Explorations of the Clinical Pathology and Prognosis of Gastric Cancer Based on Systemic Immune Inflammation Index and Ferritin Expression
-
摘要:
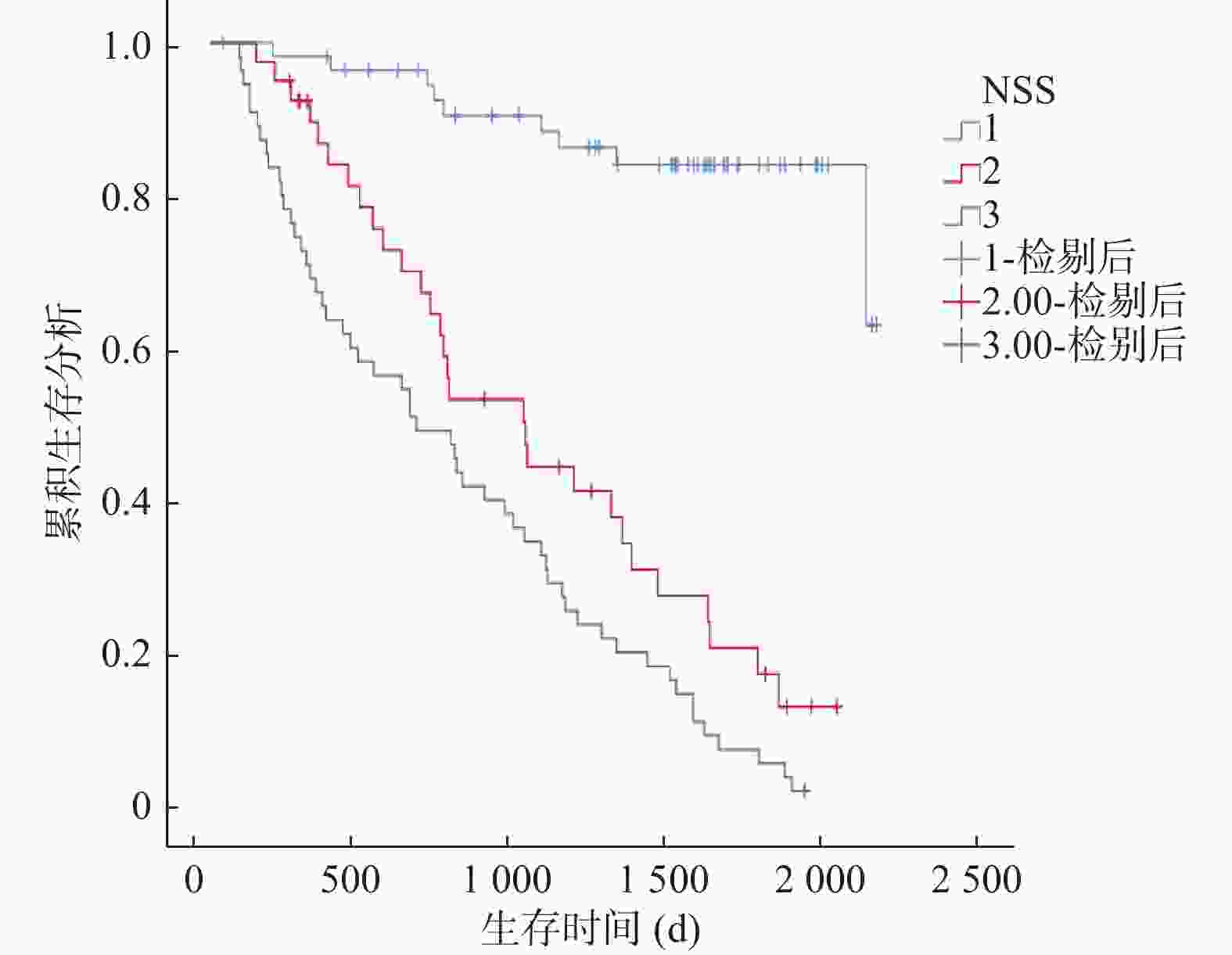
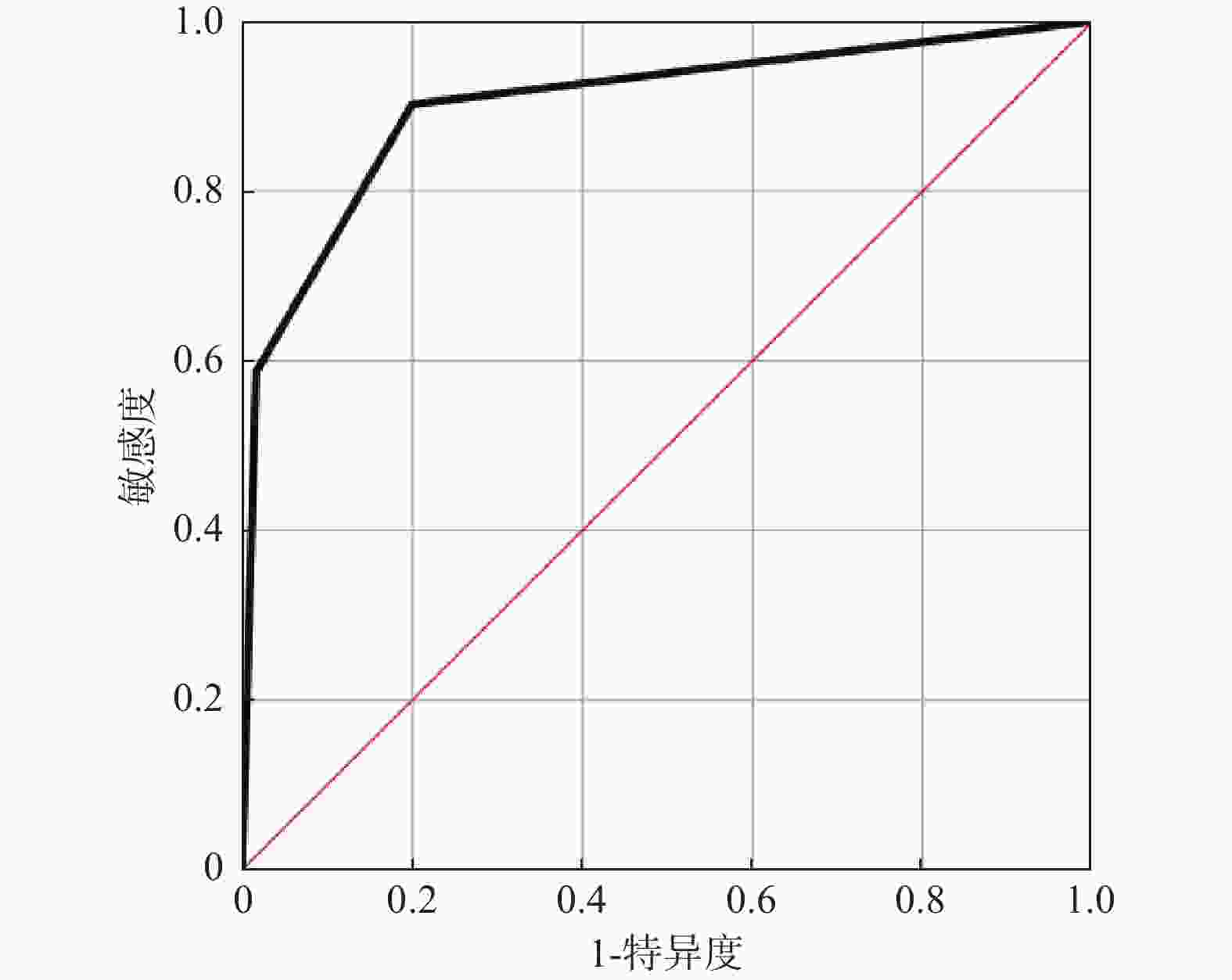
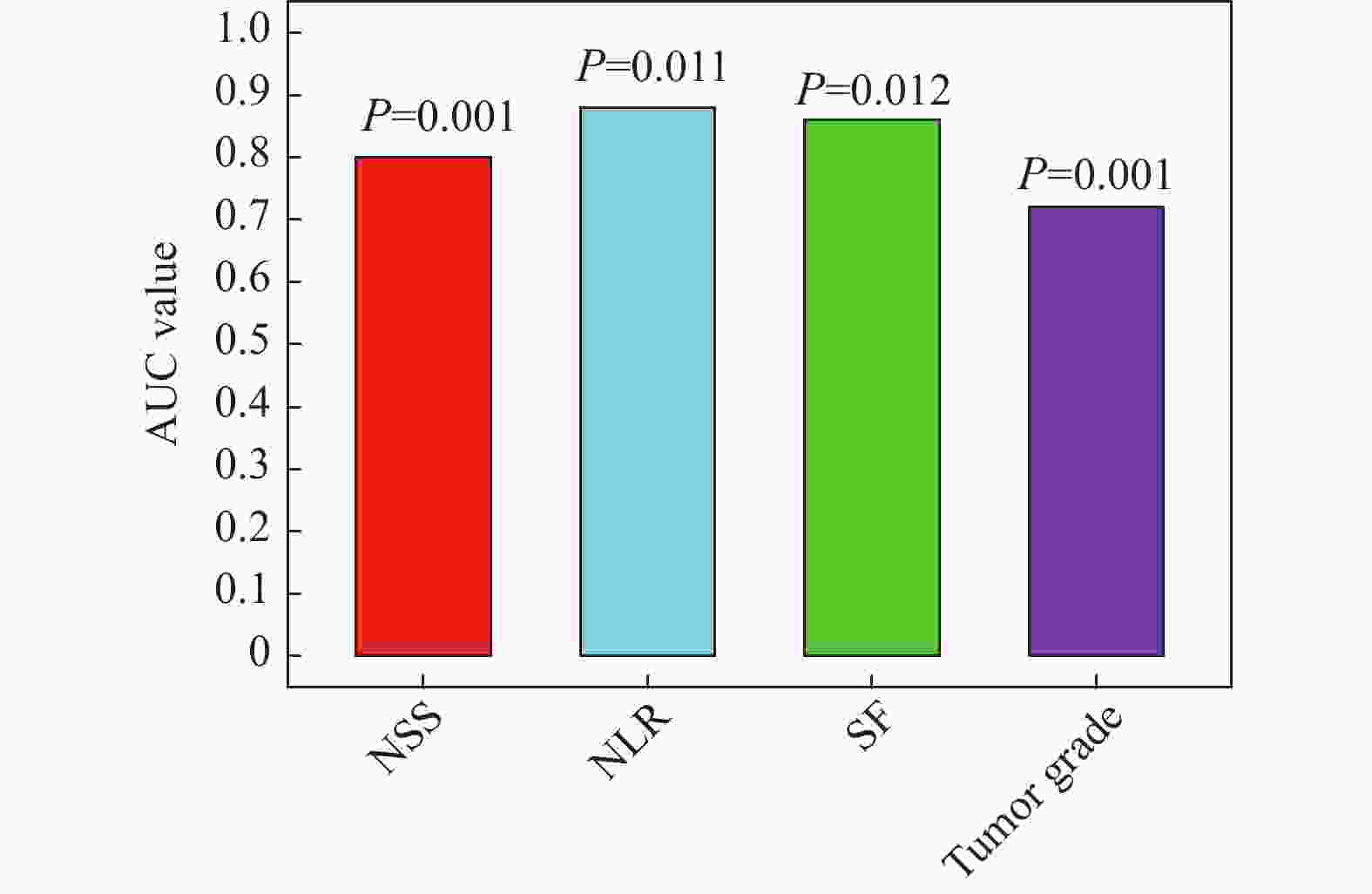
目的 探讨全身免疫炎症指数和铁蛋白(serum ferritin,SF)与胃癌(gastric cancer,GC)临床病理和预后的关系。 方法 对2017年1月至2020年12月在成都市青白江区人民医院肿瘤放疗科接受D2胃癌根治术的152例胃癌患者进行回顾性分析。通过将嗜中性粒细胞计数除以淋巴细胞计数来计算NLR。使用ELISA试剂盒评估血浆中SF水平。根据多变量分析的结果,建立一个包括NLR-SF评分(NSS)的新型预后预测系统。 结果 Cox回归分析显示,NLR(HR = 1.053,95%CI = 1.014~1.094,P = 0.007),肿瘤等级(HR = 1.944,95%CI = 1.279~2.955,P = 0.002)和SF(HR = 1.005,95%CI = 1.002~1.008,P = 0.002)是GC患者预后的独立影响因素。ROC分析显示,当SF的截止值为215.5时,其预测GC患者死亡的AUC为0.800(95%CI = 0.731~0.869),敏感度为68.5%,特异度为86.7%。NLR的截止值为3.36时,其预测GC患者死亡的AUC为0.869(95%CI = 0.810~0.928),敏感度为81.5%,特异度为91.7%。较高的NSS与淋巴结转移数量>3例数、死亡例数、嗜中性粒细胞、血小板、SF、NLR、PLR水平增加显著相关(P < 0.05),和与淋巴细胞降低显著相关(P < 0.05)。Kaplan-Meier生存曲线分析显示NSS越高,生存期越短(χ2 = 75.168,P < 0.001)。ROC分析显示,NSS预测GC患者死亡的AUC为0.902(95%CI = 0.852~0.952),敏感度为90.2%,特异度为80.0%。 结论 本研究证实了NLR和SF是GC患者的独立预后因素,将二者结合构建的NSS在预测术后总生存期方面具有重要价值。 -
关键词:
- 中性粒细胞与淋巴细胞比率 /
- 铁蛋白 /
- 胃癌 /
- 预后
Abstract:Objective To explore the relationship between systemic immune inflammatory index and ferritin (SF) and the clinicopathologic features and prognosis of gastric cancer (GC). Methods A retrospective analysis was conducted on 152 gastric cancer patients who underwent D2 gastrectomy at the Radiation Oncology Department of Qingbaijiang District People's Hospital in Chengdu from January 2017 to December 2020. Neutrophil count was divided by lymphocyte count to calculate Neutrophil to lymphocyte ratio (NLR). The SF level in plasma was evaluated by ELISA kit. According to the results of multivariate analysis, a new prognosis prediction system including NLR-SF score (NSS) was established. Results Multivariate Cox regression analysis showed that NLR (HR = 1.053, 95%CI = 1.014~1.094, P = 0.007), tumor grade (HR = 1.944, 95%CI = 1.279~2.955, P = 0.002) and SF (HR = 1.005, 95%CI = 1.002~1.008, P = 0.002) were independent factors affecting the prognosis of GC patients. ROC analysis showed that when the cutoff value of SF was 215.5, the AUC of predicting the death of GC patients was 0.800 (95%CI = 0.731~0.869), the sensitivity was 68.5%, and the specificity was 86.7%. When the cutoff value of NLR was 3.36, the AUC of predicting the death of GC patients was 0.869 (95%CI = 0.810~0.928), the sensitivity was 81.5%, and the specificity was 91.7%. Higher NSS was significantly correlated with the number of lymph node metastasis > 3 cases, the number of deaths, the increase of neutrophil, platelet, SF, NLR and PLR levels (P < 0.05), and the decrease of lymphocytes (P < 0.05). Kaplan-Meier survival curve analysis showed that the higher the NSS, the shorter the survival time (χ2 =75.168, P < 0.001). ROC analysis showed that the AUC of NSS in predicting the death of GC patients was 0.902 (95%CI = 0.852~0.952), the sensitivity was 90.2%, and the specificity was 80.0%. Conclusions This study confirms that NLR and SF are independent prognostic factors of GC patients, and the NSS constructed by combining them is of great value in predicting the overall postoperative survival. -
Key words:
- Neutrophil to lymphocyte ratio /
- Ferritin /
- Gastric cancer /
- Prognosis
-
表 1 GC患者的人口统计学数据、实验室检查和临床特征结果[n(%)/M(P25,P75),n = 152]
Table 1. Demographic data,laboratory tests,and clinical characteristics of GC patients [n(%)/M(P25,P75),n = 152]
特征 计量数据 年龄(岁) 63 (32,88) 性别 男性 105 (69.1) 女性 47 (30.9) 肿瘤等级 Ⅰ 6 (3.9) Ⅱ 58 (38.2) Ⅲ 88 (57.9) 神经侵犯 28 (18.4) 脉管内癌栓 35 (23.0) 肿瘤大小>5(cm) 73 (48.0) 淋巴结转移数量>3(个) 79 (52.0) 死亡 92 (60.5) CEA(ng/mL) 4.27 (0.10,174) CA19-9(U/mL) 33.00 (0.51, 9868 )C-反应蛋白(mg/L) 2.31 (0.11,29.02) 嗜中性粒细胞(×109/L) 5.84 (1.3,26) 淋巴细胞(×109/L) 1.52 (0.15,4.63) 血小板(×109/L) 306.5 (34, 1157 )血红蛋白(g/dL) 11.40 (4.51,16.42) NLR 3.62 (2.22,6.17) PLR 205.20 (131.74,292.99) SF(ng/mL) 210.00 (160.00,268.75) *P < 0.05。 表 2 单因素Cox比例风险回归模型分析GC患者预后的影响因素
Table 2. Univariate Cox proportional hazards regression analysis of factors influencing the prognosis of GC patients
指标 β SE Waldχ2 P HR 95%CI 下限 上限 年龄 0.002 0.010 0.032 0.857 1.002 0.983 1.021 CEA −0.001 0.001 1.286 0.257 0.999 0.996 1.001 CA19-9 0.000 0.000 3.475 0.062 1.000 1.000 1.000 CRP −0.008 0.021 0.159 0.690 0.992 0.953 1.033 NLR 0.073 0.037 3.946 0.047* 1.076 1.001 1.157 肿瘤等级 0.692 0.219 10.012 0.002* 1.997 1.301 3.065 肿瘤大小>5 (cm) −0.078 0.230 0.114 0.736 0.925 0.589 1.453 神经侵犯 0.328 0.271 1.467 0.226 1.388 0.817 2.359 脉管内癌栓 −0.384 0.270 2.014 0.156 0.681 0.401 1.158 淋巴结转移数量>3 (个) 0.541 0.275 3.860 0.049* 1.717 1.001 2.945 SF(ng/mL) 0.005 0.002 9.976 0.002* 1.005 1.002 1.009 PLR −0.001 0.001 0.416 0.519 0.999 0.998 1.001 *P < 0.05。 表 3 多因素Cox比例风险回归模型分析GC患者预后的影响因素
Table 3. Multivariate Cox proportional hazards regression analysis of factors influencing the prognosis of GC patients
指标 β SE Waldχ2 P HR 95%CI 下限 上限 CA19-9 0.000 0.000 3.253 0.071 1.000 1.000 1.000 NLR 0.052 0.019 7.268 0.007* 1.053 1.014 1.094 肿瘤等级 0.665 0.214 9.696 0.002* 1.944 1.279 2.955 淋巴结转移数量>3 0.431 0.234 3.383 0.066 1.539 0.972 2.435 SF 0.005 0.002 9.483 0.002* 1.005 1.002 1.008 *P < 0.05。 表 4 GC患者的NSS评分和临床病理特征之间的关系[n(%)/M(P25,P75)/($\bar x \pm s $)]
Table 4. The relationship between NSS score and clinical-pathological characteristics in GC patients [n(%)/M(P25,P75)/($\bar x \pm s $)]
特征 NSS 0(n = 57) NSS 1(n = 40) NSS 2(n = 55) χ2/t P 年龄(岁) 63.42 ± 9.85 60.63 ± 11.42 59.87 ± 13.48 1.5 0.247 性别 0.3 0.829 男性 38 (66.7) 29 (72.5) 38 (69.1) 女性 19 (33.3) 11 (27.5) 17 (30.9) 肿瘤等级 12.0 0.349 Ⅰ 3(5.3) 2(5.0) 1(1.8) Ⅱ 36(63.2) 24(60.0) 27(49.1) Ⅲ 18(31.6) 14(35.0) 27(49.1) 神经侵犯 10(17.5) 4(10.0) 14(25.5) 2.2 0.155 脉管内癌栓 11(19.3) 7(17.5) 17(30.9) 1.8 0.216 肿瘤大小>5 (cm) 26(45.6) 19(47.5) 28(50.9) 0.5 0.852 淋巴结转移数量>3 23(40.4) 18(45.0) 38(69.1) 2.7 0.006* 死亡 9(15.8) 29(72.5) 92(98.2) 15.5 <0.001* CEA(ng/mL) 3.14 (1.65,9.50) 4.45 (2.15,16.00) 5.70 (1.90,54.00) 1.92 0.057 CA19-(U/mL) 20.70(4.55,100.00) 52.54 (8.23,237.80) 33.000 (3.86,203.00) 0.28 0.760 C-反应蛋白(mg/L) 1.70 (0.50,4.86) 4.87(0.96,9.14) 2.31(0.90,9.32) 1.68 0.095 嗜中性粒细胞(×109/L) 4.39 ± 1.86 5.69 ± 2.80 8.67 ± 3.76 −7.59 <0.001* 淋巴细胞(×109/L) 2.08 ± 0.85 1.68 ± 0.81 1.23 ± 0.50 6.48 <0.001* 血小板(×109/L) 299.72 ± 91.65 306.55 ± 120.62 398.89 ± 201.07 −3.34 0.001* 血红蛋白(g/dL) 11.19 ± 2.29 11.61 ± 1.91 11.22 ± 2.00 −0.07 0.941 NLR 2.26 ± 0.74 4.11 ± 2.58 9.03 ± 7.24 −6.90 <0.001* PLR 161.83 ± 67.60 208.92 ± 107.26 400.40 ± 289.88 −5.95 <0.001* SF(ng/mL) 164.35 ± 42.54 188.88 ± 55.04 303.22 ± 77.60 −11.69 <0.001* *P < 0.05。 -
[1] Frick C, Rumgay H, Vignat J, et al. Quantitative estimates of preventable and treatable deaths from 36 cancers worldwide: A population-based study[J]. Lancet Glob Health, 2023, 11(11): e1700-e1712. doi: 10.1016/S2214-109X(23)00406-0 [2] López M J, Carbajal J, Alfaro A L, et al. Characteristics of gastric cancer around the world[J]. Crit Rev Oncol Hematol, 2023, 181: 103841. doi: 10.1016/j.critrevonc.2022.103841 [3] Matsas S, Aguiar Jr P N, Del Giglio A. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as biomarkers to prognosticate survival in advanced gastric cancer patients in the era of immunotherapy: A systematic review and meta-analysis[J]. J Gastrointest Oncol, 2024, 15(1): 33-51. doi: 10.21037/jgo-23-808 [4] Lazzati A, Poghosyan T, Touati M, et al. Risk of esophageal and gastric cancer after bariatric surgery[J]. JAMA Surg, 2023, 158(3): 264-271. doi: 10.1001/jamasurg.2022.6998 [5] Hirano T. IL-6 in inflammation, autoimmunity and cancer[J]. Int Immunol, 2021, 33(3): 127-148. doi: 10.1093/intimm/dxaa078 [6] Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation-have anti-inflammatory therapies come of age?[J]. Nat Rev Clin Oncol, 2021, 18(5): 261-279. doi: 10.1038/s41571-020-00459-9 [7] Lin G S, Lu J, Lin J, et al. Value of the preoperative D-dimer to albumin ratio for survival and recurrence patterns in gastric cancer[J]. Ann Surg Oncol, 2023, 30(2): 1132-1144. doi: 10.1245/s10434-022-12625-7 [8] Saboorifar H, Zafarani Y, Gholampour G, et al. Serum inflammatory markers as prognostic marker for nasopharyngeal carcinoma with liver metastasis: A multi-center retrospective study[J]. Eur Arch Otorhinolaryngol, 2024, 281(8): 4315-4324. [9] Ramírez-Carmona W, Díaz-Fabregat B, Yuri Yoshigae A, et al. Are serum ferritin levels a reliable cancer biomarker? A systematic review and meta-analysis[J]. Nutr Cancer, 2022, 74(6): 1917-1926. doi: 10.1080/01635581.2021.1982996 [10] Zhou D, Zeng C, Zhang L, et al. Serum ferritin is associated with sarcopenia and predicts long-term survival for gastric cancer undergoing radical gastrectomy[J]. Eur J Gastroenterol Hepatol, 2023, 35(12): 1341-1348. doi: 10.1097/MEG.0000000000002659 [11] Wang F H, Zhang X T, Tang L, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2023[J]. Cancer Commun, 2024, 44(1): 127-172 doi: 10.1002/cac2.12516 [12] Ma X, Zhou X H, Guo J X, et al. CA19‑9 is a significant prognostic factor in stage III gastric cancer patients undergoing radical gastrectomy[J]. BMC Surg, 2024, 24(1): 31. doi: 10.1186/s12893-024-02324-3 [13] Gao C, Tong Y X, Zhu L, et al. Short-term prognostic role of peripheral lymphocyte subsets in patients with gastric cancer[J]. Int Immunopharmacol, 2023, 115: 109641. doi: 10.1016/j.intimp.2022.109641 [14] Liu M, Hu Z, Wang C, et al. The TLR/MyD88 signalling cascade in inflammation and gastric cancer: The immune regulatory network of Helicobacter pylori[J]. J Mol Med, 2023, 101(7): 767-781. doi: 10.1007/s00109-023-02332-5 [15] Wang H, Gong H, Tang A, et al. Neutrophil/lymphocyte ratio predicts lymph node metastasis in patients with gastric cancer[J]. Am J Transl Res, 2023, 15(2): 1412. [16] Yang S, Sun B, Li J, et al. Neutrophil extracellular traps promote angiogenesis in gastric cancer[J]. Cell Commun Signal, 2023, 21(1): 176. doi: 10.1186/s12964-023-01196-z [17] Ito S, Ohgaki K, Kawazoe T, et al. Survival benefits of laparoscopic gastrectomy in elderly patients with gastric cancer: Focusing on preoperative nutritional and inflammatory status[J]. Anticancer Res, 2023, 43(5): 2055-2067. doi: 10.21873/anticanres.16366 [18] Hirahara N, Matsubara T, Kaji S, et al. Novel inflammation-combined prognostic index to predict survival outcomes in patients with gastric cancer[J]. Oncotarget, 2023, 14: 71-82. doi: 10.18632/oncotarget.28353 [19] Zhang S, Qiu C, Yu H, et al. Prognostic value of neutrophil to lymphocyte ratio in gastric cancer patients receiving immune checkpoint inhibitors: A systematic review and meta-analysis[J]. Front Oncol, 2023, 13: 1070019. doi: 10.3389/fonc.2023.1070019 [20] Nouri Z, Choi S W, Choi I J, et al. Exploring connections between oral microbiota, short-chain fatty acids, and specific cancer types: A study of oral cancer, head and neck cancer, pancreatic cancer, and gastric cancer[J]. Cancers, 2023, 15(11): 2898. doi: 10.3390/cancers15112898 [21] Wang F, Deng G, Liang N, et al. Serum ferritin level is an effective prognostic factor for lung cancer immunotherapy[J]. Cancer Biol Ther, 2023, 24(1): 2285367. doi: 10.1080/15384047.2023.2285367 [22] Zhao Y, Zhao J, Ma H, et al. High hepcidin levels promote abnormal iron metabolism and ferroptosis in chronic atrophic gastritis[J]. Biomedicines, 2023, 11(9): 2338. doi: 10.3390/biomedicines11092338 [23] Chun J H, Kim D J. Risk factors for ferritin depletion in patients with post-gastrectomy gastric cancer and normal baseline ferritin levels[J]. Foregut Surgery, 2024, 4(1): 17-26. doi: 10.51666/fs.2024.4.e1 [24] Chen Z, Liang Z, Chen K, et al. Serum ferritin predicted prognosis in patients with nasopharyngeal carcinoma[J]. Sci Rep, 2024, 14(1): 4311. doi: 10.1038/s41598-024-54627-3 [25] Skórzewska M, Pikuła A, Gęca K, et al. Systemic inflammatory response markers for prediction of response to neoadjuvant chemotherapy in patients with advanced gastric cancer[J]. Cytokine, 2023, 172: 156389. doi: 10.1016/j.cyto.2023.156389 [26] Deng D, Zhang Y, Zhang R, et al. Circulating proteins and metabolite biomarkers in gastric cancer: A systematic review and meta-analysis[J]. Arch Med Res, 2023, 54(2): 124-134. doi: 10.1016/j.arcmed.2022.12.012 -






 下载:
下载: