Preoperative Fasting Triglyceride-glucose Index as a Predictor of Contralateral New Silent Ischemic Brain Lesions after Carotid Artery Stenting
-
摘要:
目的 探讨术前空腹甘油三酯-血糖指数(triglyceride-glucose index,TyG)对颈内动脉支架置入术(carotid artery stenting,CAS)后对侧新发无症状缺血性脑损伤(contralateral new silence ischemic brain lesions,CNSIBL)的预测价值。 方法 回顾性分析行颈内动脉支架置入术患者183例,根据是否存在CNSIBL发生的情况分为CNSIBL组50例和non- CNSIBL组133例,收集患者基线资料、实验室检查及影像学检查指标,计算TyG。以是否发生CNSIBL为因变量,控制混杂因素后,以TyG为自变量进行多因素Logistic回归分析和受试者工作特征曲线评价TyG对CAS后CNSIBL的预测价值。 结果 (1)CNSIBL组患者糖尿病病史例数、入院时收缩压及舒张压高于non-CNSIBL组(P < 0.05);(2)CNSIBL组甘油三酯高于non-CNSIBL组(P < 0.05);CNSIBL组TyG高于non-CNSIBL组(P < 0.05);(3)多因素Logistic回归分析结果提示TyG [矫正OR = 1.125,95%CI (1.042−1.214),P < 0.001]是颈内动脉支架置入术后对侧新发无症状缺血性脑损伤的独立影响因素;(4)ROC曲线提示,TyG预测颈内动脉支架置入术后对侧新发无症状缺血性脑损伤的AUC为0.77[95%CI (0.71−0.84),P < 0.001],cut-off值为1.93,灵敏度86.0%,特异度63.9%。 结论 TyG是颈内动脉支架置入术后对侧新发无症状缺血性脑损伤的独立影响因素。 -
关键词:
- 颈内动脉支架置入术 /
- 对侧新发无症状缺血性脑损伤 /
- 甘油三酯-血糖指数 /
- 动脉粥样硬化
Abstract:Objective To investigate the predictive value of the preoperative fasting Triglyceride-Glucose index (TyG) for contralateral new silence ischemic brain lesions (CNSIBL) following carotid artery stenting (CAS). Methods A retrospective study was conducted to analyze the clinical data of 183 patients who underwent carotid CAS. The patients were divided into a CNSIBL group (50 cases) and a non-CNSIBL group (133 cases) based on the occurrence of CNSIBL. Baseline data, laboratory tests, and imaging indicators were collected, and TyG was calculated. Using the occurrence of CNSIBL as the dependent variable, multivariate logistic regression analysis was performed with TyG as the independent variable after controlling for confounding factors, and the predictive value of TyG for CNSIBL post-CAS was evaluated using receiver operating characteristic (ROC) curves. Results (1)The number of patients with a history of diabetes mellitus, as well as systolic and diastolic blood pressure on admission in CNSIBL group were statistically significantly higher than that in non-CNSIBL group(P < 0.05).(2) Triglyceride (TC) levels were higher in the CNSIBL group compared to the non-CNSIBL group(P < 0.05); TyG was also higher in the CNSIBL group than in the non-CNSIBL group (P < 0.05); (3) Multivariate Logistic regression analysis results showed that TyG [a OR = 1.125, 95%CI (1.042−1.214), P < 0.001]was an independent risk factor for contralateral new silent ischemic brain lesions after carotid artery stenting;(4) The ROC curve suggested that the AUC for TyG predicting contralateral new silent ischemic brain lesions post-CAS was 0.77 [95%CI (0.71−0.84), P < 0.001], with a cut-off value of 1.93, sensitivity of 86.0%, and specificity of 63.9%. Conclusion TyG is an independent influencing factor for contralateral new silent ischemic brain lesions following carotid artery stenting. -
颈动脉粥样硬化狭窄是缺血性脑血管病的主要诱因,血脂代谢紊乱导致动脉粥样硬化的重要危险因素[1]。颈动脉支架置入术(carotid artery stenting,CAS)是一种预防缺血性脑血管病的血管内治疗方法。尽管栓子保护装置(embolic protection devices,EPD)广泛使用,然而颈动脉支架置入术后颅脑核磁共振成像扩散加权相(magnetic resonance imaging diffusion-weighted imaging,MRI-DWI)提示新发无症状缺血性脑损伤(new silence ischemic brain lesions,NSIBL)发生率为18%~57%,同时CAS-NSIBL可增加未来脑血管病事件、认知障碍等风险 [2−3]。目前NSIBL仍是CAS术后一项重要的临床难题。众所周知,胰岛素抵抗(insulin resistance,IR)与血脂代谢异常呈正相关,IR通过增加血管炎性反应、改变凝血功能从而影响血脂代谢,加重动脉粥样硬化程度。甘油三酯-葡萄糖指数(riglyceride-glucose index,TyG)作为评估IR的重要指标,与血脂代谢异常、冠状动脉粥样硬化、颈动脉硬化、脑血管病有关[4−6]。因此,深入研究TyG与CAS后对侧新发无症状缺血性脑损伤(contralateral new silence ischemic brain lesions,CNSIBL)的关系并筛查评估治疗的指标尤为重要。然而目前有关TyG与CAS后发生CNSIBL的研究甚少。基于TyG指数与动脉粥样硬化相关且易得的优势,本研究通过评估TyG与CAS-CNSIBL的关系,旨在分析TyG对CAS后CNSIBL的预测价值。
1. 资料与方法
1.1 研究对象
选取2018年10月至2022年12月首都医科大学附属北京友谊医院神经内科住院的颈内动脉支架置入术患者183例,根据CNSIBL发生的情况分为CNSIBL组50例和non-CNSIBL组133例。
1.2 纳入与排除标准
纳入标准[3]:(1)行颈内动脉颅外段置入术者,无临床症状者数字减影血管造影显示颈内动脉管腔直径狭窄率≥70%,有神经系统相关临床症状者数字减影血管造影显示颈内动脉管腔直径狭窄≥50%;行颈内动脉颅内段支架置入术者,有临床症状者数字减影血管造影显示颈内动脉管腔直径狭窄率≥70%;(2)颈动脉狭窄均为大动脉粥样硬化型所致;(3)患者入院时行头CT、血常规、血生化等检查,术后24 h内完善头MRI;(4)患者及家属均签署知情同意。
排除标准:(1)首诊影像学提示脑出血、大面积脑梗死;(2)感染、自身免疫性疾病、肿瘤、肝功能异常、肾功能异常、血液系统疾病、急性心肌梗死;(3)曾行颅脑血管内治疗;(4)动脉炎、动静脉畸形等所致颈内动脉狭窄者。本研究经首都医科大学附属北京友谊医院伦理委员会审核批准(批准号2021-P2-167-01)。
1.3 方法
所有拟行颈内动脉支架患者入院时收集基线资料、临床资料、实验室检查指标及数字减影血管造影检查,包括年龄、性别、高血压病史、糖尿病病史、心血管病病史、高脂血症病史、冠状动脉粥样硬化性心脏病病史,脑卒中病史、吸烟史、饮酒史、入院时收缩压、入院时舒张压;实验室检查指标采用入院后第2天晨5点(空腹至少8 h),包括空腹血糖(fasting blood glucose,FBG)、总胆固醇(total cholesterol,TC)。其中TyG 指数=ln[空腹甘油三酯(mg/dL)×空腹血糖(mg/dL)/2]。
颈内动脉分为颅内段和颅外段,根据Bouthillier提出颈内动脉分段法,颅内段包括:C1颈段、C2岩段、C3破裂孔段、C4海绵窦段;颅外段包括:C5床突段、C6眼段、C7交通段[7]。数字减影血管造影检查明确患者狭窄位置及狭窄性状,从而指导颈内动脉支架置入术操作。
患者行CAS前至少连续使用抗血小板聚集药物(拜阿斯匹林100 mg/d+吡格雷75 mg/d)7 d,所有手术均由同一颅内血管介入团队医师在局麻下行经皮股动脉入路完成,术中给予肝素(100 U/kg)抗凝、EPD,植入支架前进行预扩张,狭窄处根据患者颈内血管情况选择放置支架类型(开环支架/闭环支架),完成支架置入术后评估残余狭窄 < 30%。所有行CAS患者在术前、术中、术后均接受神经功能评估(图1)。
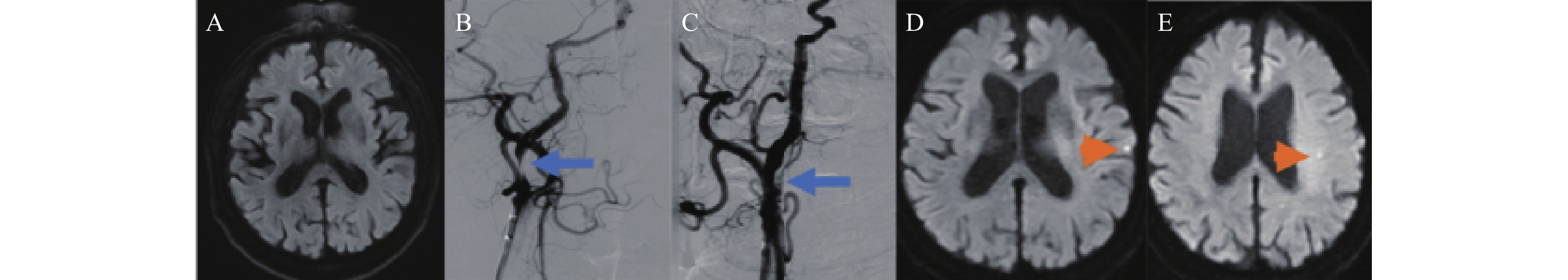 图 1 67岁男性右侧颈内动脉起始段重度狭窄行颈动脉起始段支架置入术A:术前头MRI-DWI,无脑梗死病灶;B:数字减影血管造影,右侧颈内动脉起始段重度狭窄(蓝色箭头所示);C:数字减影血管造影,右侧颈内动脉起始段支架置入术后(蓝色箭头所示);D:术后头MRI-DWI,左侧颞叶新发DWI高信号(橘色箭头所示);E:术后头MRI-DWI,左侧半卵圆中心新发DWI高信号(橘色箭头所示)。Figure 1. A 67-year-old male with severe stenosis in the right internal carotid artery origin (C1) undergoing stenting of the carotid artery origin
图 1 67岁男性右侧颈内动脉起始段重度狭窄行颈动脉起始段支架置入术A:术前头MRI-DWI,无脑梗死病灶;B:数字减影血管造影,右侧颈内动脉起始段重度狭窄(蓝色箭头所示);C:数字减影血管造影,右侧颈内动脉起始段支架置入术后(蓝色箭头所示);D:术后头MRI-DWI,左侧颞叶新发DWI高信号(橘色箭头所示);E:术后头MRI-DWI,左侧半卵圆中心新发DWI高信号(橘色箭头所示)。Figure 1. A 67-year-old male with severe stenosis in the right internal carotid artery origin (C1) undergoing stenting of the carotid artery origin1.4 统计学分析
采用SPSS 23.0 统计学软件进行数据统计分析。符合正态分布的计量资料以平均数±标准差($ \bar x \pm s $)表示,比较采用独立样本t检验分析;非正态分布的连续性变量以M(Q1,Q3)表示;计数资料分析采用χ2检验;采用多因素Logistic回归探索颈内动脉支架置入术后对侧新发无症状缺血性脑损伤的影响因素;ROC曲线评价TyG对颈内动脉支架置入术后新发对侧无症状缺血性脑损伤的预测价值。以P < 0.05为差异有统计学意义。
2. 结果
2.1 患者一般资料和实验室检查指标比较
本研究一共纳入183例行颈内动脉支架置入术患者,平均年龄(66.4±12.8)岁,男性137例(74.9%)。CAS后发生CNSIBL共50例,non-CNSIBL共133例。与non-CNSIBL组相比,CNSIBL组糖尿病病史例数、入院时收缩压及舒张压较高,甘油三酯、TyG较高(P < 0.05),见表1。
表 1 行颈内动脉支架置入术患者基线资料和实验室检查数据[$ \bar x \pm s $/M(P25,P75)/n(%)]Table 1. Baseline data and laboratory test results of patients undergoing carotid artery stenting [$ \bar x \pm s $/M(P25,P75)/n(%)]基线信息 non-CNSIBL组
(n) = 133CNSIBL组
(n = 50)t/χ2/z P 年龄(岁) 65.9 ± 12.7 67.7 ± 13.6 0.683 0.495 男性 99(74.4) 38(76.0) 0.047 0.828 高血压病史 95(71.4) 37(74.0) 0.120 0.730 糖尿病病史 62(46.6) 32(64.0) 4.396 0.036* 高脂血症病史 60(45.1) 22(44.0) 0.018 0.893 缺血性脑卒中病史 25(18.8) 11(22.0) 0.236 0.627 吸烟史 58(43.6) 18(36.0) 0.866 0.352 饮酒史 61(45.9) 24(48.0) 0.067 0.796 冠状动脉粥样硬化性心脏病病史 22(16.5) 12(24.0) 1.336 0.248 入院时收缩压(mmHg) 137.9 ± 23.1 146.8 ± 22.2 5.094 0.031* 入院时舒张压(mmHg) 85.4 ± 15.3 88.6 ± 16.4 3.741 0.049* 入院时NIHSS评分 4[2,7] 4[2,8] 0.496 0.506 静脉rt-PA治疗 27(20.3) 10(20.0) 0.002 0.964 颈内动脉颅内段 78(58.6) 32(64.0) 0.434 0.510 右侧颈内动脉 65(48.9) 23(46.0) 0.120 0.729 开环支架 87(65.4) 34(68.0) 0.109 0.742 空腹血糖(mmol/L) 4.94[4.29−8.11] 6.81[5.29−9.01] 3.734 0.068 甘油三酯 (mmol/L) 1.20[0.57−2.21] 1.94[1.14−3.06] 8.864 0.004* TyG 1.11[0.36−2.23] 1.87[1.06−2.44] 12.241 0.001* 注:CAS: 颈内动脉支架置入术;CNSIBL:对侧新发无症状缺血性脑损伤;NIHSS:美国国立卫生研究院卒中量表; rt-PA:重组组织型纤溶酶原激活剂; TyG: 甘油三酯-血糖指数;*P < 0.05。 2.2 多因素Logistic分析
矫正糖尿病病史、入院时收缩压、入院时舒张压因素,以糖尿病病史、入院时收缩压、入院时舒张压、TyG为自变量,以CAS-CNSIBL为因变量进行多因素Logistic回归分析,结果提示,TyG是CAS-CNSIBL的独立影响因素[矫正OR = 1.125,95%CI (1.042~1.214),P < 0.001],见表2。
表 2 多因素Logistic回归分析TyG和颈内动脉支架植入术后对侧新发无症状缺血性脑损伤的相关性Table 2. Multivariate analysis for the association between TyG and CAS-CNSIBL变量 wald P Exp(B) 95%CI 因变量 自变量 上限 下限 CAS-
CNSIBLTyG 9.871 0.001* 1.125 1.042 1.214 糖尿病病史 3.090 0.057 1.138 0.923 1.273 入院时收缩压 3.524 0.051 1.037 0.986 1.068 入院时舒张压 2.530 0.109 1.200 0.897 1.825 常量 10.126 0.001* 0.017 *P < 0.05。 2.3 ROC曲线分析
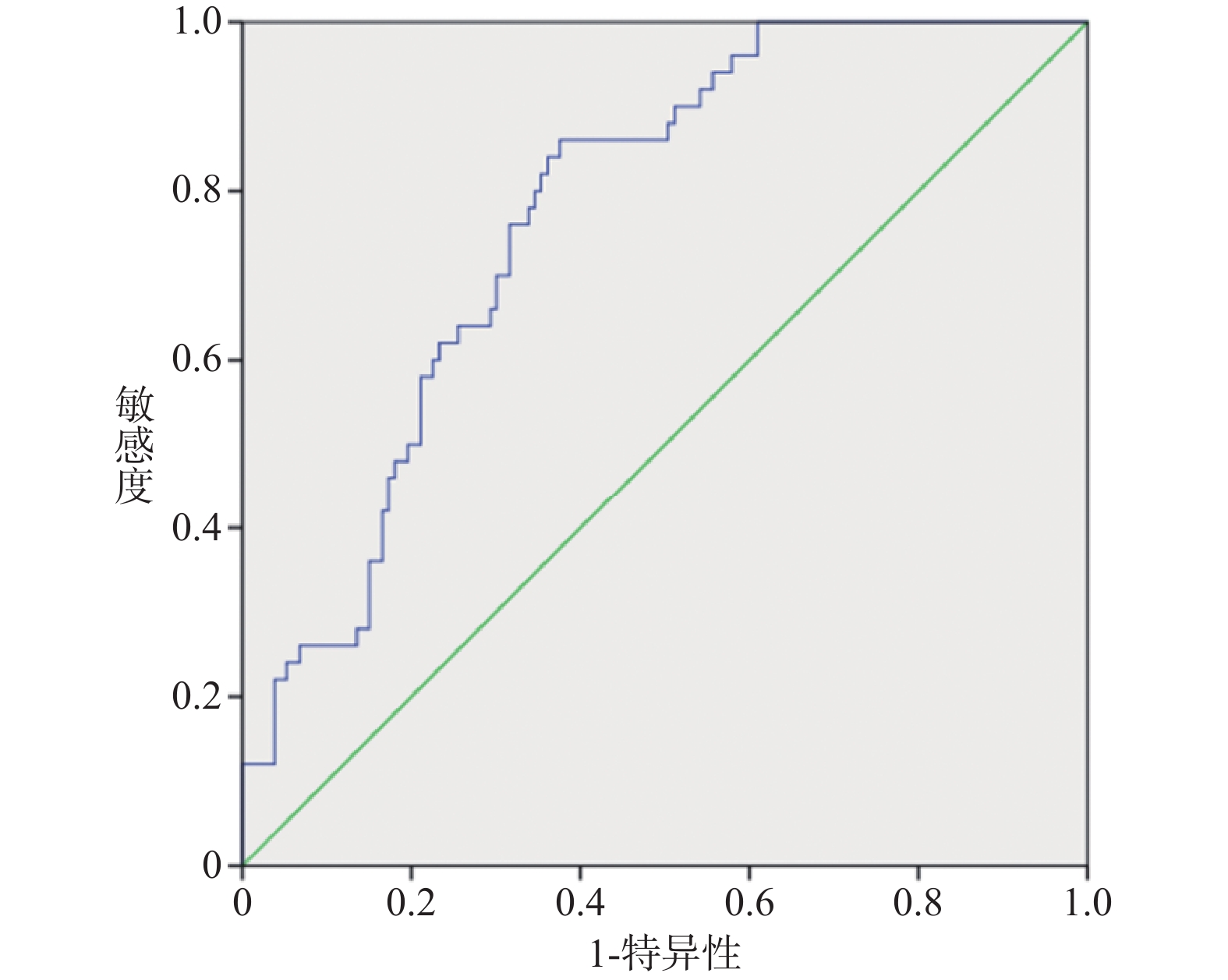
TyG预测颈内动脉支架置入术后对侧新发无症状缺血性脑损伤的ROC曲线下面积(area under curve,AUC)为0.77[95%CI (0.71~0.84),P < 0.001],cut-off值为1.93,灵敏度86.0%,特异度63.9%,见图2。
3. 讨论
颈动脉粥样硬化所致狭窄是缺血性脑卒中的主要原因之一,治疗和预防此类缺血性脑卒中的重要治疗策略是降低血栓风险及改善脑灌注。伴随近些年EPD的广泛使用,颈动脉支架置入术已被广泛应用于实现有效血管再通,其优点是创伤性小、术后恢复快、效果明确。新发颅内DWI高信号脑组织损伤是颈动脉支架置入术后常见的并发症之一。大量研究提示CAS后出现新发无症状脑组织损伤可能与高血压病史、吸烟史、主动脉弓解剖结构、颈内动脉及颈总动脉夹角、动脉粥样硬化斑块性质等相关[8−11]。众所周知,IR与颈动脉粥样硬化严重程度呈正相关,TyG指数是评估IR的一项重要标准,然而目前尚无TyG指数与CAS后CNSIBL的研究,本研究以此为契机,研究TyG指数与CAS-CNSIBL的相关性。本研究共纳入183例行颈动脉支架置入术患者,其中50例(27.3%)患者为CAS-CNSIBL,与既往文献报道CAS-CNSIBL发生率相似[12]。CAS后出现对侧颅内DWI高信号的危险因素与CAS后同侧颅内DWI高信号的危险因素不同。动脉粥样硬化斑块溃疡为CAS后同侧病变的常见危险因素,然而CAS-CNSIBL的危险因素目前尚无研究。EPD可有效降低CAS后同侧新发脑组织损伤的发生,但无法降低CAS后对侧新发脑组织损伤事件。本研究显示,与non-CNSIBL组对比,CNSIBL组糖尿病病史例数、入院时收缩压、舒张压、甘油三酯、TyG均高于对照组且差异具有统计学意义。本研究还评估了TyG指数预测CAS-CNSIBL的能力,校正混杂因素后,TyG指数是CAS-CNSIBL独立影响因素。这项研究首次证明,TyG指数是CAS-CNSIBL的潜在预测指标。
3.1 机制研究
TyG指数是空腹血糖和甘油三酯结合的新指标,被认为是IR简单且可靠的替代指标[13−14]。来自一项真实世界的研究表明,IR是缺血性脑卒中发病机制中的关键因素,同时IR可以预测脑血管病结局[14]。可能的机制是,首先,IR干扰血管内巨噬细胞、内皮细胞和血管平滑肌细胞胰岛素受体和胰岛素受体介导的信号传导,促进内皮功能障碍、斑块破裂、加速动脉硬化,从而诱发CAS-CNSIBL [16−17]。其次,IR引起糖代谢失衡,导致血糖升高,引发慢性炎症,并诱导氧化应激,导致CAS后颅内小血管缺血形成CAS-CNSIBL [18−19]。此外,在已建立的缺血性神经元中,胰岛素活性降低限制了葡萄糖向脂肪酸代谢的生物利用度,最终导致颅内神经细胞耗氧量增加和脑组织损伤,换句话说,IR加速病理性脂质代谢紊乱诱发CAS-CNSIBL[15,20]。最后,TyG指数已被认为是动脉粥样硬化所致脑血管病的独立影响因子[21−25]。多项横断面及回顾性研究表明,TyG指数与冠状动脉粥样硬化斑块、斑块性质、血管狭窄及评估的亚临床动脉粥样硬化风险增加相关,这些均被认为TyG指数是卒中发生和复发的危险机制[26−28]。CAS术后Willis环血液动力学重建,Ma等[29]研究提示TyG指数与经皮冠状动脉介入治疗患者血运重建显著相关。本研究结论和既往研究的结论相似,TyG指数与颈动脉粥样硬化存在密切关系,且TyG指数与Willis环血运重建相关,所以TyG指数与CAS术后CNSIBL存在密切关系。
3.2 研究优点及局限性
据笔者所知,本项研究是TyG指数与CAS术后CNSIBL的首次研究。在目前的研究中,通过严格的质量把控及多因素Logistics回归分析,笔者得到TyG指数是CAS术后CNSIBL独立影响因素,TyG指数升高可预测CAS术后CNSIBL。因此,TyG指数有望在临床实践中得到广泛应用,以提高识别CAS术后CNSIBL发生率。本研究对于更好地理解CAS术后CNSIBL的发病机制和优化治疗策略具有重要的临床意义。本研究也存在以下几点局限性:首先,本研究是横断面研究,纳入样本数量有限,需要将来大规模前瞻性研究验证目前结论;其次,没有对TyG指数进行动态监测,无法研究TyG指数变化与CAS术后CNSIBL的关联;最后,本次研究仅观察了TyG指数与CAS术后CNSIBL的关系,并没有纳入CAS术后同侧新发无症状脑组织损伤患者,笔者会在今后的研究中进行分析。
综上所述,本研究结果显示,TyG指数与对CAS后发生CNSIBL呈正相关,提示TyG指数升高可能有助于识别CAS后发生无症状脑组织损伤事件。
-
图 1 67岁男性右侧颈内动脉起始段重度狭窄行颈动脉起始段支架置入术
A:术前头MRI-DWI,无脑梗死病灶;B:数字减影血管造影,右侧颈内动脉起始段重度狭窄(蓝色箭头所示);C:数字减影血管造影,右侧颈内动脉起始段支架置入术后(蓝色箭头所示);D:术后头MRI-DWI,左侧颞叶新发DWI高信号(橘色箭头所示);E:术后头MRI-DWI,左侧半卵圆中心新发DWI高信号(橘色箭头所示)。
Figure 1. A 67-year-old male with severe stenosis in the right internal carotid artery origin (C1) undergoing stenting of the carotid artery origin
表 1 行颈内动脉支架置入术患者基线资料和实验室检查数据[$ \bar x \pm s $/M(P25,P75)/n(%)]
Table 1. Baseline data and laboratory test results of patients undergoing carotid artery stenting [$ \bar x \pm s $/M(P25,P75)/n(%)]
基线信息 non-CNSIBL组
(n) = 133CNSIBL组
(n = 50)t/χ2/z P 年龄(岁) 65.9 ± 12.7 67.7 ± 13.6 0.683 0.495 男性 99(74.4) 38(76.0) 0.047 0.828 高血压病史 95(71.4) 37(74.0) 0.120 0.730 糖尿病病史 62(46.6) 32(64.0) 4.396 0.036* 高脂血症病史 60(45.1) 22(44.0) 0.018 0.893 缺血性脑卒中病史 25(18.8) 11(22.0) 0.236 0.627 吸烟史 58(43.6) 18(36.0) 0.866 0.352 饮酒史 61(45.9) 24(48.0) 0.067 0.796 冠状动脉粥样硬化性心脏病病史 22(16.5) 12(24.0) 1.336 0.248 入院时收缩压(mmHg) 137.9 ± 23.1 146.8 ± 22.2 5.094 0.031* 入院时舒张压(mmHg) 85.4 ± 15.3 88.6 ± 16.4 3.741 0.049* 入院时NIHSS评分 4[2,7] 4[2,8] 0.496 0.506 静脉rt-PA治疗 27(20.3) 10(20.0) 0.002 0.964 颈内动脉颅内段 78(58.6) 32(64.0) 0.434 0.510 右侧颈内动脉 65(48.9) 23(46.0) 0.120 0.729 开环支架 87(65.4) 34(68.0) 0.109 0.742 空腹血糖(mmol/L) 4.94[4.29−8.11] 6.81[5.29−9.01] 3.734 0.068 甘油三酯 (mmol/L) 1.20[0.57−2.21] 1.94[1.14−3.06] 8.864 0.004* TyG 1.11[0.36−2.23] 1.87[1.06−2.44] 12.241 0.001* 注:CAS: 颈内动脉支架置入术;CNSIBL:对侧新发无症状缺血性脑损伤;NIHSS:美国国立卫生研究院卒中量表; rt-PA:重组组织型纤溶酶原激活剂; TyG: 甘油三酯-血糖指数;*P < 0.05。 表 2 多因素Logistic回归分析TyG和颈内动脉支架植入术后对侧新发无症状缺血性脑损伤的相关性
Table 2. Multivariate analysis for the association between TyG and CAS-CNSIBL
变量 wald P Exp(B) 95%CI 因变量 自变量 上限 下限 CAS-
CNSIBLTyG 9.871 0.001* 1.125 1.042 1.214 糖尿病病史 3.090 0.057 1.138 0.923 1.273 入院时收缩压 3.524 0.051 1.037 0.986 1.068 入院时舒张压 2.530 0.109 1.200 0.897 1.825 常量 10.126 0.001* 0.017 *P < 0.05。 -
[1] Ryu J C,Bae J H,Ha S H,et al. In-stent restenosis and stented-territory infarction after carotid and vertebrobasilar artery stenting[J]. BMC Neurol,2023,23(1):79-88. doi: 10.1186/s12883-023-03110-z [2] Gensicke H,Worp H B,Nederkoorn P J,et al. Ischemic brain lesions after carotid artery stenting increase future cerebrovascular risk[J]. J Am Coll Cardiol,2015,65(6):521-529. doi: 10.1016/j.jacc.2014.11.038 [3] Feng Y,Bai X,Zhang X,et al. Risk factors for new ischemic cerebral lesions after carotid artery stenting: A systematic review and meta-analysis[J]. Ann Vasc Surg,2021,77(1):296-305. [4] Kim M K,Ahn C W,Kang S,et al. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults[J]. Cardiovasc Diabetol,2017,16(1):108-115. doi: 10.1186/s12933-017-0589-4 [5] Tahapary D L,Pratisthita L B,Fitri N A,et al. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and Triglyceride/glucose index[J]. Diabetes Metab Syndr,2022,16(8):102581-102586. doi: 10.1016/j.dsx.2022.102581 [6] da Silva A, Caldas A P S, Hermsdorff H H M,et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care[J]. Cardiovasc Diabetol,2019,18(1):1-8. doi: 10.1186/s12933-019-0806-4 [7] Bouthillier A, van Loveren H R, Keller J T. Segments of the internal carotid artery: A new classification[J]. Neurosurgery,1996,38(3):432-433. [8] Brott T G, Hobson R W, Howard G,et al. CREST Investigators: Stenting versus endarterectomy for treatment of carotid-artery stenosis[J]. N Engl J Med,2010,363(1):11-23. doi: 10.1056/NEJMoa0912321 [9] Setacci C,Chisci E,Setacci F,et al. Siena carotid artery stenting score: Arisk modeling study for individual patients[J]. Stroke,2010,41(6):1259-1265. doi: 10.1161/STROKEAHA.110.578583 [10] Elqasass A,Hobeika L E,Al-Dawoud Y,et al. Bilateral internal carotid artery agenesis coexisting with bovine aortic arch: Case report and literature review[J]. Anat Sci Int,2024,9(4):1-10. [11] Faggioli G,Ferri M,Rapezzi C,et al. Atherosclerotic aortic lesions increase the risk of cerebral embolism during carotid stenting in patients with complex aortic arch anatomy[J]. J Vasc Surg,2009,49(1):80-85. doi: 10.1016/j.jvs.2008.08.014 [12] Miccichè E,Condello F,Cao D,et al. Procedural embolic protection strategies for carotid artery stenting: current status and future prospects[J]. Expert Rev Med Devices,2023,20(5):373-391. doi: 10.1080/17434440.2023.2198124 [13] Wang S,Shi J,Peng Y,et al. Stronger association of triglyceride glucose index than the HOMA-IR with arterial stiffness in patients with type 2 diabetes: A real-world single-center study[J]. Cardiovasc Diabetol,2021,20(1):1-10. doi: 10.1186/s12933-020-01183-5 [14] Lee S H,Kwon H S,Park Y M,et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chung ju Metabolic Disease Cohort (CMC) study[J]. PLoS ONE,2014,9(2):e90430-e90430. doi: 10.1371/journal.pone.0090430 [15] Wang F,Han L,Hu D. Fasting insulin,insulin resistance and risk of hypertension in the general population: a meta-analysis[J]. Clin Chim Acta,2017,464(3):57-63. [16] Joshua K B, Matthew J B. Atherosclerosis: Pathophysiology of insulin resistance,hyperglycemia,hyperlipidemia,and inflammation[J]. J Diabetes,2020,12(2):102-104. doi: 10.1111/1753-0407.12970 [17] Reardon C A,Lingaraju A,Schoenfelt K Q,et al. Obesity and insulin resistance promote atherosclerosis through an IFNγ-regulated macrophage protein network[J]. Cell Rep,2018,23(10):3021-3030. doi: 10.1016/j.celrep.2018.05.010 [18] Kim G R,Choi D W,Nam C M,et al. Synergistic association of high-sensitivity C-reactive protein and body mass index with insulin resistance in non-diabetic adults[J]. Sci Rep,2020,10(1):18417-18425. doi: 10.1038/s41598-020-75390-1 [19] Jeong S,Lee J H. The verification of the reliability of a triglyceride glucose index and its availability as an advanced tool[J]. Metabolomics,2021,17(11):97-109. doi: 10.1007/s11306-021-01837-9 [20] Yu H X,Tao L Y,Li Y G,et al. Association between triglyceride-glucose index trajectories and carotid atherosclerosis progression[J]. Cardiovasc Diabetol,2023,22(1):130-139. doi: 10.1186/s12933-023-01847-y [21] Luo E,Wang D,Yan G,et al. High triglyceride–glucose index is associated with poor prognosis in patients with acute ST elevation myocardial infarction after percutaneous coronary intervention[J]. Cardiovasc Diabetol,2019,18(1):150-162. doi: 10.1186/s12933-019-0957-3 [22] Su W Y,Chen S C,Huang Y T,et al. Comparison of the effects of fasting glucose,hemoglobin A1C,and triglyceride glucose index on cardiovascular events in type 2 diabetes mellitus[J]. Nutrients,2019,11(11):2838-2851. doi: 10.3390/nu11112838 [23] Won K B,Kim Y S,Lee B K,et al. The relationship of insulin resistance estimated by triglyceride glucose index and coronary plaque characteristics[J]. Medicine,2018,97(21):e10726-e10726 doi: 10.1097/MD.0000000000010726 [24] Lee S B,Ahn C W,Lee B K,et al. Association between triglyceride glucose index and arterial stiffness in Korean adults[J]. Cardiovasc Diabetol,2018,17(1):41-47. doi: 10.1186/s12933-018-0692-1 [25] Wang A,Tian X,Zuo Y,et al. Association of triglyceride-glucose index with intra- and extra-cranial arterial stenosis: A combined cross-sectional and longitudinal analysis[J]. Endocrine,2021,74(2):308-317. doi: 10.1007/s12020-021-02794-y [26] Liu F,Ling Q,Xie S,et al. Association between triglyceride glucose index and arterial stiffness and coronary artery calcification: A systematic review and exposure-effect meta-analysis[J]. Cardiovasc Diabetol,2023,22(1):111-130. doi: 10.1186/s12933-023-01819-2 [27] Li J,Dong Z,Wu H,et al. The triglyceride-glucose index is associated with atherosclerosis in patients with symptomatic coronary artery disease,regardless of diabetes mellitus and hyperlipidemia[J]. Cardiovasc Diabetol,2023,22(1):224-239. doi: 10.1186/s12933-023-01919-z [28] Chen T,Qian Y,Deng X. Triglyceride glucose index is a significant predictor of severe disturbance of consciousness and all-cause mortality in critical cerebrovascular disease patients[J]. Cardiovasc Diabetol,2023,22(1):156-169. doi: 10.1186/s12933-023-01893-6 [29] Ma X T,Dong L S,Shao Q Y,et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type2 diabetes mellitus and acute coronary syndrome[J]. Cardiovasc Diabetol,2020,19(1):31-45. doi: 10.1186/s12933-020-01006-7 -






 下载:
下载:
 下载:
下载:

