M2 Macrophage-derived Exosome miR-1246 Regulates the Growth and Invasion of Gastric Cancer Cells
-
摘要:
目的 探讨M2巨噬细胞来源的外泌体miR-1246对胃癌AGS细胞增殖,凋亡和侵袭的影响。 方法 采用IL-4和IL-13诱导M2巨噬细胞后,分离其外泌体,并通过透射电镜和免疫印迹法进行鉴定。M2巨噬细胞分别转染NC inhibitor和miR-1246 inhibitor后,分离对应外泌体与AGS细胞共培养,并采用CCK-8,Annexin V-FITC/PI和Transwell分别检测AGS细胞增殖,凋亡和侵袭。TargetScan数据库预测miR-1246下游靶标,并通过双荧光素酶报告基因实验对miR-1246和GSK3B的靶向关系进行验证。 结果 M2巨噬细胞中分离的外泌体大小为50~150 nm,且表达ALIX,CD63和TSG101。M2巨噬细胞来源外泌体增加AGS细胞活力(P < 0.05)和侵袭细胞数(P < 0.01),并降低其凋亡比例(P < 0.01)。敲低外泌体中miR-1246的表达,AGS细胞的表型变化得到回复(P < 0.01)。外泌体miR-1246靶向GSK3B,并调控β-catenin和c-Myc的表达,M2巨噬细胞来源外泌体miR-1246靶向GSK3B促进胃癌细胞增殖和侵袭、并抑制其凋亡(P < 0.001)。 结论 M2巨噬细胞来源外泌体miR-1246靶向GSK3B介导Wnt通路激活促进胃癌细胞增殖和侵袭,并抑制其凋亡。 Abstract:Objective To investigate the effects of M2 macrophage-derived exosome miR-1246 on the proliferation, apoptosis and invasion of AGS cells. Methods After induction of M2 macrophages using LPS and IFN-γ, their exosomes were isolated and identified by transmission electron microscopy and western blot. After M2 macrophages were transfected with NC inhibitor and miR-1246 inhibitor respectively, the corresponding exosomes were isolated and co-cultured with AGS cells, and proliferation, apoptosis, and invasion of AGS cells were detected by CCK-8, Annexin V-FITC/PI and Transwell, respectively. TargetScan database predicted miR-1246 downstream targets and the targeting relationship between miR-1246 and GSK3B was validated by dual-luciferase reporter gene assays. Results The exosomes isolated from M2 macrophages were 30-150 nm in size and expressed ALIX, CD63 and TSG101. M2 macrophage-derived exosomes increased the viability and invasive cell count of AGS cells and decreased their apoptotic ratio. Phenotypic changes in AGS cells were reverted by knocking down the expression of miR-1246 in exosomes. Exosome miR-1246 targets GSK3B and upregulated the expression of β-catenin and c-Myc. Conclusion M2 macrophage-derived exosome miR-1246 mediates Wnt pathway activation to promote proliferation and invasion of gastric cancer cells and inhibit their apoptosis via targeting GSK3B. -
Key words:
- Gastric cancer /
- Macrophages /
- Exosomes /
- miR-1246 /
- Wnt signaling pathway
-
肝内胆管癌是一种具有高度侵袭性的恶性肿瘤[1],预后差,近年来,其发病率逐年升高[2]。腺苷酸激酶4(Adenylate kinase4,AK4)是腺苷酸激酶家族的一员,其分子量为25kDa,别称为AK3L1。多项研究显示AK4促进恶性肿瘤的发生发展[3-5]。但其对肝内胆管癌的作用尚无报道。本实验通过小干扰RNA手段,就AK4对肝内胆管癌细胞HUCCT1增殖、迁移能力的影响作出探究。
1. 资料与方法
1.1 细胞系
本实验所用肝内胆管癌细胞HUCCT1购自上海誉驰生物科技有限公司,该细胞已通过中国科学院昆明动物研究所鉴定明确。该细胞作为肝内胆管癌细胞株之一,常用于肿瘤增殖、迁移的研究。
1.2 小干扰RNA(Small interfering RNA,siRNA)转染细胞
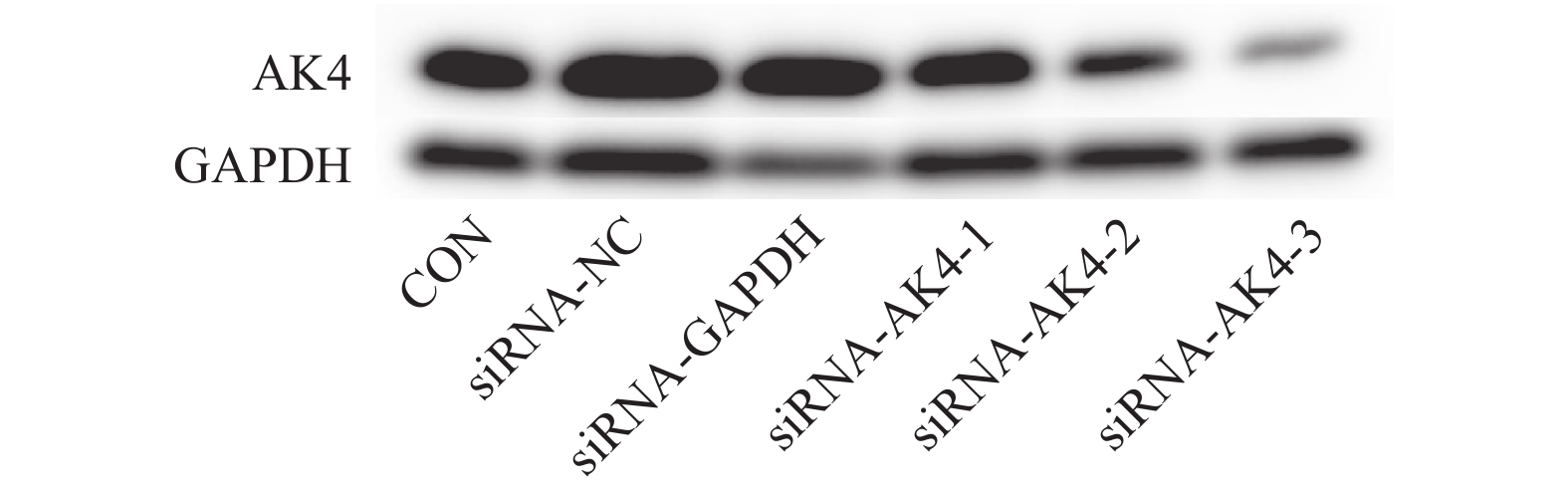
本实验采用siRNA技术以沉默AK4。siRNA购自上海市吉玛基因生物技术有限公司,共计构建6条si-RNA序列,序列设计见表1。本次实验分组为空白对照组(CON)、阴性对照组(siRNA-NC)、阳性对照组(siRNA-GAPDH)、实验组1(siRNA-AK4-1),实验组2(siRNA-AK4-2),实验组3(siRNA-AK4-3)。转染试剂采用上海市吉玛基因生物技术有限公司GP-transfect-Mate。采用免疫印迹法(Western Blot,WB)对实验组1、实验组 实验组3进行si-RNA筛选,其中空白对照组不添加siRNA,阴性对照组基因序列与目的基因序列无同源性,阳性对照组基因序列与内参GAPDH同源。各组间其余实验操作步骤(转染方法、WB、EdU、细胞划痕实验)均一致。
表 1 si-RNA序列Table 1. Sequence of si-RNA built by siRNA technology组别 序列 Antisense Negative control 5′-UUCUCCGAACGUGUCACGUTT-3′ 5′-ACGUGACACGUUCGGAGAATT-3′ GAPDH Positive control 5′-UGACCUCAACUACAUGGUUTT-3′ 5′-AACCAUGUAGUUGAGGUCATT-3′ siRNA-AK4-1 5′-GCGGAAGGGUAUAUAACCUTT-3′ 5′-AGGUUAUAUACCCUUCCGCCT-3′ siRNA-AK4-2 5′-CAGGCUAAGACAGUACAAATT-3′ 5′-UUUGUACUGUCUUAGCCUGTT-3′ siRNA-AK4-3 5′-CACCUAUUCAGUCCAAAGATT-3′ 5′-UCUUUGGACUGAAUAGGUGTT-3′ 转染前1 d将HUCCT1细胞接种至6孔板中,以次日细胞融合度达到60%~80%为宜,用无抗生素的完全培养基进行培养。转染:(1)转染试剂室温备用;(2)按照每孔200 µL无血清培养基(1640培养基)加5~8 µL转染试剂配置转染试剂混合物。静置5 min;(3)按照每孔200 µL无血清培养基(1640培养基)加150/pmol siRNA配置siRNA混合物。静置5 min;(4)将转染试剂混合物滴加到siRNA混合物中,混匀,静置20 min;(5)20 min后将混合液均匀加至6孔板各孔中;(6)各孔另外加入1 600 µL无抗生素完全培养基;(7)孔板放入细胞培养箱中培养48 h,使用免疫印迹检测转染效率。
1.3 免疫印迹实验检测转染效率及siRNA筛选
(1)裂解细胞:使用PMSF(品牌:Beyotime,货号:ST506-2 PMSF)与RIPA裂解液(强)品牌:Beyotime,货号:P0013B)按照200∶1配置细胞裂解液。于冰上充分裂解细胞;(2)测蛋白浓度:取裂解产物于4 ℃离心机12000 r/min,离心30 min。吸取86 µL上清液,使用BCA蛋白浓度测定试剂盒(品牌:Beyotime,货号:P0012 500次)测定蛋白浓度;(3)取80 µL上清液加20 µL SDS-PAGE蛋白上样缓冲液(品牌:Beyotime,货号:P0015L)沸水加热15 min;(4)电泳:配置12%下层胶,5%上层胶,蛋白上样量为2.5 µg,上层胶60 V恒压电泳,下层胶100 V恒压电泳;(5)转膜:甲醇浸泡PVDF膜,胶、膜放置妥当后以300 mA恒流条件下湿转发转膜,时间为30 min;(6)封闭:PVDF膜置于5%脱脂牛奶中封闭,缓慢摇晃,室温封闭2 h;(7):孵育一抗:兔单克隆抗体[EPR7678] to AK3L1抗体,(1∶7000,品牌:Abcam,货号:ab131327)。一抗孵育过夜,4 ℃;(8):孵育二抗:山羊抗兔IgG(H+L)(品牌:Proteintech,货号:SA00001-2,1∶10000),室温孵育2 h;(9)显影:ECL化学发光液孵育1 min后,于成像仪中曝光显影。
1.4 增殖EdU实验
(1)转染细胞:6孔板培养细胞。免疫印迹实验已明确siRNA-AK4-3的沉默效果最好,故本次实验使用siRNA-NC及siRNA-AK4-3做细胞转染。分组亦同。转染完成24 h开始进行EdU实验。(2)工作液孵育:使用BeyoclickTM Edu-555配置2XEdU工作液后(品牌:Beyotime,货号:C0075s),与无抗生素完全培养基等体积加入六孔板中,孵育2 h。(3):固定、染色:每孔1 mL固定液固定15 min,洗涤后加入通透液孵育15 min,Click反应液孵育15 min,1X Hoechst 33342溶液孵育10 min。(4):荧光检测:洗涤液清洗后于倒置荧光显微镜观察。
1.5 细胞划痕实验
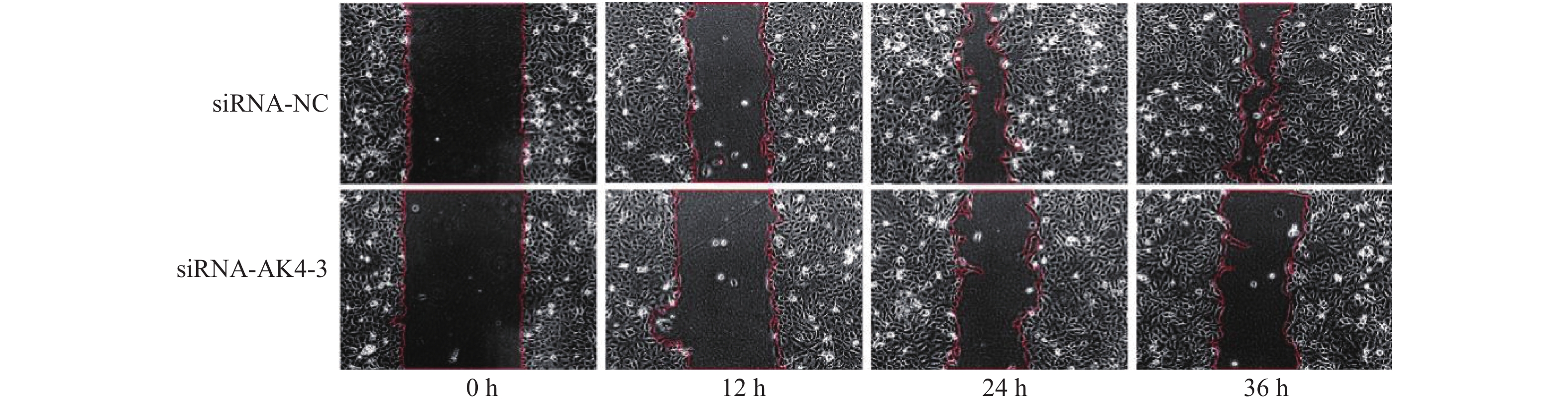
(1)转染细胞:6孔板培养细胞。本次实验分组为siRNA-NC及siRNA-AK4-3。转染完成24 h开始细胞划痕实验。(2)画线:使用直尺于6孔板背面作横行画线。(3)划痕:待细胞融合度为95%~100%时,使用200 µL枪头沿直尺作垂直于画线的划痕。(4)冲洗:使用PBS冲洗孔板3次,动作轻柔,吸净PBS后,加入无血清1640培养液。(5)拍照:每孔以“十”字交叉处上下为定点,于0 h,12 h,24 h,36 h拍照,对比不同时间下各组细胞迁移能力,并进行统计分析。
1.6 统计学处理
数据分析使用GraphPad Prism 8及SPSS 26统计软件,计量资料采用(
$\bar x \pm s $ ),计数资料用t检验,3组及多组计量资料采用单因素方差分析。所有检验取两端。P < 0.05为差异有统计学意义。2. 结果
2.1 siRNA转染效率及筛选
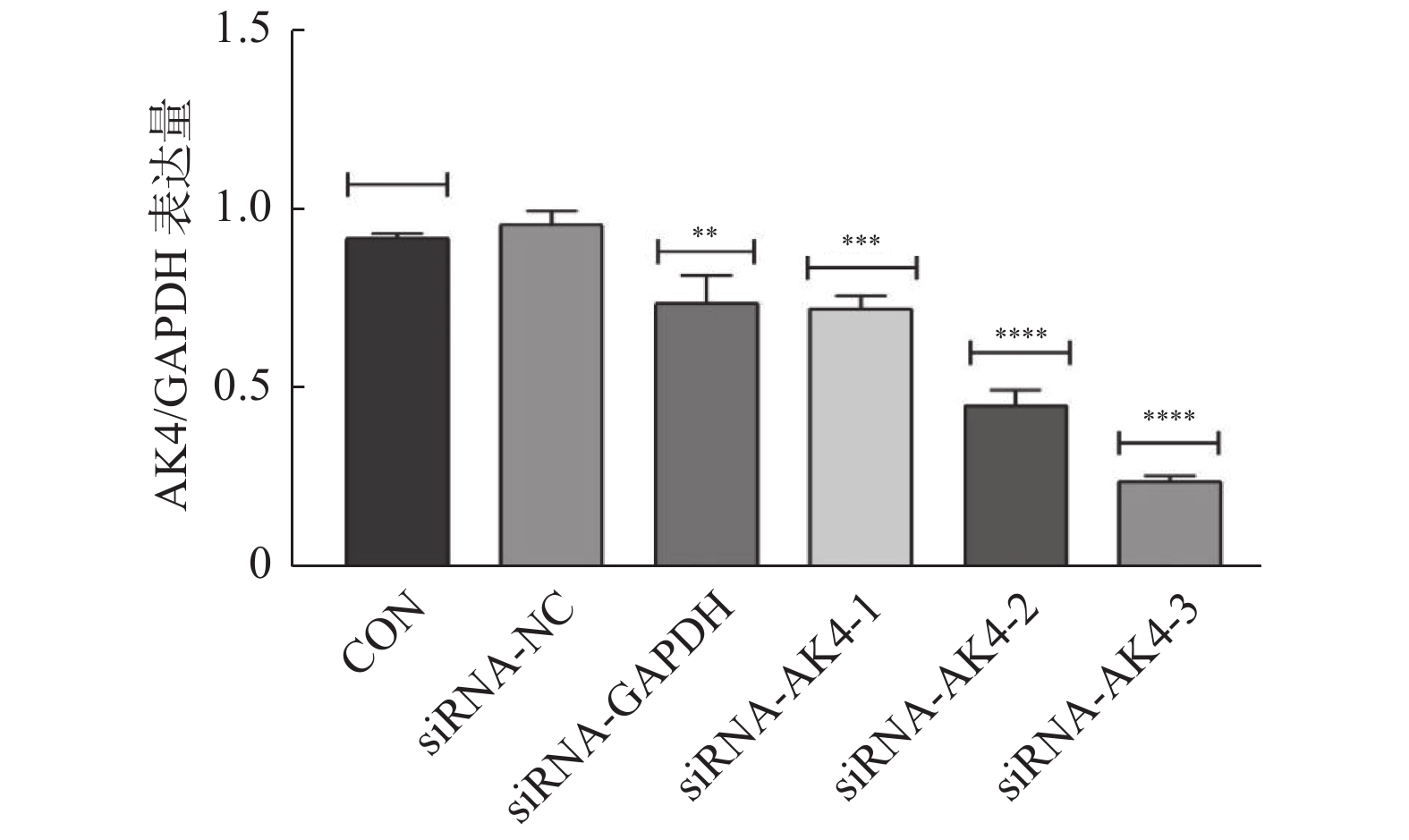
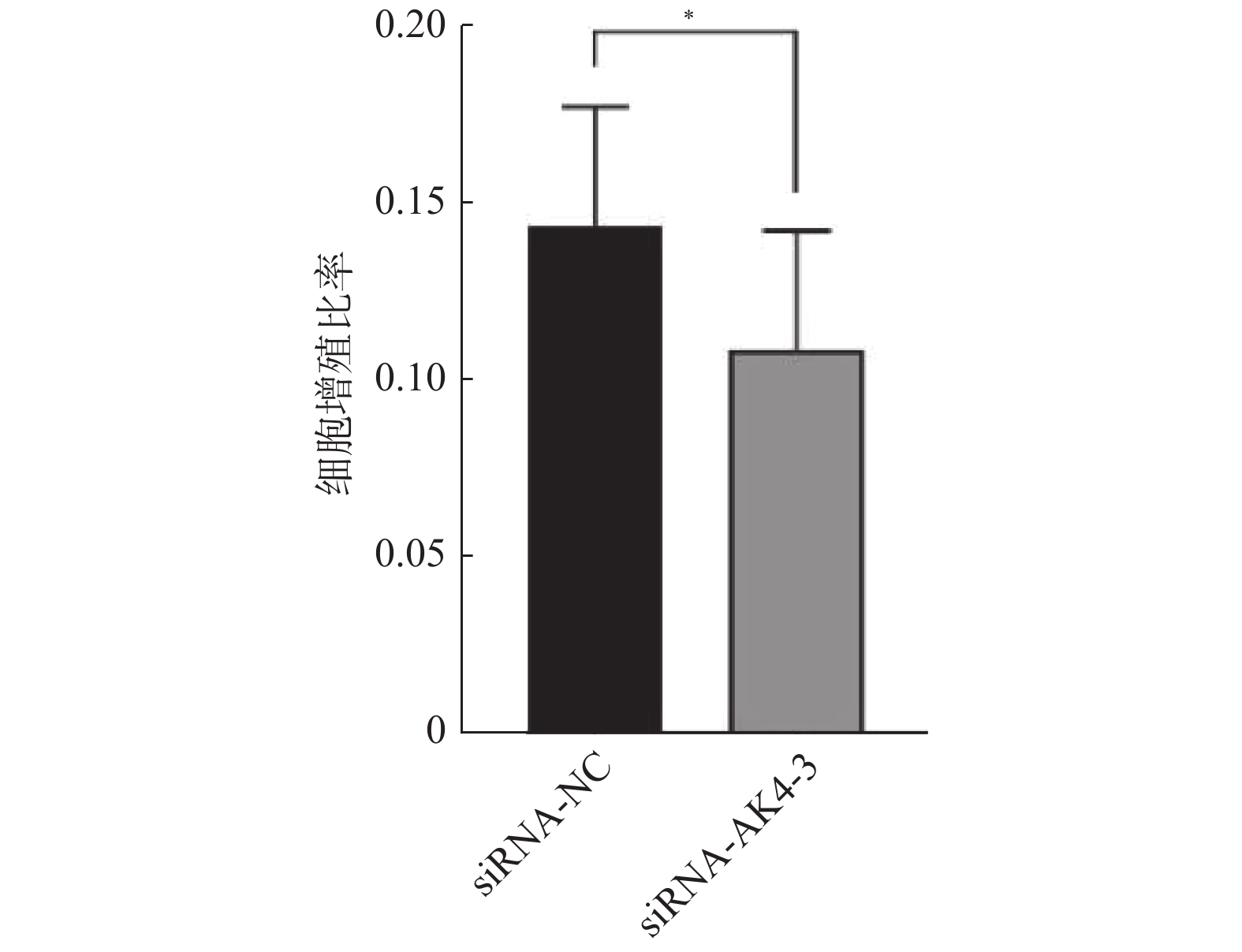
免疫印迹法检测各组后量化结果分别为:空白对照组(CON):(0.9±0.01,)阴性对照组(siRNA-NC):(0.92±0.01),阳性对照组(siRNA-GAPDH):(0.95±0.04),实验组1(siRNA-AK4-1):(0.74±0.08),实验组2(siRNA-AK4-2):(0.45±0.04),实验组3(siRNA-AK4-3):(0.24±0.02)。免疫印迹结果显示各组内参齐,沉默效果较好,其中阳性对照组对GAPDH的沉默效果显著。各siRNA组中siRNA-AK4-3对AK4的沉默效果最好,见图1,2。因此,后期实验使用siRNA-NC作为对照组,siRNA-AK4-3作为实验组。
2.2 增殖EdU实验
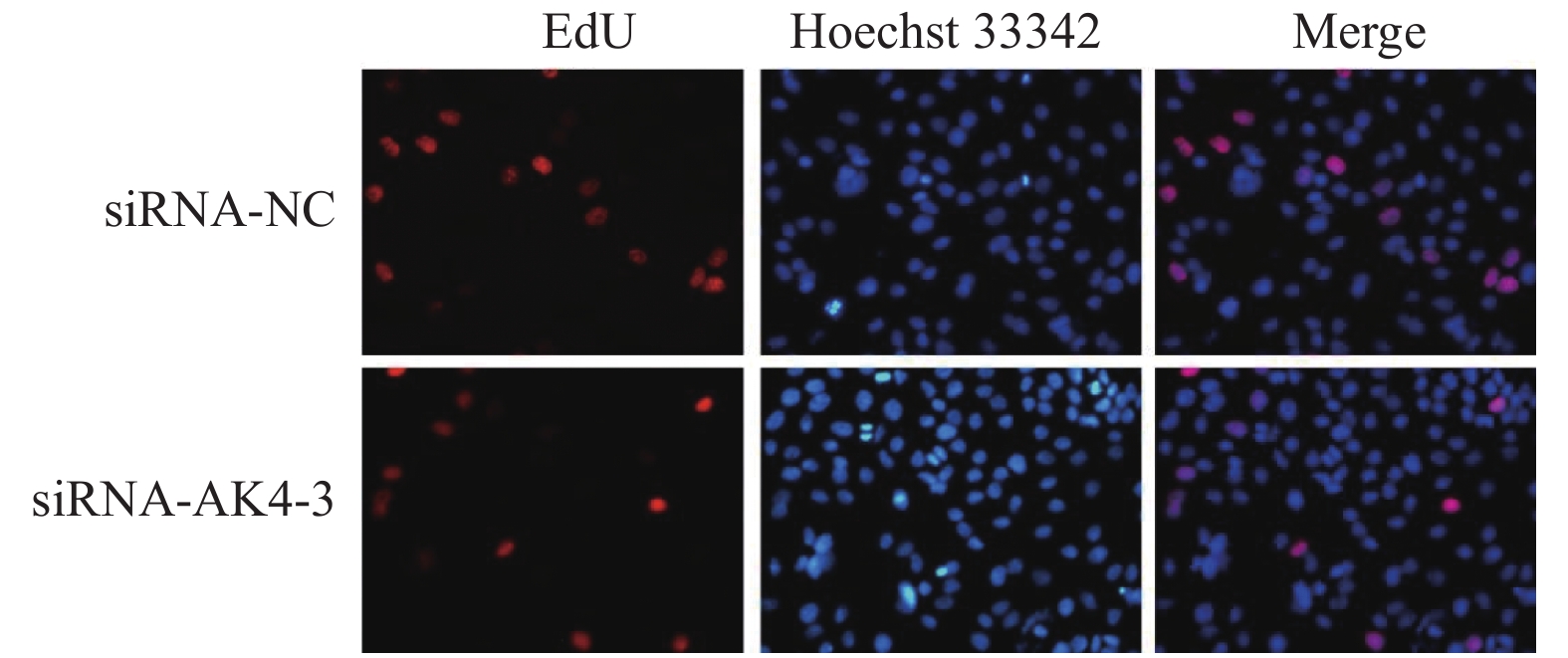
Edu实验结果显示:siRNA-NC组增殖细胞(15.9±4.4)/视野,细胞总数(110.9±22.4)/视野。siRNA-AK4-3组增殖细胞数目(12.8±5.0)/视野,细胞总数(116.7±22.1)/视野。两组增殖比率有差异,差异有统计学意义。siRNA-NC组增殖细胞多于siRNA-AK4-3组,AK4促进细胞增殖,见图3,4。
图3中EdU中增殖的HUCCT1细胞被标记为红色荧光,Hoechst 33342中蓝色荧光标记的为视野下所有活细胞,Merge图由EdU和Hoechst 33342图像合并后得到。
2.3 细胞划痕实验
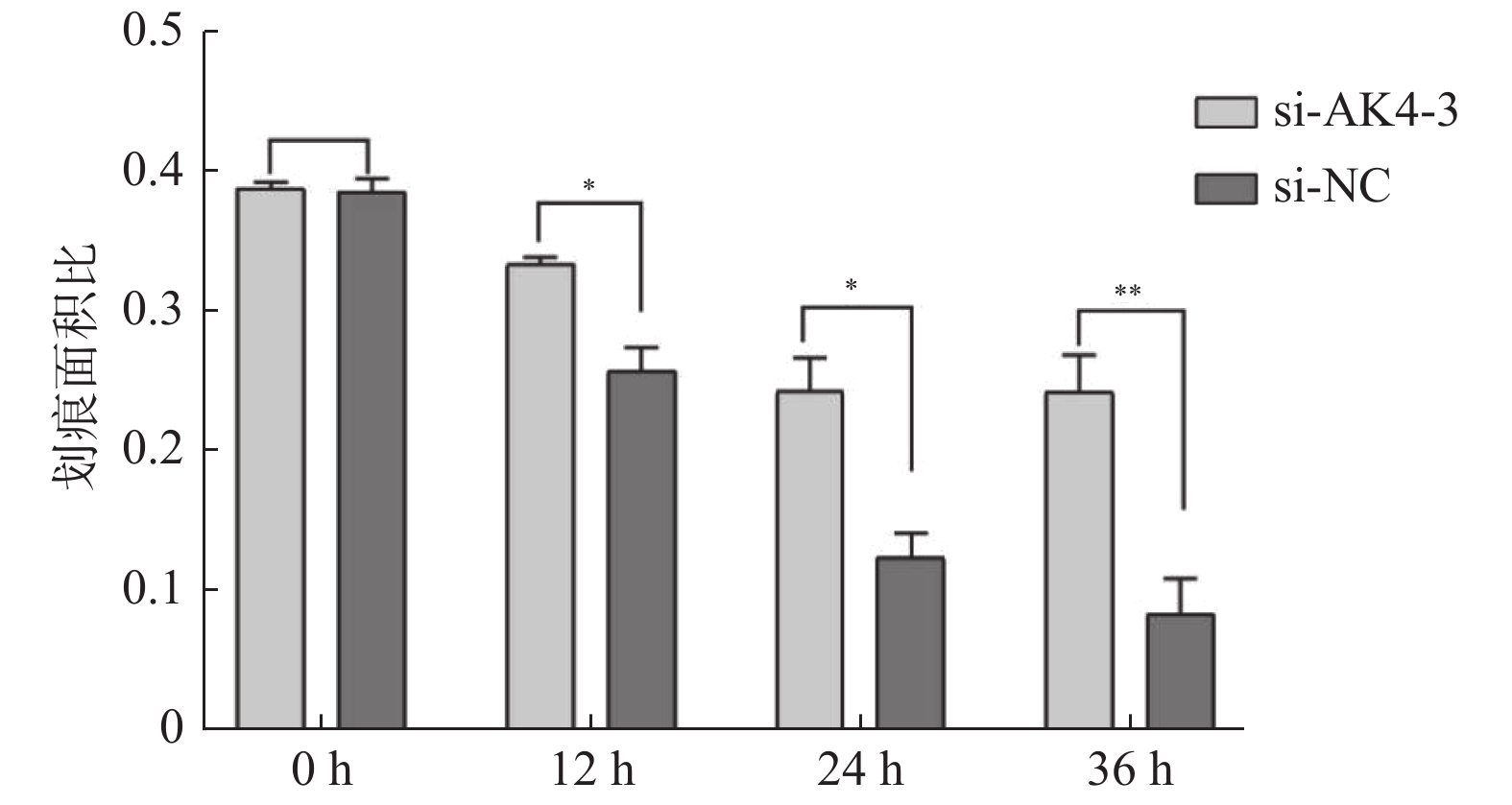
划痕实验取划痕面积/视野总面积,各组面积比见表2,差异有统计学意义,见图5,6。siRNA-NC组细胞迁移面积较siRNA-AK4-3组更大,AK4促进细胞迁移。
表 2 划痕面积比Table 2. Scratch area ratio项目 0 h 12 h 24 h 36 h siRNA-NC 0.39 ± 0.01 0.26 ± 0.017 0.12 ± 0.017 0.08 ± 0.026 siRNA-AK4-3 0.39 ± 0.005 0.33 ± 0.001 0.24 ± 0.024 0.24 ± 0.027 3. 讨论
肝内胆管癌的手术治疗是极其重要的,目前全球范围内术后其平均无瘤生存时间( DFS) 为12 ~ 36 个月[6-7],但胆管癌起病隐匿,多数患者在发现疾病时已属晚期[8],据报道,肝内胆管癌的根治切除率为30%~40% ,其他肝胆恶性肿瘤均比此数据高[1,9]。肝内胆管癌的辅助化疗(吉西他滨联合铂类)在一定程度上实现肿瘤将期,从而获得手术机会[10-12],但其中位生存期仍然不到1 a时间[13-15]。根据Andrew X Zhu的RCT临床研究,靶向药物Ivosidenib使得患者有10.3个月的中位生存期,而安慰剂组仅有7.5个月[16],Abou-Alfa GK等学者也通过RCT实验研究了Ivosidenib在胆管癌中的作用,其结果是实验组的无病生存期为2.7个月(95% CI:1.6~4.2),而安慰剂组的无病生存期为1.4个月(95% CI:1.4~1.6)[17]。A Demols也在胆管癌的靶向治疗上进行了RCT研究,他研究了靶向药Regorafenib对胆管癌作用,最终得出实验组无病生存期为3.0个月(95% CI:2.3~4.9),而对照组无病生存期为1.5个月(95% CI:1.2~2.0),然而实验组的总生存时间和对照组的总生存时间并无统计学差异[18]。可以看出,靶向药能提高患者生存时间,但有研究显示部分靶向药并不能显著提升患者的总生存率[18, 19]。因此需寻找一个更有效的作用靶点。
1944年,美国学者Kalckar在实验中首次发现了肌苷酸[20]。在随后的科学实践中,更名为腺苷酸激酶AK。AK4定位于细胞线粒体基质内,在肝脏、心脏、脑、肾脏、胃肠道组织中富于表达[21],AK4在细胞能量代谢方面表现出特异性[22],因此他与肿瘤应该存在相关性。2012年由台湾地区学者Yi-Hua Jan完成了首例AK4与癌症关系论证的研究:体外试验中shRNA沉默肺癌细胞CL1-5和A549中的AK4后,两株细胞的侵袭能力下降约50%,从而证实AK4促进肺癌细胞的侵袭[3]。连云港市的学者MinMin HUANG在研究中显示AK4在人浆液性卵巢癌组织中高表达。在体内试验中,AK4敲低组的肿瘤体积明显减小,肿瘤重量明显减轻。这些试验均显示AK4促进了人浆液性卵巢癌的发生发展[4]。Jie Zhang等在Her-2阳性乳腺癌中的研究显示AK4的表达水平与肿瘤TNM分期(P = 0∶017)和淋巴结转移(P = 0∶046) 显著相关。体外试验中,MTT法、细胞划痕实验和Transwell试验都显示敲低AK4组的癌细胞增值、迁移、侵袭能力减弱。体外试验也显示敲低了AK4的肿瘤组织体积减小,重量减轻[5]。此后AK4与肿瘤的研究未曾断绝,田华等学者通过免疫组化等方法证实了AK4在肺腺癌中高表达[23]。李辰运的研究显示AK4在胰腺导管腺癌中高表达,并且与肿瘤分期、淋巴结转移、神经受侵、脉管内瘤栓有相关性(P < 0.05)[24]。李绍军等也证实AK4在食管鳞状细胞中高表达[25],夏林等也通过免疫组化的方式证实了AK4在胃癌中高表达[26],尽管我国的多数学者都明确了AK4在大多数肿瘤中的表达,但是很遗憾,仅有少数人通过体内、体外实验论证AK4对相关癌症的影响,无法将他们的研究进一步转化为临床制定治疗方案的依据。
本实验首次论证AK4对肝内胆管癌细胞HUCCT1增殖、迁移的影响。首先予siRNA沉默AK4的表达,再通过EdU检测细胞增殖能力,细胞划痕实验检测细胞迁移能力。本次实验的结论与前人的研究结论相似,即AK4促进肝内胆管癌细胞HUCCT1的增殖、迁移。本实验的不足在于,笔者尚未完成AK4对肝内胆管癌细胞其他生物学能力的影响,如EMT、侵袭等等。因此,笔者接下来即将完成其他细胞生物学行为实验,并且探究在机制方面的变化以及通过体内实验验证结论。本实验结论将为肝内胆管癌的临床分子靶向治疗提供基础实验数据,采用生物医学工程技术干预肝内胆管癌组织中AK4的表达,将有助于肿瘤的综合治疗。
-
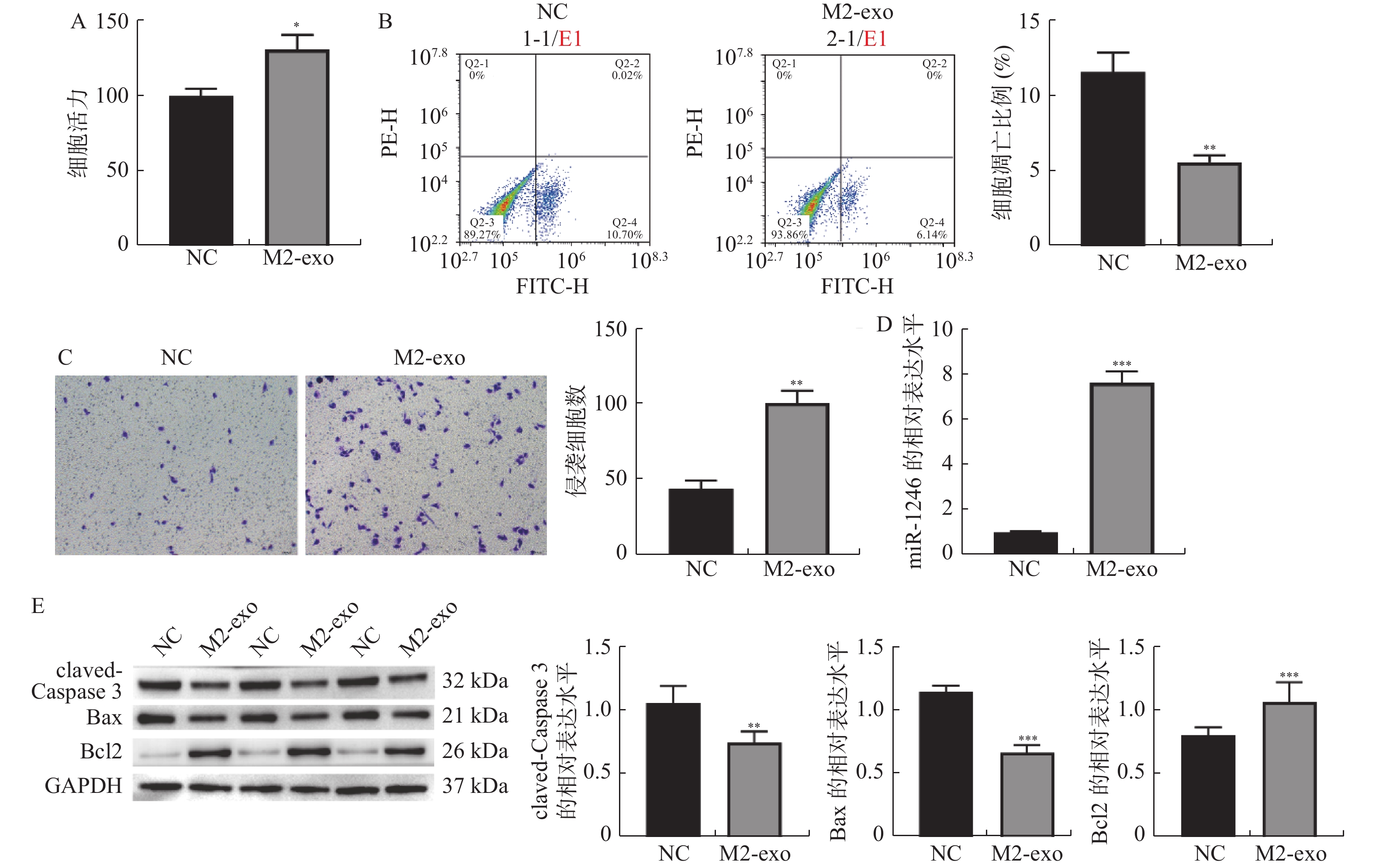
图 2 M2巨噬细胞来源外泌体促进AGS细胞增殖和侵袭,并抑制其凋亡
A:CCK-8检测M2巨噬细胞来源外泌体对AGS细胞活力的影响;B:Annexin V-FITC/PI检测AGS细胞凋亡的流式结果;C:Transwell检测AGS细胞侵袭的代表性图片和统计分析(×40);D:RT-qPCR检测M2巨噬细胞来源外泌体对AGS细胞中miR-1246表达的影响;E:Western blot 检测凋亡相关蛋白claved-caspase3,BAX以及BCL2表达。相较于NC组,*P < 0.05,**P < 0.01,***P < 0.001。
Figure 2. M2 macrophage-derived exosomes promoted the proliferation and invasion of AGS cells,and inhibited their apoptosis
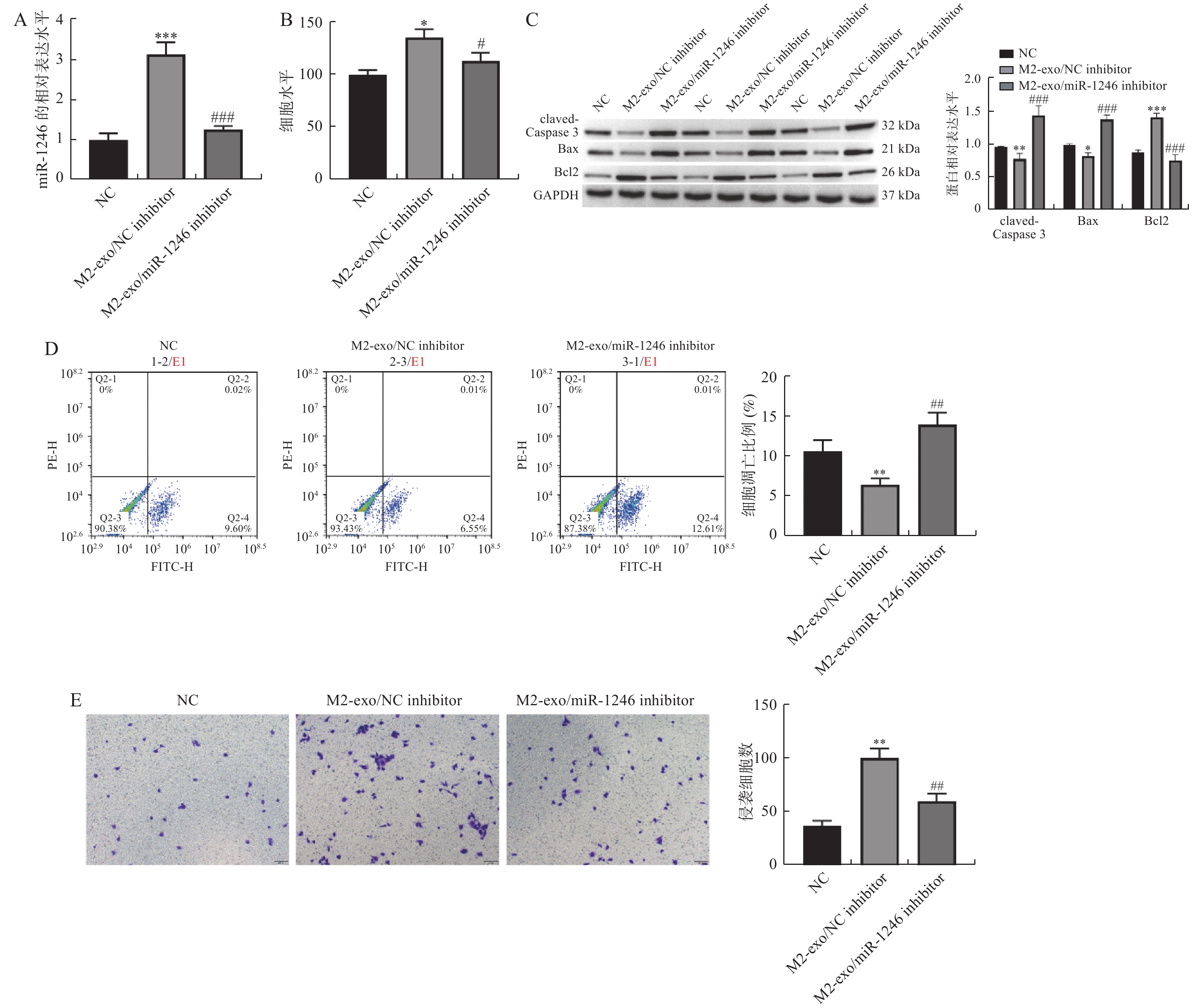
图 3 M2巨噬细胞来源外泌体miR-1246促进AGS细胞增殖和侵袭,并抑制其凋亡
A:RT-qPCR检测各组AGS细胞中miR-1246的表达变化;B:由CCK-8试剂盒检测得到的AGS细胞活力变化;C:Western blot 检测凋亡相关蛋白claved-caspase3,BAX以及BCL2表达;D:AGS细胞凋亡比例的流式代表性图片和统计分析结果;E:Transwell检测AGS细胞的侵袭变化(×40)。相较于NC组,*P < 0.05,**P < 0.01,***P < 0.001;相较于M2-exo/NCinhibitor组,#P < 0.05,##P < 0.01,###P < 0.001。
Figure 3. M2 macrophage-derived exosome miR-1246 promoted the proliferation and invasion of AGS cells,and inhibited their apoptosis
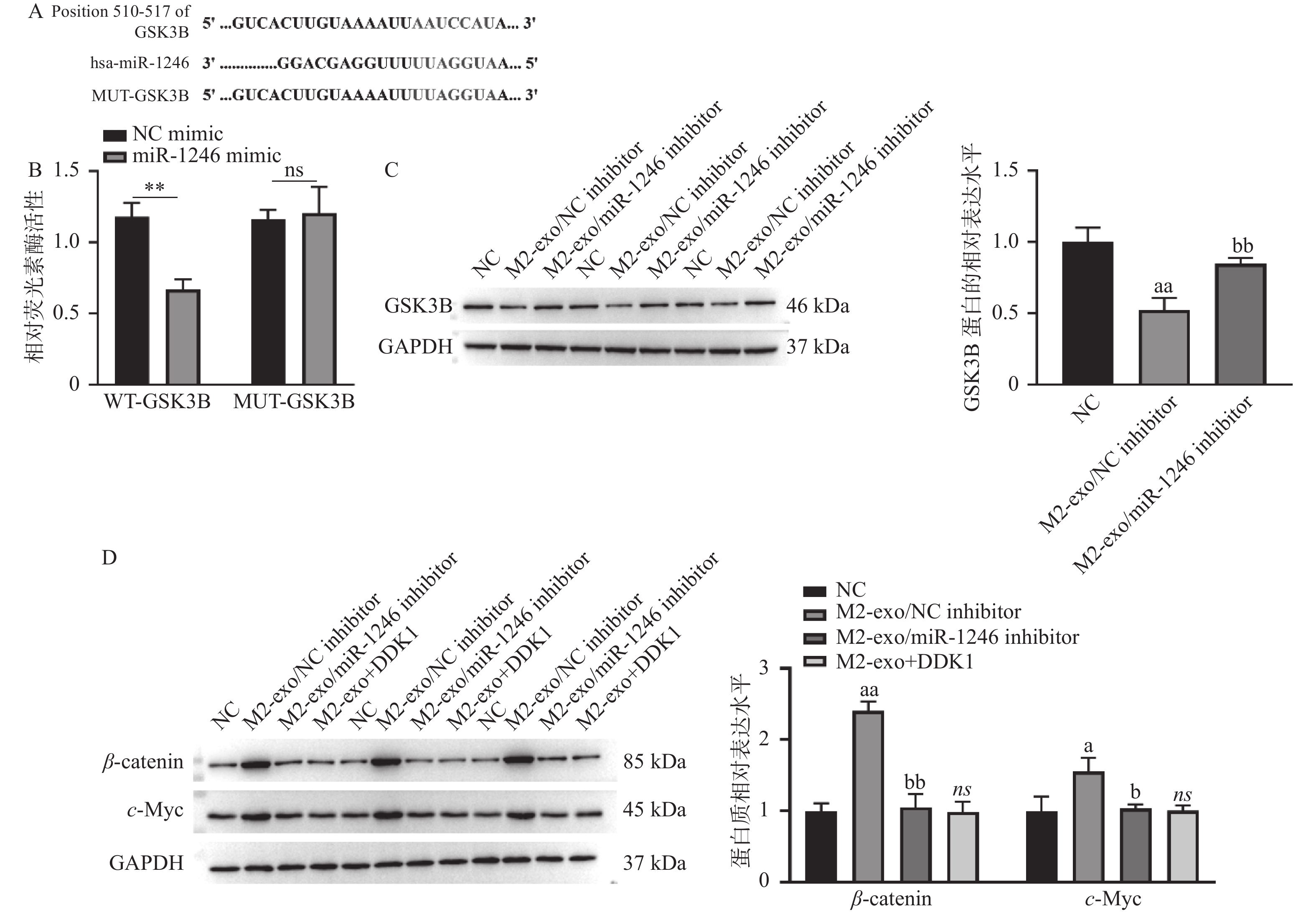
图 4 miR-1246靶向调控Wnt信号通路
A:TargetScan数据库预测得到的miR-1246与GSK3B的潜在结合序列;B:双荧光素酶报告基因实验验证miR-1246与GSK3B的靶向关系;C:WB检测M2巨噬细胞来源外泌体miR-1246对AGS细胞中GSK3B蛋白表达的影响;D:WB检测M2巨噬细胞来源外泌体miR-1246对AGS细胞中Wnt信号通路的影响。相较于NCmimic组,**P < 0.01;相较于NC组,aP < 0.05,aaP < 0.01;相较于M2-exo/NCinhibitor组,bP < 0.05,bbP < 0.01;ns表示差异无统计学意义。
Figure 4. miR-1246 targets the Wnt signaling pathway
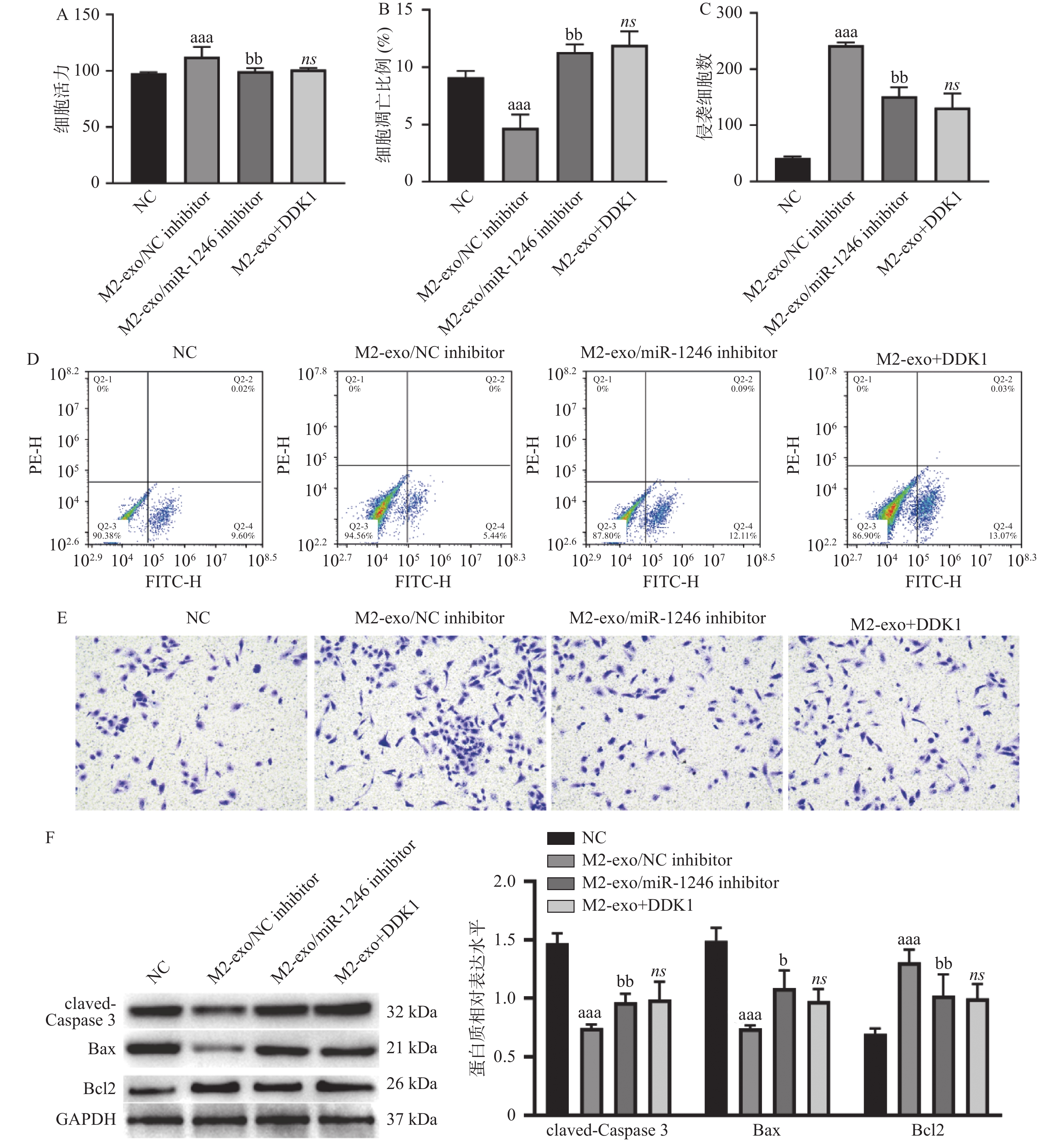
图 5 M2巨噬细胞来源外泌体miR-1246靶向GSK3B促进胃癌细胞增殖和侵袭、并抑制其凋亡
A:由CCK-8试剂盒检测得到的AGS细胞活力变化;B:AGS细胞凋亡比例的统计分析结果;C:Transwell检测AGS细胞的侵袭变化统计分析结果;D:AGS细胞凋亡比例的流式代表性图片;E:Transwell检测AGS细胞的侵袭变化代表性图片(×40);F:Western blot 检测凋亡相关蛋白claved-caspase3,BAX以及BCL2表达。相较于NC组,aP < 0.05,aaP < 0.01,aaaP < 0.001;相较于M2-exo/NCinhibitor组,bP < 0.05,bbP < 0.01;ns表示差异无统计学意义。
Figure 5. M2 macrophage-derived exosome miR-1246 targets GSK3B to promote proliferation and invasion and inhibit apoptosis of gastric cancer cells
-
[1] Thrift A P,El-Serag H B. Burden of gastric cancer[J]. Clin Gastroenterol Hepatol,2020,18(3):534-542. doi: 10.1016/j.cgh.2019.07.045 [2] Fitzmaurice C,Abate D,Abbasi N,et al. Global,regional,and national cancer incidence,mortality,years of life lost,years lived with disability,and disability-adjusted life-years for 29 cancer groups,1990 to 2017: A systematic analysis for the global burden of disease study[J]. JAMA Oncol,2019,5(12):1749-1768. doi: 10.1001/jamaoncol.2019.2996 [3] 曹毛毛,李贺,孙殿钦,等. 2000—2019年中国胃癌流行病学趋势分析[J]. 中华消化外科杂志,2021,20(1):8. [4] Sung H,Ferlay J,Siegel R L,et al. Global cancer statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2021,71(3):209-249. doi: 10.3322/caac.21660 [5] Arnold M,Abnet C C,Neale R E,et al. Global burden of 5 major types of gastrointestinal cancer[J]. Gastroenterology,2020,159(1):335-349.e15. doi: 10.1053/j.gastro.2020.02.068 [6] Hegde P S,Chen D S. Top 10 challenges in cancer immunotherapy[J]. Immunity,2020,52(1):17-35. doi: 10.1016/j.immuni.2019.12.011 [7] Vitale I,Manic G,Coussens L M,et al. Macrophages and metabolism in the tumor microenvironment[J]. Cell Metab,2019,30(1):36-50. doi: 10.1016/j.cmet.2019.06.001 [8] 王一晨,杨文山,董宪喆,等. 肿瘤相关巨噬细胞的作用综述[J]. 解放军医学院学报,2021,42(12):1315-1321. doi: 10.3969/j.issn.2095-5227.2021.12.017 [9] Xia Y,Rao L,Yao H,et al. Engineering macrophages for cancer immunotherapy and drug delivery[J]. Adv Mater,2020,32(40):e2002054. doi: 10.1002/adma.202002054 [10] Mohapatra S,Pioppini C,Ozpolat B,et al. Non-coding RNAs regulation of macrophage polarization in cancer[J]. Mol Cancer,2021,20(1):24. doi: 10.1186/s12943-021-01313-x [11] 谢玙玙,段昕所. 肿瘤微环境下巨噬细胞的极化和靶向治疗意义[J]. 华西医学,2021,36(5):679-685. [12] Kalluri R,Lebleu V S. The biology,function,and biomedical applications of exosomes[J]. Science,2020,367(6478):eaau6977. [13] 王莹,杨婷蓉,陈雅,等. 外泌体的生物学特征及其作为靶向药物载体在恶性肿瘤的应用[J]. 肿瘤代谢与营养电子杂志,2021,8(6):6. [14] 徐锋,张真发. 肿瘤源性外泌体调节肺癌免疫微环境及作为肺癌生物标志物的应用[J]. 癌症,2021,40(9):6. [15] Mao X,Xu J,Wang W,et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives[J]. Mol Cancer,2021,20(1):131. doi: 10.1186/s12943-021-01428-1 [16] Wei L,Sun J,Zhang N,et al. Noncoding RNAs in gastric cancer: implications for drug resistance[J]. Mol Cancer,2020,19(1):62. doi: 10.1186/s12943-020-01185-7 [17] Shi Y,Wang Z,Zhu X,et al. Exosomal miR-1246 in serum as a potential biomarker for early diagnosis of gastric cancer[J]. Int J Clin Oncol,2020,25(1):89-99. doi: 10.1007/s10147-019-01532-9 [18] Qian X,Xie F,Wei H,et al. Identification of key circulating exosomal microRNAs in gastric cancer[J]. Front Oncol,2021,11(1):693360. doi: 10.3389/fonc.2021.693360 [19] Yang Y,Guo Z,Chen W,et al. M2 macrophage-derived exosomes promote angiogenesis and growth of pancreatic ductal adenocarcinoma by targeting E2F2[J]. Mol Ther,2021,29(3):1226-1238. doi: 10.1016/j.ymthe.2020.11.024 [20] Wortzel I,Dror S,Kenific C M,et al. Exosome-mediated metastasis: communication from a distance[J]. Dev Cell,2019,49(3):347-360. doi: 10.1016/j.devcel.2019.04.011 [21] Doyle L M,Wang M Z. Overview of extracellular vesicles,their origin,composition,purpose,and methods for exosome isolation and analysis[J]. Cells,2019,8(7):727. [22] Pittet M J,Michielin O,Migliorini D. Clinical relevance of tumour-associated macrophages[J]. Nat Rev Clin Oncol,2022,19(6):402-421. doi: 10.1038/s41571-022-00620-6 [23] Zheng P,Chen L,Yuan X,et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells[J]. J Exp Clin Cancer Res,2017,36(1):53. doi: 10.1186/s13046-017-0528-y [24] Yang X,Cai S,Shu Y,et al. Exosomal miR-487a derived from m2 macrophage promotes the progression of gastric cancer[J]. Cell Cycle,2021,20(4):434-444. doi: 10.1080/15384101.2021.1878326 [25] Lin S S,Peng C Y,Liao Y W,et al. MiR-1246 targets CCNG2 to enhance cancer stemness and chemoresistance in oral carcinomas[J]. Cancers (Basel),2018,10(8):272. [26] Xu X,Cao L,Zhang Y,et al. MicroRNA-1246 inhibits cell invasion and epithelial mesenchymal transition process by targeting CXCR4 in lung cancer cells[J]. Cancer Biomark,2018,21(2):251-260. doi: 10.3233/CBM-170317 [27] Zhang Y,Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer[J]. J Hematol Oncol,2020,13(1):165. doi: 10.1186/s13045-020-00990-3 [28] Yu F,Yu C,Li F,et al. Wnt/β-catenin signaling in cancers and targeted therapies[J]. Signal Transduct Target Ther,2021,6(1):307. doi: 10.1038/s41392-021-00701-5 [29] Zhang X,Zhong S,Xu Y,et al. MicroRNA-3646 contributes to docetaxel resistance in human breast cancer cells by GSK-3β/β-catenin signaling pathway[J]. PLoS One,2016,11(4):e0153194. doi: 10.1371/journal.pone.0153194 [30] Yang F,Xiong H,Duan L,et al. MiR-1246 promotes metastasis and invasion of A549 cells by targeting GSK-3β‒mediated Wnt/β-catenin pathway[J]. Cancer Res Treat,2019,51(4):1420-1429. doi: 10.4143/crt.2018.638 期刊类型引用(4)
1. 穆雨,周亚辉. 不同孕周分娩的新生儿颅脑超声评估特点及临床意义. 罕少疾病杂志. 2024(01): 19-20 .  百度学术
百度学术2. 陈慧慧. 颅脑超声在诊断新生儿颅脑疾病的临床应用价值. 现代医用影像学. 2024(06): 1159-1161 .  百度学术
百度学术3. 李艳,刘芬,谢桢,陶琦,田袁静. 高频超声联合低频超声检查诊断新生儿颅脑病变的临床价值研究. 现代生物医学进展. 2022(10): 1932-1936 .  百度学术
百度学术4. 史晶,于宁. 颅脑超声检查联合血清D二聚体、乳酸脱氢酶在不同胎龄早产儿颅脑损伤诊断中的应用. 医学影像学杂志. 2022(12): 2054-2057 .  百度学术
百度学术其他类型引用(2)
-






 下载:
下载:
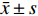

 下载:
下载: