大脑松弛评分(BRS)。 结果 T3、T4、T5时刻,M组患者的ONSD值、血清中VEGF-A 、MMP-9、ET-1的浓度明显低于C组患者(P < 0.05);M组患者的BRS评分明显低于C组患者(P < 0.05)。 结论 对术前存在颅内高压的神经胶质瘤患者,在视神经鞘直径的指导下提前给予脱水治疗,可有效减轻脑水肿。 Abstract:Objective To investigate the feasibility of ultrasonic measurement of optic nerve sheath diameter (ONSD) in the treatment of dehydration of intracranial hypertension (ICH) in patients undergoing glioma surgery. Methods A total of 40 patients with cranial hypertension who underwent elective frontal glioma resection were randomly divided into 2 groups (n = 20). After 10 minutes of endotracheal intubation, Group M was given 20% mannitol 0.5 g/kg intravenously within 15 minute; Group C was given the same amount of normal saline intravenously within 15 minutes. ONSD values of both eyes were measured before anesthesia induction (T0), immediately after endotracheal intubation (T1), at the beginning of drug infusion (T2), 30 min after drug infusion (T3), immediately after surgery (T4), and 24 h after surgery (T5). The levels of VEGF-A, MMP-9 and ET-1 in serum at each time were detected. Cerebral relaxation score (BRS) was recorded when the dura was opened. Results At T3, T4 and T5, the ONSD value and serum concentrations of VEGF-A, MMP-9 and ET-1 in group M were significantly lower than those in group C (P < 0.05). BRS score in M group was significantly lower than that in C group (P < 0.05). Conclusion For glioma patients with intracranial hypertension before operation, dehydration treatment in advance under the guidance of optic nerve sheath diameter can effectively reduce cerebral edema. -
Key words:
- Optic nerve sheath diameter /
- Glioma /
- Intracranial pressure /
- Dehydration
-
妊娠女性作为一个特殊的群体,其叶酸的充足补充对于胎儿的生长发及其自身的身体健康都非常重要。研究表明,亚甲基四氢叶酸还原酶(methylenetetrahydrofolate reductase,MTHFR)是体内叶酸代谢过程中涉及的重要酶,其基因多态性将影响妊娠女性体内叶酸及同型半胱氨酸(homocysteine,HCY)的水平,从而诱发神经管畸形[1]、复发性流产[2]、妊娠高血压疾病[3]等。其中妊娠期高血压疾病(hypertensive disorder complication pregnancy,HDCP)发病率高达12%,主要包括妊娠期高血压、子痫前期(轻度、重度)、子痫、妊娠合并慢性高血压、慢性高血压并发子痫前期等,且近几年呈上升趋势[4],严重影响了母婴的健康。但其发病原因至今尚未明确阐明,有研究表明HDCP的发生与妊娠女性MTHFR基因的个体化差异密切相关[5]。而MTHFR基因多态性的分布存在种族及地域差异。因此,了解本地区妊娠女性MTHFR基因多态性的群体遗传特征及遗传因素在妊娠期高血压疾病中的作用,指导孕期女性科学补服叶酸,防治妊娠高血压疾病具有必要性。
因此,本研究通过整理分析昆明市延安医院妊娠期女性的MTHFR 677 C > T基因多态性分布情况,并初步推测其与HDCP的关系,旨在阐明叶酸代谢关键酶基因多态性的分布特征及其与HDCP的相关性,为因地制宜的制定妊娠女性叶酸补充方案,并同时为预防妊娠女性妊娠期疾病的发生发展制定针对性的个体化干预措施提供科学依据,从而确保母婴健康安全。
1. 资料与方法
1.1 研究对象
资料来源于2016年10月至2017年9月到昆明市延安医院进行孕期检查的2 476例妊娠女性,其中妊娠期高血压疾病患者90例,且均在延安医院确诊。患者平均年龄(30.5±5.0)岁,平均收缩压(146.1±17.0)mmHg,平均舒张压(98.4±12.7)mmHg;而同时期在昆明市延安医院孕检的其余2386例患者,平均年龄(29.3±4.6)岁,平均收缩压(113.6±10.0)mmHg,平均舒张压(75.1±7.7)mmHg。全部纳入研究对象均未患有遗传性疾病、血液性疾病或生殖器畸形,且和年龄等一般资料无明显差异(P > 0.05),具有可比性。
1.2 主要试剂与仪器
本研究中检测目的基因分型的试剂包括核酸纯化试剂及测序反应通用试剂盒,分别为耀金保、耀金分(商品名)。离心机型号:湘仪TDZ5-WS。检测仪器:RT-CyclerTM 436 / TL998A荧光检测仪(博奥生物科技有限公司/西安天隆科技有限公司),检测结果的运行软件系统是 V2.0.713/V1.0.28。
1.3 检测方法
本研究检测MTHFR 677C > T基因型方法:数字荧光分子杂交(DFMH)检测。
主要操作方法如下:(1)裂解:1 mL 的NH4Cl预处理液稀释,用于裂解红细胞;(2)提取:静置5 min,期间需对血液样本反复上下颠倒,而后3 000 r/min,5 min离心可得到沉淀在离心管底的丰富的白细胞;(3)混匀:加入耀金保,吹打使其与白细胞混匀。室温静置20 ~ 30 min,期间需要颠倒混匀2次,放置待检测;(4)上样:据需检测的目的基因,取相对应的耀金分测试试剂,短暂离心后,向该测试试剂中加入1.5 μL处理后的白细胞样本,搅拌均匀,便可上机检测;(5)结果判读:按说明书操作荧光检测仪进行目的基因位点的检测,并利用个体化药学服务平台V2.0.713/V1.0.283进行检测结果分析与判读。
1.4 统计学处理
采用SPSS软件对所有数据进行统计差异学分析。对收集到的基因分型数据统计后进行Hardy-Weinberg遗传平衡检验,对基因多态性及等位基因频率进行χ2 检验,P < 0.05为差异有统计学意义。
2. 结果
2.1 Hardy-Weinberg遗传平衡分析
2 476例妊娠女性MTHFR 677C > T检测数据进行Hardy-Weinberg遗传平衡检验,所得结果差异无统计学意义(χ2 = 4.50,P > 0.05,表明MTHFR 677C > T基因型分布频率符合Hardy-Weinberg遗传平衡,样本具有本区域群体代表性,见表1。
表 1 妊娠女性基因分型的Hardy-Weinberg遗传平衡分析[n(%)]Table 1. Hardy-weinberg genetic balance analysis of the recruited pregnant women [n(%)]分析项 n 基因分型 等位基因频率 CC(a2) CT(2ab) TT(b2) C(a) T(b) 实际频数 2476 1005(40.59) 1106(44.67) 365(14.74) 3116/4952 1 836/4952 理论频数 2476 980.25(39.59) 1155.30(46.66) 340.45(13.75) 62.92 37.08 2.2 妊娠女性MTHFR 677C > T基因多态性及其与其他地区的相比较
本研究中MTHFR 677C > T基因型频率CC 40.59%,CT 44.67%,TT 14.74%,等位基因频率C62.92%,T37.08%;其与淄博、郑州、琼海等地区,差异有统计学意义(P < 0.05),与武汉、惠州、镇江、德阳等地区,差异无统计学意义(P > 0.05),见表2、表3。
表 2 不同地区MTHFR 677C > T基因型分布情况比较[n(%)]Table 2. Comparison of MTHFR 677C > T genotype distribution and frequencies by areas [n(%)]地区 MTHFR 677C > T χ2 P CC CT TT 昆明(本研究) 1005(40.59) 1106(44.67) 365(14.74) 淄博[6] 130(12.5) 457(43.9) 454(43.6) 28.784 < 0.05 郑州[7] 198(18.1) 493(45.1) 402(36.8) 18.269 < 0.05 武汉[8] 1069(36.9) 1367(47.2) 463(16.0) 0.276 > 0.05 惠州[9] 186(51.8) 134(37.3) 39(10.9) 2.692 > 0.05 琼海[10] 756(61.9) 390(31.9) 75(6.1) 10.329 < 0.05 镇江[11] 877(30.4) 1378(47.8) 630(21.8) 3.120 > 0.05 德阳[12] 1047(40.7) 1171(45.5) 355(13.8) 0.045 > 0.05 表 3 不同地区MTHFR 677C > T等位基因频率比较[n(%)]Table 3. Comparison of MTHFR 677C > T allele frequencies by areas [ n(%)]地区 MTHFR 677C > T χ2 P C T 昆明(本研究) 3116(62.92) 1836(37.08) 淄博[6] 717(34.4) 1365(65.6) 16.835 < 0.05 郑州[7] 889(40.7) 1297(59.3) 9.696 < 0.05 武汉[8] 3505(60.5) 2293(39.5) 0.144 > 0.05 惠州[9] 506(70.5) 212(29.5) 1.204 > 0.05 琼海[10] 1 902(77.9) 540(22.1) 5.409 < 0.05 镇江[11] 3132(54.3) 2638(45.7) 1.668 > 0.05 德阳[12] 3265(63.4) 1 881(36.6) 0.00 > 0.05 2.3 不同HDCP类型的妊娠女性MTHFR 677C > T 基因多态性分析
90例HDCP妊娠期女性,包括妊娠期高血压(63例)和子痫前期(27例),见表4、表5,妊娠期高血压和子痫前期妊娠女性MTHFR 677C > T基因型及等位基因频率,差异无统计学意义(P > 0.05)。
表 4 不同HDCP的妊娠女性MTHFR 677C > T 基因型分布情况比较[n(%)]Table 4. Comparison of MTHFR 677C > T genotype distribution and frequencies between gestational hypertension and preeclampsia [n(%)]妊娠期高血压疾病组别 MTHFR 677C > T n χ2 P CC CT TT 妊娠高血压组 24(38.09) 31(42.21) 8(12.69) 63 4.831 > 0.05 子痫前期组 9(33.33) 16(59.26) 2(7.41) 27 表 5 不同HDCP的妊娠女性MTHFR 677C > T 等位基因基因频率比较[n(%)]Table 5. Comparison of MTHFR 677C > T allele frequencies between gestational hypertension and preeclampsia [n(%)]妊娠期高血压疾病组别 等位基因频率 n χ2 P C T 妊娠高血压组 79(62.70) 47(37.30) 126 0.001 > 0.05 子痫前期组 34(63.00) 20(37.00) 54 2.4 妊娠高血压疾病(病例组)与非妊娠高血压疾病女性(对照组)MTHFR 677C > T基因多态性比较
如下图所示,妊娠高血压疾病组与同期非妊娠高血压疾病组比较,MTHFR 677C > T基因型频率,差异无统计学意义(P > 0.05);两组等位基因频率比较,差异无统计学意义(P > 0.05),见表6。
表 6 HDCP妊娠女性与非HDCP妊娠女性MTHFR 677C > T 基因分型及等位基因频率的比较[n(%)]Table 6. Comparison of MTHFR 677C > T genotype distribution and frequencies between HDCP(case)and control [n(%)]组别 n MTHFR 677C > T 等位基因频率 CC CT TT C T 病例组 90 33(36.67) 47(52.22) 10(11.11) 62.78 37.22 对照组 2386 972(40.74) 1059(44.38) 355(14.88) 62.93 37.07 P > 0.05 > 0.05 > 0.05 > 0.05 > 0.05 3. 讨论
亚甲基四氢叶酸还原酶主要作用是将体内的 5,10-亚甲基四氢叶酸还原为 5-甲基四氢叶酸,为血液中的Hcy再甲基化生成蛋氨酸提供甲基供体,从而参与DNA、RNA、蛋白质的甲基化,同时维持体内正常的同型半胱氨酸水平,是叶酸代谢的关键限速酶。其编码基因MTHFR基因位于染色体 1p36.3,整个编码区长1 970 bp,且包含了13个外显子。1995 年Front等[13]发现MTHFR 677C > T是该基因常见的多态性位点,其编码的蛋白质位于酶的活性区域内,而该位点的突变将导致MTHFR酶活性下降30%~70%,致使Hcy向蛋氨酸转化发生障碍,引发DNA等甲基化不足,同时Hcy在血液中堆积,增加血液中Hcy水平,从而产生一系列级联反应。已有研究证明在妊娠期高血压疾病发病过程中,患者体内往往伴随着Hcy水平不同程度的增高[14]。因此,可推测妊娠期高血压疾病的发病与MTHFR 677C > T基因多态性具有一定的关系。
本研究结果显示,云南昆明地区MTHFR 677C > T等位基因频率C62.92%,T37.08%,与莱芜、郑州、惠州、琼海等相比,等位基因频率,差异有统计学意义(P < 0.05)。表明昆明地区MTHFR 677C > T基因多态性频率分布不同于其他地区,且具有地域特异性,但云南省内该基因多态性地域性差异不明显[15]。病例组MTHFR 677C > T等位基因频率分别为C62.78%,T37.22%;对照组MTHFR 677C > T等位基因频率C62.93%,T37.07%,组间比较差异无统计学意义(P > 0.05)。本研究结果表明MTHFR 677C > T基因多态性与HDCP的发生之间并无显著性的相关性。但本研究作为回顾性研究,具有其局限性,可能产生数据偏差,从而影响研究结果。而且HDCP除了受遗传方面的影响,还受到多方面的影响,如免疫方面、氧化应激方面、胎盘或滋养细胞缺血,还有营养缺乏等[16]。因此,MTHFR 677C > T基因多态性与妊娠期高血压疾病的相关性仍需要系统的、全面的的研究。
MTHFR基因多态性与叶酸代谢吸收利用的个体化差异密切相关,而叶酸对于妊娠女性及胎儿的健康起着非常重要的作用。叶酸利用率降低将会增加胎儿畸形、先天性心脏病、妊娠高血压等多种疾病的发病风险[17-18]。理论上体内正常的叶酸水平能弥补因 MTHFR 基因多态性导致的酶活性降低引发的甲基化不足、Hcy水平过高等,从而降低出生缺陷、妊娠期高血压疾病等一系列疾病的发生率。因此,据MTHFR 677C > T多态性检测的结果,对风险较高基因型的女性进行早期干预。这样可提高体内叶酸水平,纠正Hcy代谢障碍,降低妊娠期孕妇及其胎儿相关疾病的发病风险,也可以为备孕女性提供个性化增补叶酸的临床指导[19]。
-
表 1 2组患者一般情况的比较(
$\bar x \pm s $ ,n = 20)Table 1. Comparison of general conditions between two groups of patients(
$\bar {\boldsymbol{x}} \pm {\boldsymbol{s}}$ ,n = 20)组别 年龄
(岁)性别
男/女(n)BMI
(kg/m2)ASAI/II
(n)肿瘤位置
左/右(n)肿瘤体积
(cm3)C组 41.7 ± 9.8 8/12 21.9 ± 1.1 10/10 11/9 44.5 ± 2.7 M组 41.4 ± 10.1 11/9 22.0 ± 1.0 11/9 13/7 45.6 ± 2.4 t/χ2 0.006 0.902 0.121 0.100 0.417 1.935 P 0.937 0.342 0.730 0.752 0.519 0.172 表 2 2组患者在不同时刻双侧ONSD值的比较(
$\bar x \pm s $ ,n = 20,mm)Table 2. Comparison of bilateral ONSD values between two groups at different time(
$\bar {\boldsymbol{x}} \pm {\boldsymbol{s}}$ ,n = 20,mm)部位 组别 T0 T1 T2 T3 T4 T5 左侧 C组 6.31 ± 0.28 7.18 ± 0.35* 6.31 ± 0.28 6.29 ± 0.30 4.99 ± 0.18* 5.51 ± 0.16* M组 6.43 ± 0.29 7.26 ± 0.37* 6.43 ± 0.28 6.00 ± 0.24*# 4.46 ± 0.19*# 5.10 ± 0.20*# F 2.410 0.729 2.128 14.948 128.119 78.090 P 0.137 0.404 0.161 < 0.01# < 0.01# < 0.01# 右侧 C组 6.22 ± 0.21 7.00 ± 0.23* 6.21 ± 0.18 6.18 ± 0.12 4.82 ± 0.10* 5.38 ± 0.08* M组 6.24 ± 0.22 7.03 ± 0.16* 6.25 ± 0.20 5.89 ± 0.19*# 4.41 ± 0.19*# 4.93 ± 0.20*# F 0.416 0.222 1.976 60.181 105.733 90.717 P 0.527 0.643 0.176 < 0.01# < 0.01# < 0.01# 与T0时刻比较,*P < 0.05;与C组比较,#P < 0.05 表 3 2组患者BRS评分的比较(
$\bar x \pm s$ ,n = 20,分)Table 3. Comparison of BRS score between two groups(
$\bar {\boldsymbol{x}} \pm{\boldsymbol{ s}}$ ,n = 20,Points)组别 BRS评分 C组 3.0 ± 0.6 M组 1.7 ± 0.5# t 5.814 P < 0.01# 与C组比较,#P < 0.05。 表 4 2组患者在不同时刻血清VEGF-A、MMP-9、ET-1浓度的比较(
$\bar x \pm s $ ,n = 20,pg/mL)Table 4. Comparison of serum VEGF-A, MMP-9 and ET-1 concentrations between 2 groups at different time points(
$\bar {\boldsymbol{x}} \pm {\boldsymbol{s}}$ ,n = 20,pg/mL)指标 组别 T0 T3 T4 T5 VEGF-A C组 162.11 ± 9.60 161.56 ± 9.52 143.29 ± 10.33* 158.15 ± 10.05* M组 160.59 ± 11.61 149.14 ± 11.32*# 123.01 ± 11.44*# 130.18 ± 10.10*# F 1.084 59.675 102.747 90.252 P 0.311 < 0.01# < 0.01# < 0.01# MMP-9 C组 23.28 ± 2.72 23.53 ± 2.51 19.15 ± 2.59* 20.71 ± 2.07* M组 23.41 ± 2.78 19.70 ± 2.43*# 15.52 ± 2.16*# 17.38 ± 1.94*# F 0.459 285.752 280.944 126.377 P 0.506 < 0.01# < 0.01# < 0.01# ET-1 C组 86.74 ± 8.30 86.34 ± 7.79 67.68 ± 8.87* 73.89 ± 8.54* M组 86.40 ± 9.14 80.08 ± 7.35*# 52.52 ± 8.70*# 64.20 ± 8.05*# F 0.245 63.927 515.625 171.555 P 0.626 < 0.01# < 0.01# < 0.01# 与T0时刻比较,*P < 0.05;与C组比较,#P < 0.05。 -
[1] Lapointe S,Perry A,Butowski NA. Primary brain tumours in adults[J]. Lancet,2018,392(10145):432-446. doi: 10.1016/S0140-6736(18)30990-5 [2] Murayi R,Chittiboina P. Glucocorticoids in the management of peritumoral brain edema:a review of molecular mechanisms[J]. Childs Nerv Syst,2016,32(12):2293-2302. doi: 10.1007/s00381-016-3240-x [3] Boer C,Franschman G,Loer SA. Prehospital management of severe traumatic brain injury:concepts and ongoing controversies[J]. Curr Opin Anaesthesiol,2012,25(5):556-562. doi: 10.1097/ACO.0b013e328357225c [4] Robba C,Santori G,Czosnyka M,et al. Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure:a systematic review and meta-analysis[J]. Intensive Care Med,2018,44(8):1284-1294. doi: 10.1007/s00134-018-5305-7 [5] Hernández-Palazón J,Fuentes-García D,Doménech-Asensi P,et al. A dose-response relationship study of hypertonic saline on brain relaxation during supratentorial brain tumour craniotomy[J]. Br J Neurosurg,2018,32(6):619-627. doi: 10.1080/02688697.2018.1508640 [6] Sharma HS,Muresanu DF,Castellani RJ,et al. Pathophysiology of blood-brain barrier in brain tumor. Novel therapeutic advances using nanomedicine[J]. Int Rev Neurobiol,2020,151(5):1-66. [7] Berhouma M,Jacquesson T,Jouanneau E,et al. Pathogenesis of peri-tumoral edema in intracranial meningiomas[J]. Neurosurg Rev,2019,42(1):59-71. doi: 10.1007/s10143-017-0897-x [8] Hanafi MG,Verki MM,Parei SN. Ultrasonic Assessment of Optic Nerve Sheath to Detect Increased Intracranial Pressure[J]. J Med Ultrasound,2019,27(2):69-74. doi: 10.4103/JMU.JMU_54_18 [9] Zhang X,Medow JE,Iskandar BJ,et al. Invasive and noninvasive means of measuring intracranial pressure:a review[J]. Physiol Meas,2017,38(8):R143-R182. doi: 10.1088/1361-6579/aa7256 [10] Wang J,Li K,Li H,et al. Ultrasonographic optic nerve sheath diameter correlation with ICP and accuracy as a tool for noninvasive surrogate ICP measurement in patients with decompressive craniotomy[J]. J Neurosurg,2019,133(7):1-7. [11] Munawar K,Khan MT,Hussain SW,et al. Optic Nerve Sheath Diameter Correlation with Elevated Intracranial Pressure Determined via Ultrasound[J]. Cureus,2019,11(2):e4145. [12] Bäuerle J,Nedelmann M. Sonographic assessment of the optic nerve sheath in idiopathic intracranial hypertension[J]. J Neurol,2011,258(11):2014-2019. doi: 10.1007/s00415-011-6059-0 [13] Demirgan S,Özcan FG,Gemici EK,et al. Reverse Trendelenburg position applied prior to pneumoperitoneum prevents excessive increase in optic nerve sheath diameter in laparoscopic cholecystectomy:randomized controlled trial[J]. J Clin Monit Comput,2021,35(1):89-99. doi: 10.1007/s10877-020-00608-6 [14] Martin M,Lobo D,Bitot V,et al. Prediction of Early Intracranial Hypertension After Severe Traumatic Brain Injury:A Prospective Study[J]. World Neurosurg,2019,127(7):e1242-e1248. [15] Rasmussen M,Bundgaard H,Cold GE. Craniotomy for supratentorial brain tumors:risk factors for brain swelling after opening the dura mater[J]. J Neurosurg,2004,101(4):621-626. doi: 10.3171/jns.2004.101.4.0621 [16] Launey Y,Nesseler N,Le Maguet P,et al. Effect of osmotherapy on optic nerve sheath diameter in patients with increased intracranial pressure[J]. J Neurotrauma,2014,31(10):984-988. doi: 10.1089/neu.2012.2829 [17] Nowacka A,Smuczyński W,Rość D,et al. Serum VEGF-A concentrations in patients with central nervous system (CNS) tumors[J]. PLoS One,2018,13(3):e0192395. doi: 10.1371/journal.pone.0192395 [18] Yang J,Wang T,Jin X,et al. Roles of Crosstalk between Astrocytes and Microglia in Triggering Neuroinflammation and Brain Edema Formation in 1,2-Dichloroethane-Intoxicated Mice[J]. Cells,2021,10(10):2647. doi: 10.3390/cells10102647 [19] Liu T,Liao XZ,Zhou MT. Ulinastatin alleviates traumatic brain injury by reducing endothelin-1[J]. Transl Neurosci,2021,12(1):1-8. -






 下载:
下载:
 下载:
下载:









