SIRT1 Agonist Treatment of Mice with Coronary Artery Disease Improves Myocardial Function by modulating Nrf2-GPX4 Ferroptosis Pathway
-
摘要:
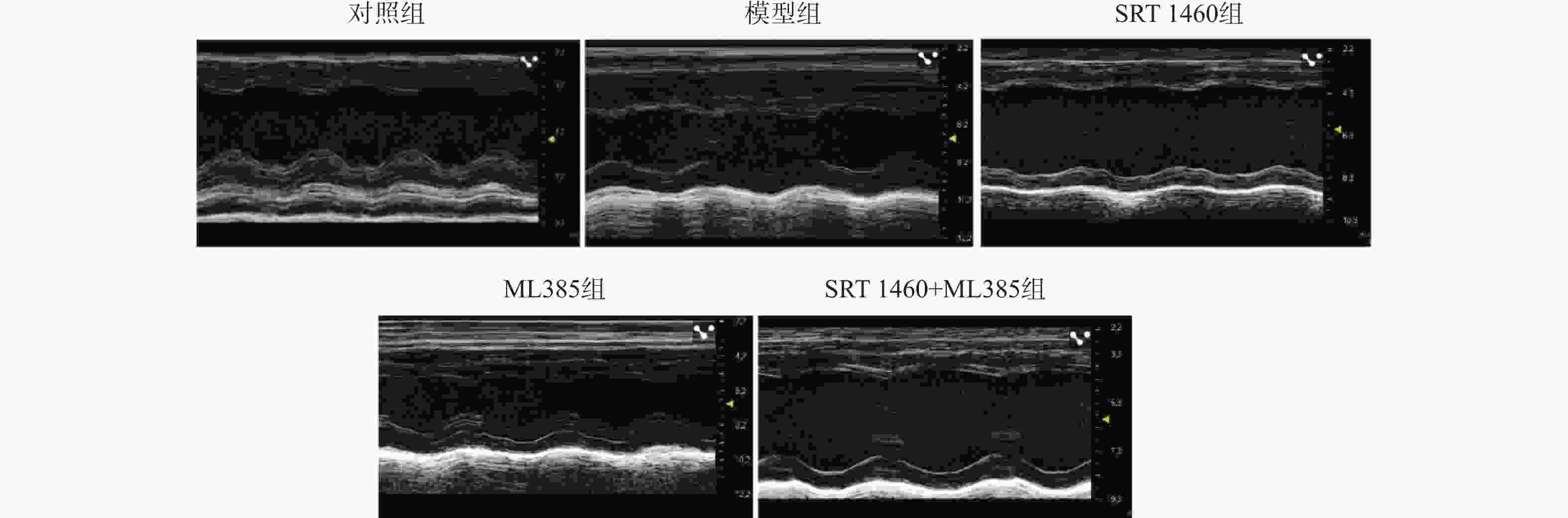
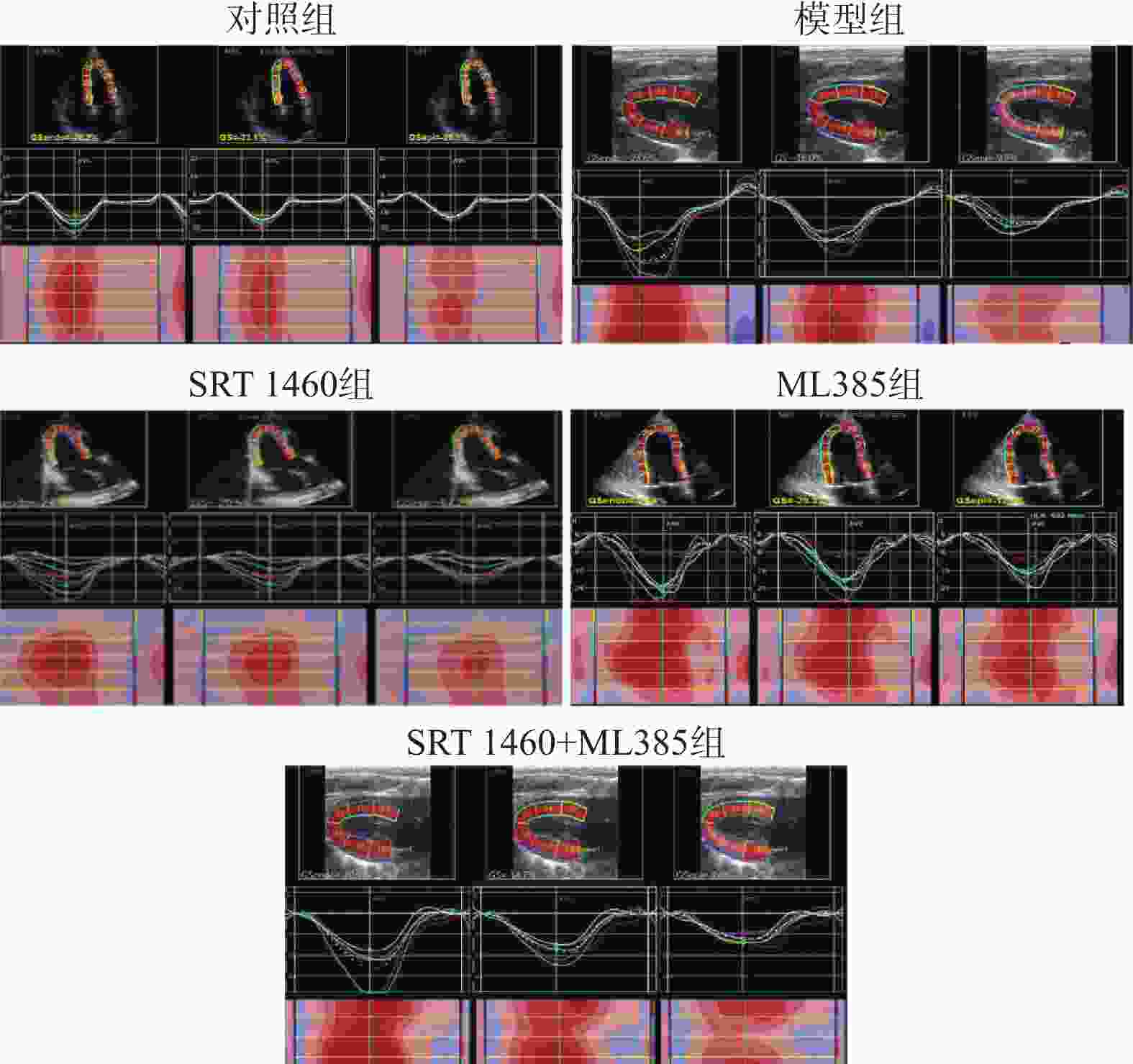
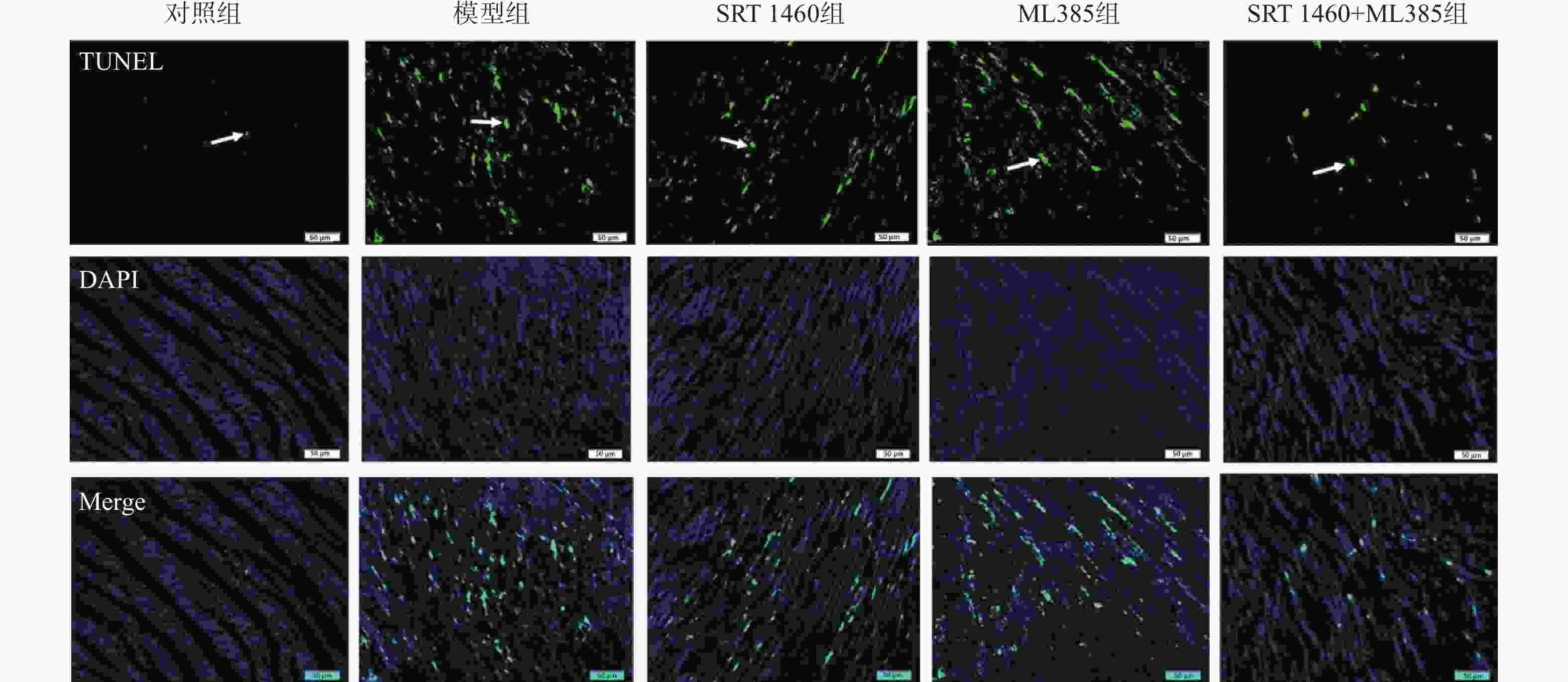
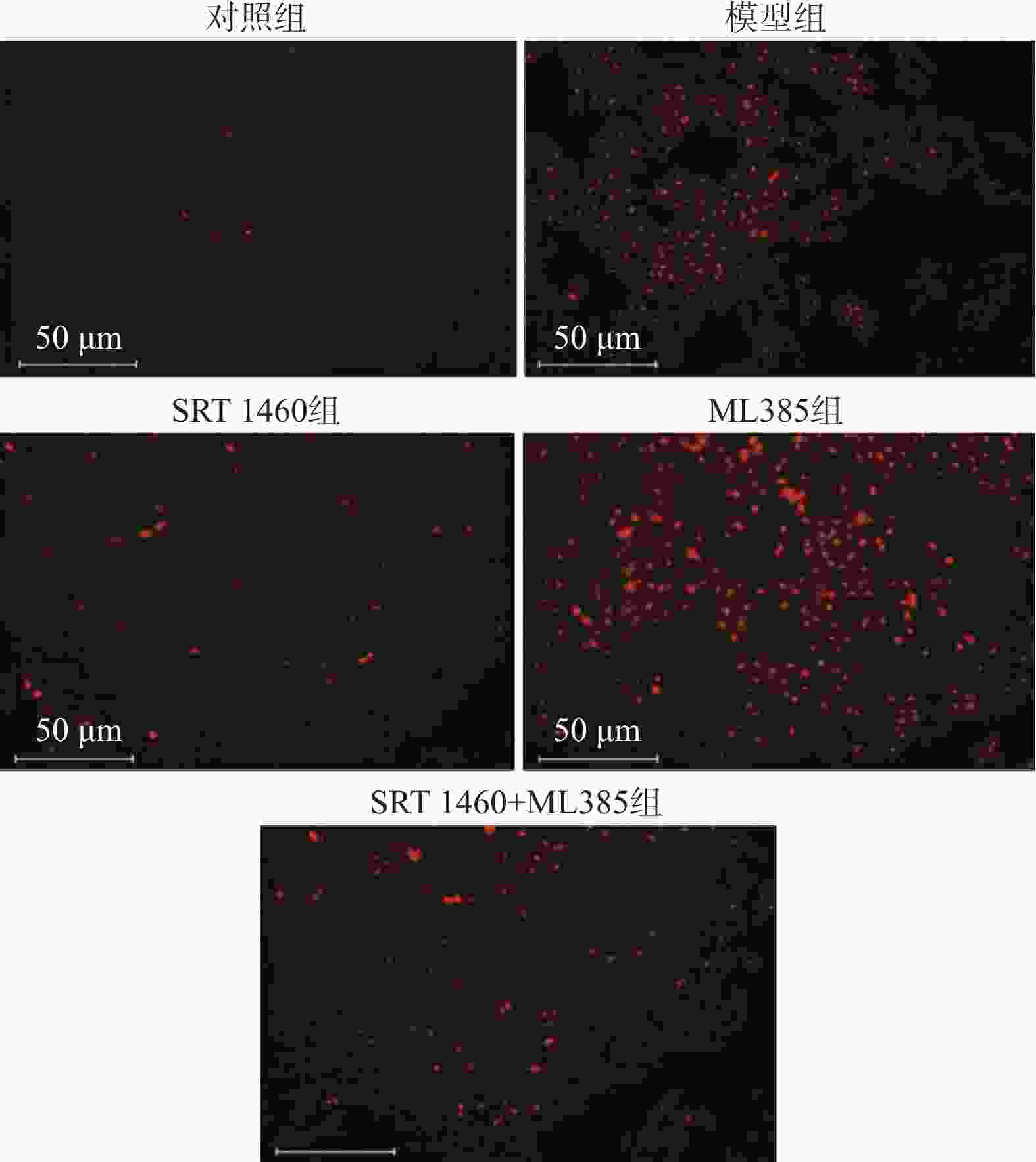
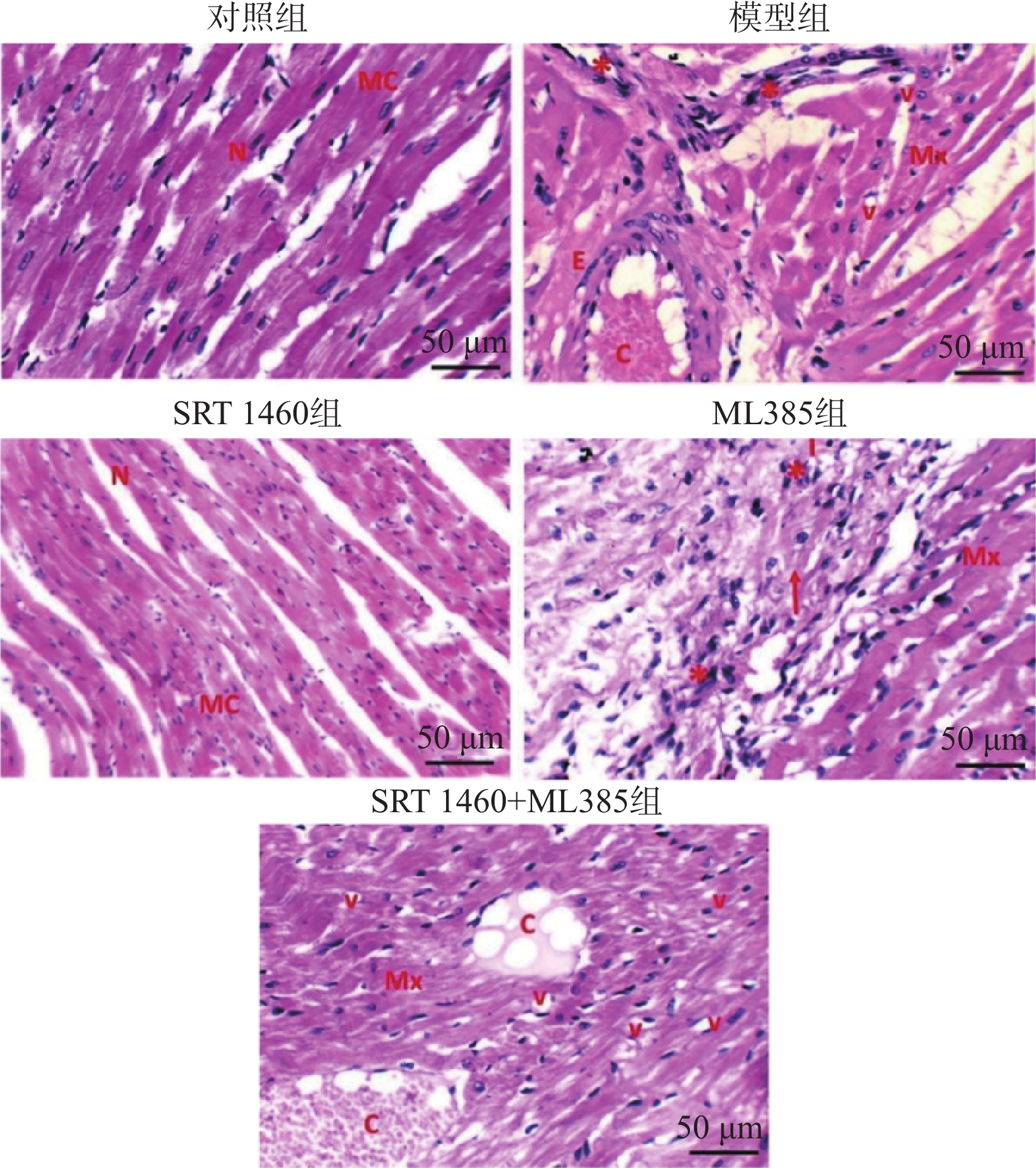
目的 探讨SIRT1激动剂对冠状动脉病变(Coronary artery disease,CAD)小鼠心肌功能的保护机制。 方法 采用随机数字表法将60只雄性C57BL/6J小鼠随机分为对照组、模型组、SRT1激动剂组(SRT 1460组,30 mg/kg)、Nrf2抑制剂组(ML385组,10 mg/kg)及SRT 1460+ML385联合治疗组,每组12只。对照组小鼠饲喂普通饲料,其余各组小鼠饲喂高脂饲料复制动脉粥样硬化小鼠模型,造模周期为12周。模型构建成功后,超声参数检测小鼠心肌功能;二维超声斑点追踪技术检测小鼠左室各层心肌应变;苏木素-伊红染色观察心肌组织病理变化;ELISA测定cTnI、LDH、CK水平; TUNEL分析心肌细胞凋亡情况;DHE荧光法检测心肌组织ROS水平;比色法检测心肌组织SOD活性、MDA、GSH和Fe2+含量;qPCR和Western Blot检测心肌组织Nrf2、GPX4、FTH1、ACSL4的基因和蛋白表达。 结果 与模型组比较,SRT 1460组LVEDd和LVPWd水平降低(P < 0.05),GLSendo、GLSmid和GCSendo升高(P < 0.05);SRT 1460组心肌细胞间隙变窄,胶原纤维沉积减少。与模型组比较,SRT 1460组小鼠血清cTnI、CK、LDH、心肌细胞凋亡率、ROS、MDA、Fe2+含量、ACSL4 mRNA及蛋白水平降低(P < 0.05)。相反,SOD活性、GSH含量、Nrf2、GPX4、FTH1 mRNA及蛋白水平均升高(P < 0.05)。Nrf2抑制剂ML385显著减弱SRT 1460对上述标记物的抑制作用(P < 0.05)。 结论 SRT 1460能提高冠状动脉病变小鼠GLSendo、GLSmid和GCSmid,改善左室重构和收缩功能,其机制可能与调控Nrf2-GPX4铁死亡途径有关。 -
关键词:
- 冠状动脉病变 /
- SIRT1激动剂 /
- 铁死亡 /
- 心肌功能 /
- 核因子E2相关因子2 /
- 谷胱甘肽过氧化物酶4
Abstract:Objective The objective of this research was to examine the cardioprotective properties of a SIRT1 agonist in mice afflicted with coronary artery disease (CAD). Methods Using the random number table method, 60 male C57BL/6J mice were randomly divided into a control group, a model group, an SRT1 agonist group (Group SRT 1460, 30 mg/kg), an Nrf2 inhibitor group (ML385 group, 10 mg/kg), and a combined treatment group of SRT 1460 + ML385, with 12 mice in each group. The control group mice were fed with normal feed, and the other groups of mice were fed with high-fat feed to create atherosclerosis mouse model. The modeling period was 12 weeks. After the successful construction of the model, ultrasonic parameters were used to detect the myocardial function of mice; two-dimensional ultrasound spot tracking technology was used to detect the strain of each layer of left ventricular myocardium. Pathological alterations in myocardial tissue were detected via HE staining, and the levels of cTnI, LDH and CK were measured using ELISA. Cardiomyocyte apoptosis was evaluated using TUNEL analysis, and levels of ROS in myocardial tissue were measured via DHE fluorescence assay. Additionally, SOD activity and MDA, GSH and Fe2+ contents in myocardial tissue were determined using colorimetry. Finally, gene and protein expressions of Nrf2, GPX4, FTH1, and ACSL4 were assessed through qRT-PCR and Western blot analysis. Results A significant decrease in LVEDd and LVPWd levels was observed in the SRT 1460 group (P < 0.05), whereas increases in GLSendo, GLSmid, and GCSendo levels were identified. A decrease in collagen fiber deposition was observed in the SRT 1460 group, in which the myocardium space narrowed. In comparison to the control group, the SRT 1460 group showed reductions in serum cTnI, CK, and LDH levels, cardiomyocyte apoptosis rate, ROS, MDA, and Fe2+ contents, as well as ACSL4 mRNA and protein levels (P < 0.05). An increase in SOD activity and GSH content as well as in Nrf2, GPX4, and FTH1 mRNA and protein levels was observed (P < 0.05). By inhibiting the nuclear translocation of Nrf2, ML385 could significantly block the regulatory effect of SRT 1460 (P < 0.05). Conclusion It has been shown that SRT 1460 enhances GLSendo, GLSmid, and GCSmid, and improves left ventricular remodeling and systolic function in mice with CAD, in part because it regulates the Nrf2-GPX4 ferroptosis pathway. -
表 1 引物序列
Table 1. Primer sequences
引物 正向引物 反向引物 Nrf2 F-5' TGATATTCCCGGTCACATCGAG-3' R-5' TGTCCTGTTGCATACCGTCT-3' GPX4 F-5' GTGGATGAAGATCCAACCC-3' R-5' TTGTCGATGAGGAACTTGG-3' FTH1 F-5' CTTCCTTCAGGATATCAAGAAACC-3' R-5' TCCAAATGTAATGCACACTCC-3' ACSL4 F-5' AGTACAACTTTCCTCTTGTGAC-3' R-5' AAGCCTCAGATTCATTTAGCC-3' GAPDH F-5' GGCATCCACGAAACTACATTCAA-3' R-5' AGCCAGAGCAGTGATCTCCTTCT-3' 表 2 各组小鼠左心室常规超声参数比较 [($\bar x \pm s $),n = 12]
Table 2. Comparison of conventional ultrasound parameters of left ventricle in each group [($\bar x \pm s $),n = 12]
组别 LVEDd(mm) LVPWd(mm) LVEF(%) E/E' 对照组 5.74 ± 0.53 1.72 ± 0.21 74.02 ± 10.64 14.51 ± 1.10 模型组 7.83 ± 2.13* 2.14 ± 0.41* 61.38 ± 11.13* 17.97 ± 3.17 SRT 1460组
ML385组6.34 ± 1.68#
9.49 ± 1.19#1.86 ± 0.20#
2.59 ± 0.68#72.13 ± 13.14#
50.36 ± 9.10#14.22 ± 5.06
21.89 ± 4.97SRT 1460+ML385组 7.81 ± 2.30& 2.10 ± 0.40& 60.53 ± 13.02& 18.43 ± 4.12 F值 8.956 7.500 8.403 7.637 P值 <0.001* <0.001* <0.001* <0.001* 与对照组相比,*P < 0.05;与模型组相比,#P < 0.05;与SRT 1460组相比,&P < 0.05。 表 3 各组小鼠内膜下层、中层及外膜下层心肌应变值比较 [($\bar x \pm s $),n = 12]
Table 3. Comparison of myocardial strain values in the lower,middle and outer membrane layers of the mice [($\bar x \pm s $),n = 12]
组别 GLSendo(%) GLSmid(%) GLSepi(%) GCSendo(%) GCSmid(%) GCSepi(%) 对照组 19.71 ± 1.66 16.98 ± 1.32 11.87 ± 1.75 26.34 ± 2.78 14.64 ± 2.17 9.51 ± 2.42 模型组 16.39 ± 2.21* 14.10 ± 2.69* 9.25 ± 1.98* 22.83 ± 4.07* 10.02 ± 3.35a 6.50 ± 1.33a SRT 1460组
ML385组19.04 ± 2.99#
12.81 ± 3.83#16.86 ± 3.16#
10.89 ± 3.68#11.35 ± 4.17#
7.33 ± 3.07#26.15 ± 6.93#
18.66 ± 3.88#13.83 ± 3.90#
7.85 ± 1.24#8.13 ± 2.39#
5.14 ± 1.13#SRT 1460+ML385组 15.96 ± 4.43& 13.41 ± 3.25& 8.96 ± 2.38& 21.93 ± 5.42& 10.23 ± 2.86& 6.31 ± 2.03& F 8.916 9.090 5.240 5.262 11.834 9.398 P <0.001* <0.001* 0.001* 0.001* <0.001* <0.001* 与对照组相比,*P < 0.05;与模型组相比,#P < 0.05;与SRT 1460组相比,&P < 0.05。 表 4 各组小鼠血清cTnI、LDH、CK水平比较 [($\bar x \pm s $),n = 6]
Table 4. Comparison of mouse serum levels of cTnI,LDH,and CK in each group [($\bar x \pm s $),n = 6]
组别 cTnI(ng/mL) LDH(U/L) CK(U/L) 对照组 3.77 ± 0.59 22.08 ± 3.22 34.60 ± 4.47 模型组 6.29 ± 1.62* 33.57 ± 6.19 58.02 ± 11.61* SRT 1460组
ML385组4.99 ± 0.74#
8.53 ± 0.85#24.57 ± 5.78
42.78 ± 6.2639.39 ± 7.09#
74.22 ± 8.98#SRT 1460+ML385组 6.52 ± 1.94& 31.17 ± 4.37 54.33 ± 9.64& F 11.906 14.223 19.747 P P < 0.001* P < 0.001* P < 0.001* 与对照组相比,*P < 0.05;与模型组相比,#P < 0.05;与SRT 1460组相比,&P < 0.05。 表 5 各组小鼠心肌细胞的凋亡率和ROS水平[($\bar x \pm s $),n = 6]
Table 5. Apoptosis rate and ROS levels of cardiomyocytes in each group [($\bar x \pm s $),n = 6]
组别 细胞凋亡率
(%)ROS
(DHE荧光强度)对照组 3.24 ± 0.74 18.28 ± 1.92 模型组 21.06 ± 4.52* 25.87 ± 4.65* SRT 1460组
ML385组14.66 ± 2.45#
28.45 ± 3.64#19.14 ± 2.31#
34.42 ± 2.90#SRT 1460+ML385组 22.41 ± 6.49& 24.38 ± 5.23& F值 33.418 18.932 P值 P < 0.001* P < 0.001* 与对照组相比,*P < 0.05;与模型组相比,#P < 0.05;与SRT 1460组相比,&P < 0.05。 表 6 各组小鼠心肌组织SOD活性、MDA、GSH和Fe2+含量比较[($\bar x \pm s $),n = 6]
Table 6. Comparison of SOD activity,MDA,GSH and Fe2 + content in myocardial tissues [($\bar x \pm s $),n = 6]
组别 SOD(U/mgprot) MDA(μmol/gprot) GSH(μmol/gprot) Fe2+ (μmol/gprot) 对照组 52.93 ± 3.84 1.96 ± 0.38 173.17 ± 19.26 4.37 ± 0.35 模型组 37.33 ± 7.07* 4.25 ± 1.05* 109.91 ± 18.02* 8.45 ± 2.52* SRT 1460组
ML385组48.55 ± 6.31#
28.17 ± 6.31#2.53 ± 0.52#
5.84 ± 0.92#161.32 ± 30.04#
74.17 ± 18.49#5.19 ± 0.93#
10.98 ± 2.26#SRT 1460+ML385组 35.55 ± 8.89& 3.52 ± 0.70& 122.07 ± 23.36& 8.55 ± 2.74& F 13.633 24.226 19.345 10.989 P <0.001* <0.001* <0.001* <0.001* 与对照组相比,*P < 0.05;与模型组相比,#P < 0.05;与SRT 1460组相比,&P < 0.05。 表 7 各组小鼠心肌组织Nrf2、GPX4、FTH1、ACSL4 mRNA水平比较[($\bar x \pm s $),n = 6]
Table 7. Comparison of mRNA levels of Nrf2,GPX4,FTH1 and ACSL4 in mouse myocardial tissues in each group [($\bar x \pm s $),n = 6]
组别 Nrf2 GPX4 FTH1 ACSL4 对照组 1.00 ± 0.14 1.01 ± 0.11 1.00 ± 0.06 1.00 ± 0.11 模型组 0.60 ± 0.11* 0.61 ± 0.13* 0.59 ± 0.16* 1.48 ± 0.17* SRT 1460组
ML385组0.95 ± 0.23#
0.45 ± 0.09#0.90 ± 0.19#
0.40 ± 0.09#0.94 ± 0.22#
0.39 ± 0.12#0.98 ± 0.42#
1.71 ± 0.21#SRT 1460+ML385组 0.56 ± 0.14& 0.61 ± 0.18& 0.63 ± 0.24& 1.61 ± 0.33& F 15.849 17.240 13.206 9.706 P <0.001* <0.001* <0.001* <0.001* 与对照组相比,*P < 0.05;与模型组相比,#P < 0.05;与SRT 1460组相比,&P < 0.05。 表 8 各组小鼠心肌组织Nrf2、GPX4、FTH1、ACSL4蛋白水平比较[($\bar x \pm s $),n = 6]
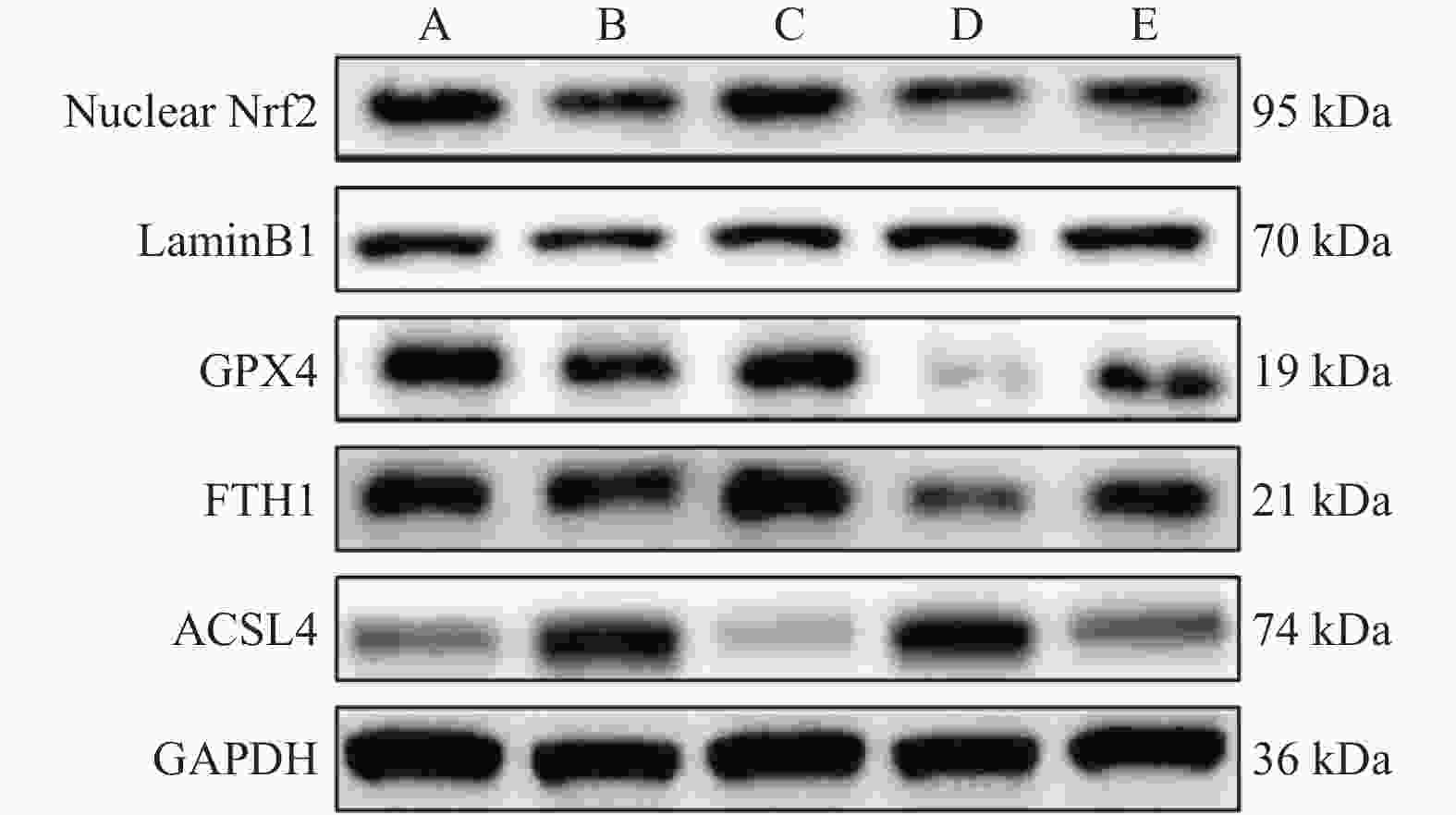
Table 8. Comparison of the protein expression of Nrf2,GPX4,FTH1 and ACSL4 in mouse myocardial tissues in each group [($\bar x \pm s $),n = 6]
组别 Nuclear Nrf2/LaminB1 GPX4/GAPDH FTH1/GAPDH ACSL4/GAPDH 对照组 1.00 ± 0.11 1.00 ± 0.12 1.00 ± 0.07 1.00 ± 0.12 模型组 0.61 ± 0.18* 0.60 ± 0.18* 0.66 ± 0.17* 1.48 ± 0.39* SRT 1460组
ML385组0.96 ± 0.23#
0.43 ± 0.11#0.86 ± 0.15#
0.40 ± 0.03#0.95 ± 0.19#
0.39 ± 0.11#1.01 ± 0.16#
2.23 ± 0.33#SRT 1460+ML385组 0.61 ± 0.19& 0.65 ± 0.18& 0.63 ± 0.13& 1.43 ± 0.31& F值 12.946 15.426 18.412 19.188 P值 <0.001* <0.001* <0.001* <0.001* 与对照组相比,*P < 0.05;与模型组相比,#P < 0.05;与SRT 1460组相比,&P < 0.05。 -
[1] Bergström G,Persson M,Adiels M,et al. Prevalence of Subclinical Coronary Artery Atherosclerosis in the General Population[J]. Circulation,2021,144(12):916-929. doi: 10.1161/CIRCULATIONAHA.121.055340 [2] Bytyçi I,Shenouda R,Wester P,et al. Carotid Atherosclerosis in Predicting Coronary Artery Disease: A Systematic Review and Meta-Analysis[J]. Arterioscler Thromb Vasc Biol,2021,41(4):e224-e237. [3] Pagliaro BR,Cannata F,Stefanini GG,Bolognese L. Myocardial ischemia and coronary disease in heart failure[J]. Heart Fail Rev,2020,25(1):53-65. [4] Savira F,Magaye R,Liew D,et al. Cardiorenal syndrome: Multi-organ dysfunction involving the heart,kidney and vasculature[J]. Br J Pharmacol,2020,177(13):2906-2922. [5] Yu Y,Yan Y,Niu F,et al. Ferroptosis: a cell death connecting oxidative stress,inflammation and cardiovascular diseases[J]. Cell Death Discov,2021,7(1):193. doi: 10.1038/s41420-021-00579-w [6] Wang Y,Zhao Y,Ye T,et al. Ferroptosis Signaling and Regulators in Atherosclerosis.[J]. Front Cell Dev Biol,2021,9:809457. doi: 10.3389/fcell.2021.809457 [7] Zhang J,Wang X,Guan B,et al. Qing-Xin-Jie-Yu Granule inhibits ferroptosis and stabilizes atherosclerotic plaques by regulating the GPX4/xCT signaling pathway[J]. J Ethnopharmacol,2023,301:115852. doi: 10.1016/j.jep.2022.115852 [8] Ma C,Zheng X,Wu X,et al. microRNA-181c-5p stimulates the development of coronary artery disease by targeting SIRT1[J]. Hellenic J Cardiol,2023,69:31-40. doi: 10.1016/j.hjc.2022.10.001 [9] Zhao S,Yu L. Sirtuin 1 activated by SRT1460 protects against myocardial ischemia/reperfusion injury[J]. Clin Hemorheol Microcirc,2021,78(3):271-281. doi: 10.3233/CH-201061 [10] Nandave M,Acharjee R,Bhaduri K,Upadhyay J,Rupanagunta GP,Ansari MN. A pharmacological review on SIRT 1 and SIRT 2 proteins,activators,and inhibitors: Call for further research[J]. Int J Biol Macromol,2023,242(Pt 1):124581. [11] Cheng P,Wang X,Liu Q,et al. LuQi formula attenuates Cardiomyocyte ferroptosis via activating Nrf2/GPX4 signaling axis in heart failure[J]. Phytomedicine,2024,125:155357. doi: 10.1016/j.phymed.2024.155357 [12] Golforoush P,Yellon DM,Davidson SM. Mouse models of atherosclerosis and their suitability for the study of myocardial infarction[J]. Basic Res Cardiol,2020,115(6):73. doi: 10.1007/s00395-020-00829-5 [13] Zhang Y,Ying F,Tian X,et al. TRPM2 Promotes Atherosclerotic Progression in a Mouse Model of Atherosclerosis[J]. Cells,2022,11(9):1423. [14] Pacholec M,Bleasdale JE,Chrunyk B,et al. SRT1720,SRT2183,SRT1460,and resveratrol are not direct activators of SIRT1[J]. J Biol Chem,2010,285(11):8340-8351. [15] Farooqui Z,Mohammad RS,Lokhandwala MF,Banday AA. Nrf2 inhibition induces oxidative stress,renal inflammation and hypertension in mice[J]. Clin Exp Hypertens,2021,43(2):175-180. doi: 10.1080/10641963.2020.1836191 [16] Libby P. The changing landscape of atherosclerosis[J]. Nature,2021,592(7855):524-533. doi: 10.1038/s41586-021-03392-8 [17] Askin L,Tibilli H,Tanriverdi O,Turkmen S. The relationship between coronary artery disease and SIRT1 protein[J]. North Clin Istanb,2020,7(6):631-635. [18] Gerdes A M. Cardiomyocyte ultrastructure [M]. Muscle. Academic Press. 2012: 47-55. [19] Endale HT,Tesfaye W,Mengstie TA. ROS induced lipid peroxidation and their role in ferroptosis[J]. Front Cell Dev Biol,2023,11:1226044. [20] Fang X,Ardehali H,Min J,et al. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease[J]. Nat Rev Cardiol,2023,20(1):7-23. doi: 10.1038/s41569-022-00735-4 [21] Li J Y,Liu S Q,Yao R Q,et al. A novel insight into the fate of cardiomyocytes in ischemia-reperfusion injury: From iron metabolism to ferroptosis[J]. Front Cell Dev Biol,2021,9:799499. doi: 10.3389/fcell.2021.799499 [22] Chen X,Yu C,Kang R,et al. Iron metabolism in ferroptosis[J]. Front Cell Dev Biol,2020,8:590226. doi: 10.3389/fcell.2020.590226 [23] Ding K,Liu C,Li L,et al. Acyl-CoA synthase ACSL4: an essential target in ferroptosis and fatty acid metabolism[J]. Chin Med J (Engl),2023,136(21):2521-2537. [24] Ma T,Du J,Zhang Y,et al. GPX4-independent ferroptosis-a new strategy in disease's therapy[J]. Cell Death Discov,2022,8(1):434. doi: 10.1038/s41420-022-01212-0 [25] Shen Y,Wang X,Shen X,et al. Geniposide possesses the protective effect on myocardial injury by inhibiting oxidative stress and ferroptosis via activation of the Grsf1/GPx4[J]. Axis. Front Pharmacol,2022,13:879870. doi: 10.3389/fphar.2022.879870 [26] Tang D,Chen X,Kang R,et al. Ferroptosis: molecular mechanisms and health implications[J]. Cell Res,2021,31(2):107-125. doi: 10.1038/s41422-020-00441-1 [27] Sykiotis G P J A. Keap1/Nrf2 signaling pathway [Z]. MDPI. 2021: 828 [28] Paramesha B,Anwar M S,Meghwani H,Maulik S K,Arava S K,Banerjee S K. Sirt1 and Sirt3 activation improved cardiac function of diabetic rats via modulation of mitochondrial function[J]. Antioxidants (Basel),2021,10(3):338. -





 下载:
下载: