-
摘要: 脓毒症是由感染引起的宿主反应失调,导致危及生命的器官功能障碍的综合征。脓毒症的免疫反应通常分为免疫亢进和免疫抑制两个阶段,免疫抑制被认为是导致患者继发感染和死亡率增加的主要原因。本文综述脓毒症免疫抑制的主要机制,包括免疫细胞的功能障碍、免疫抑制细胞因子的释放、免疫调节细胞的过度活化、免疫检查点的表达增加以及近年来备受关注的表观遗传失调等。同时,也总结了目前现有的及一些新的免疫治疗策略,包括免疫刺激性细胞因子(如GM-CSF、IL-7、IL-15)、免疫检查点抑制剂(如PD-1/PD-L1抗体、CTLA-4抗体、TIM-3抗体)及新兴免疫治疗方法(如间充质干细胞、钙卫蛋白抑制剂、髓样细胞触发受体1抑制剂)。本文聚焦表观遗传调控机制和间充质干细胞等新兴免疫治疗,旨在为脓毒症患者个体化精准免疫治疗提供理论支持。Abstract: Sepsis is a syndrome caused by a dysregulated host response to infection that leads to life-threatening organ dysfunction. The immune response in sepsis is usually divided into two stages: hyperimmunity and immunosuppression. Immunosuppression is considered as the main cause of secondary infection and increased mortality in patients. This article reviews the main mechanisms of immunosuppression in sepsis, including dysfunction of immune cells, release of immunosuppressive cytokines, excessive activation of immunomodulatory cells, increased expression of immune checkpoints, and epigenetic dysregulation that have attracted much attention in recent years. At the same time, it also summarizes the existing and some new immunotherapy strategies. It includes immunostimulatory cytokines (such as GM-CSF, IL-7, IL-15), immune checkpoint inhibitors (such as PD-1/PD-L1 antibody, CTLA-4 antibody, TIM-3 antibody) and emerging immunotherapy methods (such as mesenchymal stem cells, calprotectin inhibitors, triggering receptor expressed on myeloid cells 1 inhibitors). This article focuses on epigenetic regulation mechanisms and emerging immunotherapies such as mesenchymal stem cells, aiming to provide theoretical support for individualized precision immunotherapy in patients with sepsis.
-
Key words:
- Sepsis /
- Immunosuppression /
- Immunotherapy /
- Epigenetic dysregulation
-
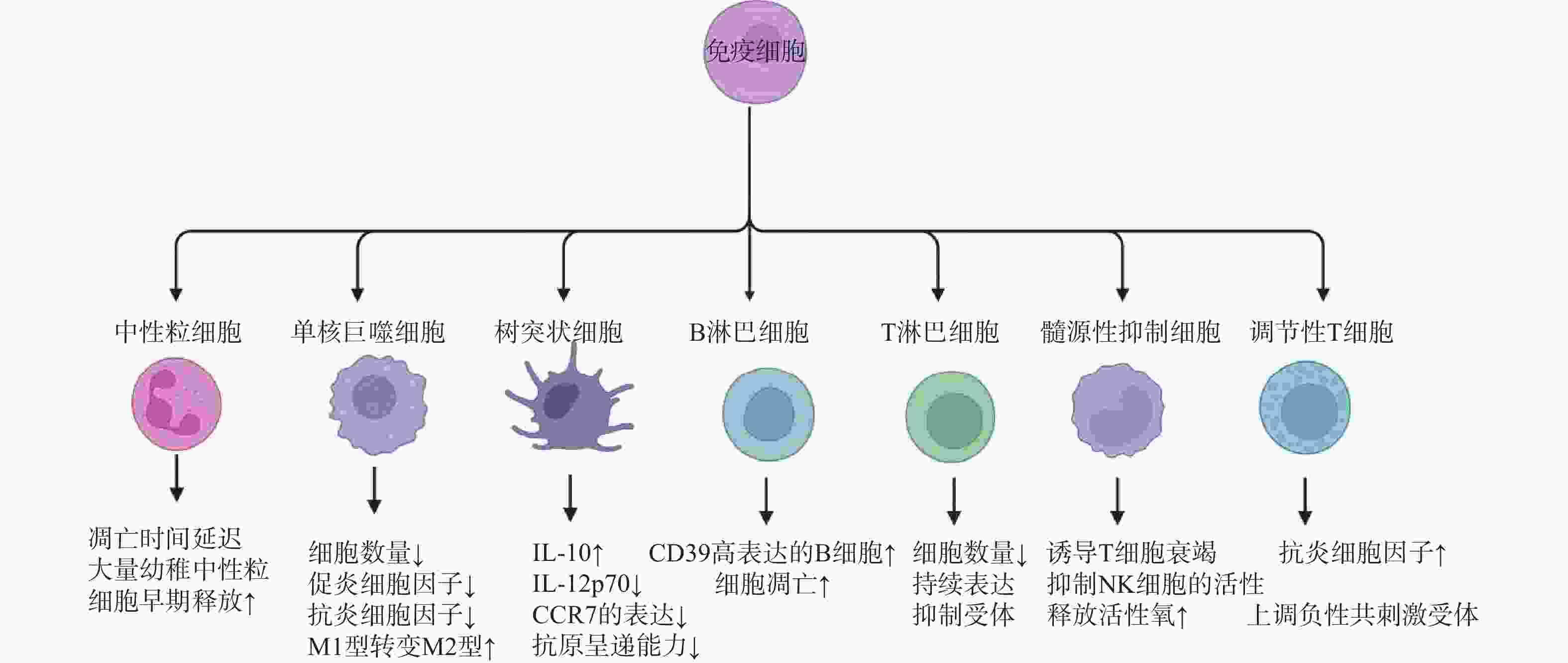
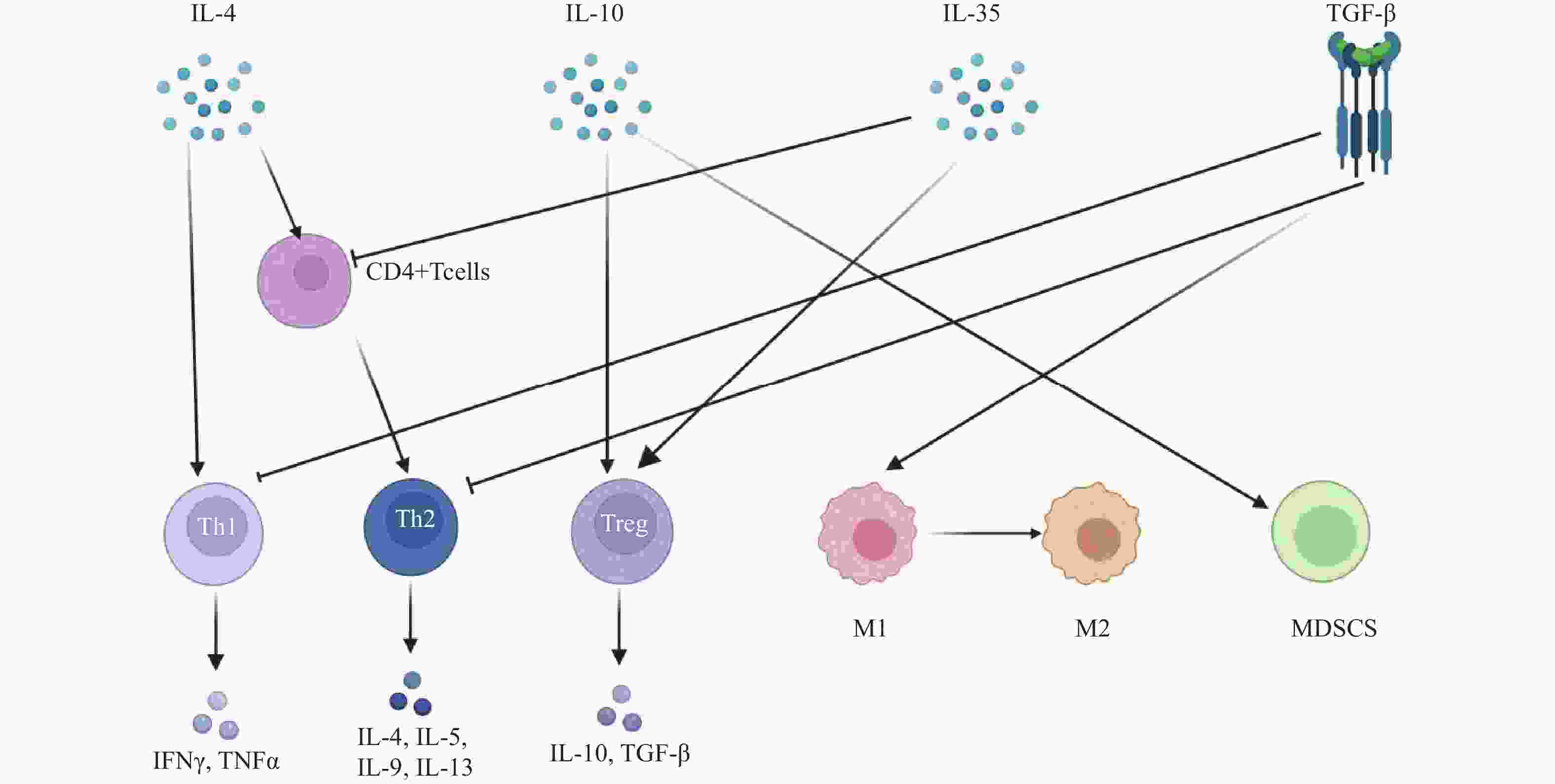
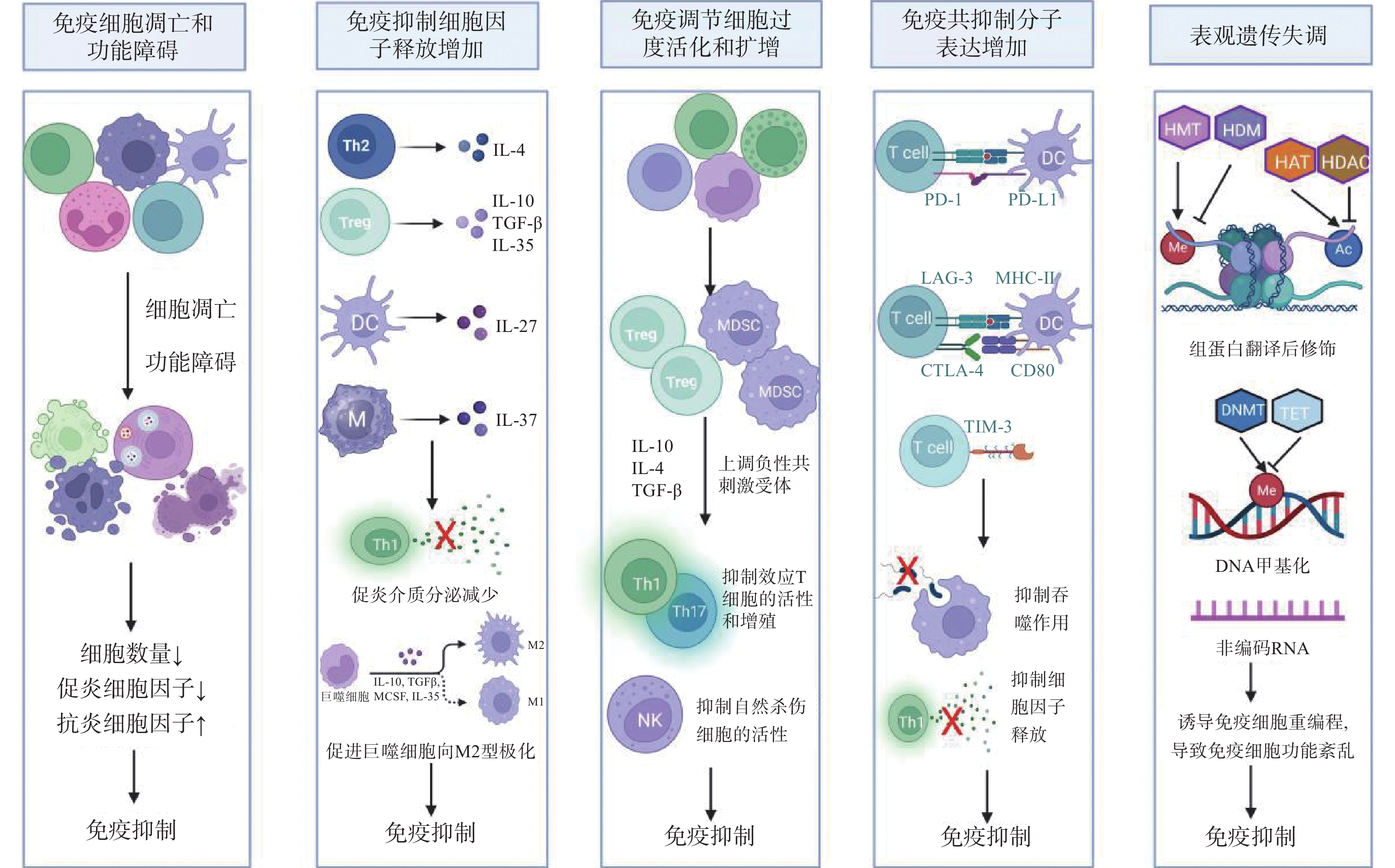
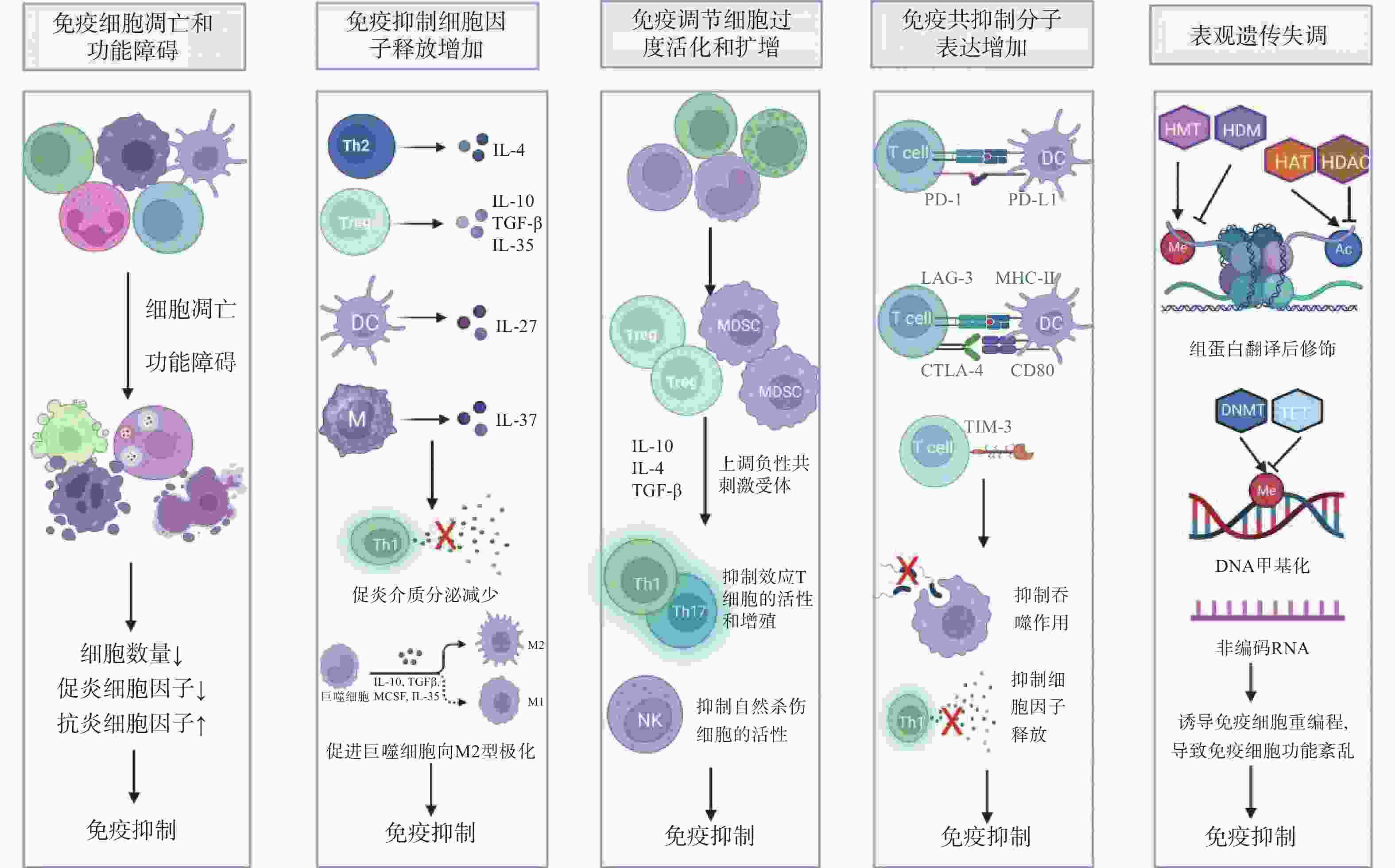
图 1 脓毒症免疫抑制的机制
注:IL-4:白细胞介素-4;TGF-β:转化生长因子-β;Th1:1型辅助性T细胞;Th2:2型辅助性T细胞;Treg:调节性T细胞;DC:树突状细胞;M:单核巨噬细胞;M2:M2型巨噬细胞;MDSCs:髓源性抑制细胞;NK:自然杀伤细胞;T cell:T淋巴细胞;PD-1/PD-L1:程序性细胞死亡受体-1/程序性死亡配体-1;LAG-3:淋巴细胞活化基因-3;CTLA-4:细胞毒性T淋巴细胞抗原-4;TIM-3:T细胞免疫球蛋白及黏蛋白分子-3;MHC-II:主要组织相容性复合体II;Me:甲基化;Ac:乙酰化;HMT:组蛋白甲基转移酶;HDM:组蛋白去甲基化酶;HAT:组蛋白乙酰转移酶;HDAC:组蛋白去乙酰化酶;DNMT:DNA甲基转移酶;TET:DNA去甲基化酶。
Figure 1. Mechanisms of immunosuppression in sepsis
-
[1] Vucelić V,Klobučar I,Đuras-Cuculić B,et al. Sepsis and septic shock - an observational study of the incidence,management,and mortality predictors in a medical intensive care unit[J]. Croatian Medical Journal,2020,61(5):429-439. doi: 10.3325/cmj.2020.61.429 [2] Joosten S C M,Wiersinga W J,Poll T van der. Dysregulation of host-pathogen interactions in sepsis: Host-related factors[J]. Seminars in Respiratory and Critical Care Medicine,2024,45(4):469-478. doi: 10.1055/s-0044-1787554 [3] Cao M,Wang G,Xie J. Immune dysregulation in sepsis: Experiences,lessons and perspectives[J]. Cell Death Discovery,2023,9(1):465. doi: 10.1038/s41420-023-01766-7 [4] Unsinger J,Walton A H,Blood T,et al. Frontline science: OX40 agonistic antibody reverses immune suppression and improves survival in sepsis[J]. Journal of Leukocyte Biology,2021,109(4):697-708. doi: 10.1002/JLB.5HI0720-043R [5] Wei Y,Kim J,Ernits H,et al. The septic neutrophil-friend or foe[J]. Shock (augusta,Ga.),2021,55(2):147-155. doi: 10.1097/SHK.0000000000001620 [6] Huang S,Chen Y,Gong F,et al. Septic macrophages induce T cells immunosuppression in a cell-cell contact manner with the involvement of CR3[J]. Heliyon,2024,10(1):e23266. doi: 10.1016/j.heliyon.2023.e23266 [7] Lu Z Q,Zhang C,Zhao L J,et al. Matrix metalloproteinase-8 regulates dendritic cell tolerance in late polymicrobial sepsis via the nuclear factor kappa-B p65/β-catenin pathway[J]. Burns & Trauma,2024,12:tkad025. [8] Yao R Q,Li Z X,Wang L X,et al. Single-cell transcriptome profiling of the immune space-time landscape reveals dendritic cell regulatory program in polymicrobial sepsis[J]. Theranostics,2022,12(10):4606-4628. doi: 10.7150/thno.72760 [9] Nascimento D C,Viacava P R,Ferreira R G,et al. Sepsis expands a CD39+ plasmablast population that promotes immunosuppression via adenosine-mediated inhibition of macrophage antimicrobial activity[J]. Immunity,2021,54(9): 2024-2041. e8. [10] Ma K,Luo L,Yang M,et al. The suppression of sepsis-induced kidney injury via the knockout of T lymphocytes[J]. Heliyon,2024,10(1):e23311. doi: 10.1016/j.heliyon.2023.e23311 [11] Reizine F,Grégoire M,Lesouhaitier M,et al. Beneficial effects of citrulline enteral administration on sepsis-induced T cell mitochondrial dysfunction[J]. Proceedings of the National Academy of Sciences of the United States of America,2022,119(8):e2115139119. [12] Gao K,Jin J,Huang C,et al. Exosomes derived from septic mouse serum modulate immune responses via exosome-associated cytokines[J]. Frontiers in Immunology,2019,10:1560. doi: 10.3389/fimmu.2019.01560 [13] Neumann C,Scheffold A,Rutz S. Functions and regulation of T cell-derived interleukin-10[J]. Seminars in Immunology,2019,44:101344. doi: 10.1016/j.smim.2019.101344 [14] Wei X,Zhang J,Cui J,et al. Adaptive plasticity of natural interleukin-35-induced regulatory T cells (Tr35) that are required for T-cell immune regulation[J]. Theranostics,2024,14(7):2897-2914. doi: 10.7150/thno.90608 [15] Bergmann C B,Beckmann N,Salyer C E,et al. Potential targets to mitigate trauma- or sepsis-induced immune suppression[J]. Frontiers in Immunology,2021,12:622601. doi: 10.3389/fimmu.2021.622601 [16] Qichuan Y,Li Y,Wang H,et al. TSLP induces a proinflammatory phenotype in circulating innate cells and predicts prognosis in sepsis patients[J]. FEBS Open Bio,2024,14(3):525. doi: 10.1002/2211-5463.13739 [17] Gaborit B J,Roquilly A,Louvet C,et al. Regulatory T cells expressing tumor necrosis factor receptor type 2 play a major role in CD4+ T-cell impairment during sepsis[J]. Journal of Infectious Diseases,2020,222(7):1222-1234. doi: 10.1093/infdis/jiaa225 [18] Shi Y,Wu D,Wang Y,et al. Treg and neutrophil extracellular trap interaction contributes to the development of immunosuppression in sepsis[J]. JCI Insight,2024,9(14):e180132. doi: 10.1172/jci.insight.180132 [19] Zhang W,Fang X,Gao C,et al. MDSCs in sepsis-induced immunosuppression and its potential therapeutic targets[J]. Cytokine & Growth Factor Reviews,2023,69:90-103. [20] Li K,Shi H,Zhang B,et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer[J]. Signal Transduction and Targeted Therapy,2021,6(1):362. doi: 10.1038/s41392-021-00670-9 [21] McBride M A,Patil T K,Bohannon J K,et al. Immune checkpoints: novel therapeutic targets to attenuate sepsis-induced immunosuppression[J]. Frontiers in Immunology,2020,11:624272. [22] Chen J,Chen R,Huang S,et al. Atezolizumab alleviates the immunosuppression induced by PD‑L1‑positive neutrophils and improves the survival of mice during sepsis[J]. Molecular Medicine Reports,2021,23(2):144. [23] Rossi A L,Le M,Chung C S,et al. A novel role for programmed cell death receptor ligand 2 in sepsis-induced hepatic dysfunction[J]. American journal of physiology. Gastrointestinal and liver physiology,2019,316(1):G106-G114. doi: 10.1152/ajpgi.00204.2018 [24] Wakeley M E,Armstead B E,Gray C C,et al. Lymphocyte HVEM/BTLA co-expression after critical illness demonstrates severity indiscriminate upregulation,impacting critical illness-induced immunosuppression[J]. Frontiers in Medicine,2023,10:1176602. doi: 10.3389/fmed.2023.1176602 [25] Lange A,Cajander S,Magnuson A,et al. Sustained elevation of soluble B- and T- lymphocyte attenuator predicts long-term mortality in patients with bacteremia and sepsis[J]. PLOS One,2022,17(3):e0265818. doi: 10.1371/journal.pone.0265818 [26] Cheng W,Zhang J,Li D,et al. CTLA-4 expression on CD4+ lymphocytes in patients with sepsis-associated immunosuppression and its relationship to mTOR mediated autophagic-lysosomal disorder[J]. Frontiers in Immunology,2024,15:1396157. doi: 10.3389/fimmu.2024.1396157 [27] THuang S,Liu D,Sun J,et al. Tim-3 regulates sepsis-induced immunosuppression by inhibiting the NF-κB signaling pathway in CD4 T cells[J]. Mol Ther.,2022,30(3):1227-1238. doi: 10.1016/j.ymthe.2021.12.013 [28] Mewes C,Alexander T,Büttner B,et al. Effect of the lymphocyte activation gene 3 polymorphism rs951818 on mortality and disease progression in patients with sepsis-a prospective genetic association study[J]. Journal of Clinical Medicine,2021,10(22):5302. doi: 10.3390/jcm10225302 [29] Falcão-Holanda R B,Brunialti M K C,Jasiulionis M G,et al. Epigenetic regulation in sepsis,role in pathophysiology and therapeutic perspective[J]. Frontiers in Medicine,2021,8:685333. doi: 10.3389/fmed.2021.685333 [30] C ó rneo E da S,Michels M,Dal-Pizzol F. Sepsis,immunosuppression and the role of epigenetic mechanisms[J]. Expert Review of Clinical Immunology,2021,17(2):169-176. doi: 10.1080/1744666X.2021.1875820 [31] Zhang Y,Sun Z,Jia J,et al. Overview of histone modification[J]. Advances in Experimental Medicine and Biology,2021,1283:1-16. [32] Denis M,Dupas T,Persello A,et al. An O-GlcNAcylomic approach reveals ACLY as a potential target in sepsis in the young rat[J]. International journal of molecular sciences,2021,22(17):9236. doi: 10.3390/ijms22179236 [33] Moreno-Yruela C,Zhang D,Wei W,et al. Class I histone deacetylases (HDAC1-3) are histone lysine delactylases[J]. Science Advances,2022,8(3):eabi6696. doi: 10.1126/sciadv.abi6696 [34] Yu K,Proost P. Insights into peptidylarginine deiminase expression and citrullination pathways[J]. Trends in Cell Biology,2022,32(9):746-761. doi: 10.1016/j.tcb.2022.01.014 [35] Lawton M L,Inge M M,Blum B C,et al. Multiomic profiling of chronically activated CD4+ T cells identifies drivers of exhaustion and metabolic reprogramming[J]. PLoS biology,2024,22(12):e3002943. doi: 10.1371/journal.pbio.3002943 [36] Zhang S,Zhan L,Li X,et al. Preclinical and clinical progress for HDAC as a putative target for epigenetic remodeling and functionality of immune cells[J]. International Journal of Biological Sciences,2021,17(13):3381-3400. doi: 10.7150/ijbs.62001 [37] Poli V,Pui-Yan Ma V,Di Gioia M,et al. Zinc-dependent histone deacetylases drive neutrophil extracellular trap formation and potentiate local and systemic inflammation[J]. Iscience,2021,24(11):103256. doi: 10.1016/j.isci.2021.103256 [38] Wu C,Li A,Hu J,et al. Histone deacetylase 2 is essential for LPS-induced inflammatory responses in macrophages[J]. Immunology & Cell Biology,2019,97(1):72-84. [39] Chen W,Liu J,Ge F,et al. Long noncoding RNA HOTAIRM1 promotes immunosuppression in sepsis by inducing T cell exhaustion[J]. Journal of Immunology (baltimore,Md. : 1950),2022,208(3): 618-632. [40] Du J,Jiang H,Wang B. Long non-coding RNA GAS5/miR-520-3p/SOCS3 axis regulates inflammatory response in lipopolysaccharide-induced macrophages[J]. Biochemical Genetics,2022,60(5):1793-1808. doi: 10.1007/s10528-021-10179-z [41] Harkless R,Singh K,Christman J,et al. Microvesicle-mediated transfer of DNA methyltransferase proteins results in recipient cell immunosuppression[J]. The Journal of Surgical Research,2023,283:368-376. doi: 10.1016/j.jss.2022.10.030 [42] Beltrán-García J,Casabó-Vallés G,Osca-Verdegal R,et al. Alterations in leukocyte DNA methylome are associated to immunosuppression in severe clinical phenotypes of septic patients[J]. Frontiers in Immunology,2023,14:1333705. [43] Tu F,Pan L,Wu W,et al. Recombinant GM-CSF enhances the bactericidal ability of PMNs by increasing intracellular IL-1β and improves the prognosis of secondary pseudomonas aeruginosa pneumonia in sepsis[J]. Journal of Leukocyte Biology,2023,114(5):443-458. doi: 10.1093/jleuko/qiad088 [44] Sehgal R,Maiwall R,Rajan V,et al. Granulocyte-macrophage colony-stimulating factor modulates myeloid-derived suppressor cells and treg activity in decompensated cirrhotic patients with sepsis[J]. Frontiers in Immunology,2022,13:828949. doi: 10.3389/fimmu.2022.828949 [45] Bhavani S V,Spicer A,Sinha P,et al. Distinct immune profiles and clinical outcomes in sepsis subphenotypes based on temperature trajectories[J]. Intensive Care Medicine,2024,50(12):2094-2104. doi: 10.1007/s00134-024-07669-0 [46] Fu C,Zhang X,Zhang X,et al. Advances in IL-7 research on tumour therapy[J]. Pharmaceuticals (basel,Switzerland),2024,17(4):415. doi: 10.3390/ph17040415 [47] Daix T,Mathonnet A,Brakenridge S,et al. Intravenously administered interleukin-7 to reverse lymphopenia in patients with septic shock: A double-blind,randomized,placebo-controlled trial[J]. Annals of Intensive Care,2023,13(1):17. doi: 10.1186/s13613-023-01109-w [48] Bidar F,Hamada S,Gossez M,et al. Correction to: recombinant human interleukin-7 reverses T cell exhaustion ex vivo in critically ill COVID-19 patients[J]. Annals of Intensive Care,2022,12(1):30. doi: 10.1186/s13613-022-01007-7 [49] Lélu K,Dubois C,Evlachev A,et al. Viral delivery of IL-7 is a potent immunotherapy stimulating innate and adaptive immunity and confers survival in sepsis models[J]. Journal of Immunology (baltimore,Md. : 1950),2022,209(1): 99-117. [50] He C,Yu Y,Wang F,et al. Pretreatment with interleukin-15 attenuates inflammation and apoptosis by inhibiting NF-κB signaling in sepsis-induced myocardial dysfunction[J]. European Journal of Histochemistry: EJH,2024,68(2):4019. [51] Saito M,Inoue S,Yamashita K,et al. IL-15 improves aging-induced persistent T cell exhaustion in mouse models of repeated sepsis[J]. Shock (augusta,Ga.),2020,53(2):228-235. doi: 10.1097/SHK.0000000000001352 [52] Yang L,Gao Q,Li Q,et al. PD-L1 blockade improves survival in sepsis by reversing monocyte dysfunction and immune disorder[J]. Inflammation,2024,47(1):114-128. doi: 10.1007/s10753-023-01897-0 [53] Zhao Z Z,Wang X L,Xie J,et al. Therapeutic effect of an anti-human programmed death-ligand 1 (PD-L1) nanobody on polymicrobial sepsis in humanized mice[J]. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research,2021,27:e926820. [54] Sun R,Huang J,Liu L,et al. Neutrophils mediate T lymphocyte function in septic mice via the CD80/cytotoxic T lymphocyte antigen-4 signaling pathway[J]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue,2021,33(7):849-854. [55] Paterson C W,Fay K T,Chen C W,et al. CTLA-4 checkpoint inhibition improves sepsis survival in alcohol-exposed mice[J]. Immunohorizons,2024,8(1):74-88. doi: 10.4049/immunohorizons.2300060 [56] Busch L M,Sun J,Cui X,et al. Checkpoint inhibitor therapy in preclinical sepsis models: A systematic review and meta-analysis[J]. Intensive Care Medicine Experimental,2020,8(1):7. doi: 10.1186/s40635-019-0290-x [57] Liu S,Wang C,Jiang Z,et al. Tim-3 blockade decreases the apoptosis of CD8+ T cells and reduces the severity of sepsis in mice[J]. Journal of Surgical Research,2022,279:8-16. doi: 10.1016/j.jss.2022.05.014 [58] Wu H,Tang T,Deng H,et al. Immune checkpoint molecule tim-3 promotes NKT cell apoptosis and predicts poorer prognosis in sepsis[J]. Clinical Immunology (orlando,Fla.),2023,254:109249. doi: 10.1016/j.clim.2023.109249 [59] Li Y,Sun Z,Li Y,et al. IL-1β-stimulated bone mesenchymal stem cell-derived exosomes mitigate sepsis through modulation of HMGB1/AKT pathway and M2 macrophage polarization[J]. Current Molecular Medicine,2025,25(1):79-89. doi: 10.2174/0115665240277763231206051401 [60] Alp E,Gonen Z B,Gundogan K,et al. The effect of mesenchymal stromal cells on the mortality of patients with sepsis and septic shock: A promising therapy[J]. Emergency Medicine International,2022,2022:9222379. [61] Zhang L,Zhang X,Liu Y,et al. CD146+ umbilical cord mesenchymal stem cells exhibit high immunomodulatory activity and therapeutic efficacy in septic mice[J]. Journal of Inflammation Research,2023,16:579-594. doi: 10.2147/JIR.S396088 [62] Wang Q,Long G,Luo H,et al. S100A8/A9: An emerging player in sepsis and sepsis-induced organ injury[J]. Biomedicine & Pharmacotherapy,2023,168:115674. [63] Shi W,Wan T T,Li H H,et al. Blockage of S100A8/A9 ameliorates septic nephropathy in mice[J]. Frontiers in Pharmacology,2023,14:1172356. doi: 10.3389/fphar.2023.1172356 [64] Boufenzer A,Carrasco K,Jolly L,et al. Potentiation of NETs release is novel characteristic of TREM-1 activation and the pharmacological inhibition of TREM-1 could prevent from the deleterious consequences of NETs release in sepsis[J]. Cellular and Molecular Immunology,2021,18(2):452-460. doi: 10.1038/s41423-020-00591-7 [65] Siskind S,Brenner M,Wang P. TREM-1 modulation strategies for sepsis[J]. Frontiers in Immunology,2022,13:907387. doi: 10.3389/fimmu.2022.907387 [66] François B,Lambden S,Fivez T,et al. Prospective evaluation of the efficacy,safety,and optimal biomarker enrichment strategy for nangibotide,a TREM-1 inhibitor,in patients with septic shock (ASTONISH): A double-blind,randomised,controlled,phase 2b trial[J]. The Lancet. Respiratory Medicine,2023,11(10):894-904. doi: 10.1016/S2213-2600(23)00158-3 [67] François B,Wittebole X,Ferrer R,et al. Nangibotide in patients with septic shock: A Phase 2a randomized controlled clinical trial[J]. Intensive Care Med.,2020,46(7):1425-1437. doi: 10.1007/s00134-020-06109-z [68] François B,Lambden S,Garaud J J,et al. Evaluation of the efficacy and safety of TREM-1 inhibition with nangibotide in patients with COVID-19 receiving respiratory support: the ESSENTIAL randomised,double-blind trial[J]. Eclinicalmedicine,2023,60:102013. doi: 10.1016/j.eclinm.2023.102013 [69] Wu Y,He Y,Liu C,et al. Histone deacetylase inhibitor (SAHA) reduces mortality in an endotoxemia mouse model by suppressing glycolysis[J]. International Journal of Molecular Sciences,2023,24(15):12448. doi: 10.3390/ijms241512448 -





 下载:
下载: