Effects of Inhibiting lncRNA H19 Expression on Neuroinflammation and Cognitive Function in Vascular Dementia Models
-
摘要:
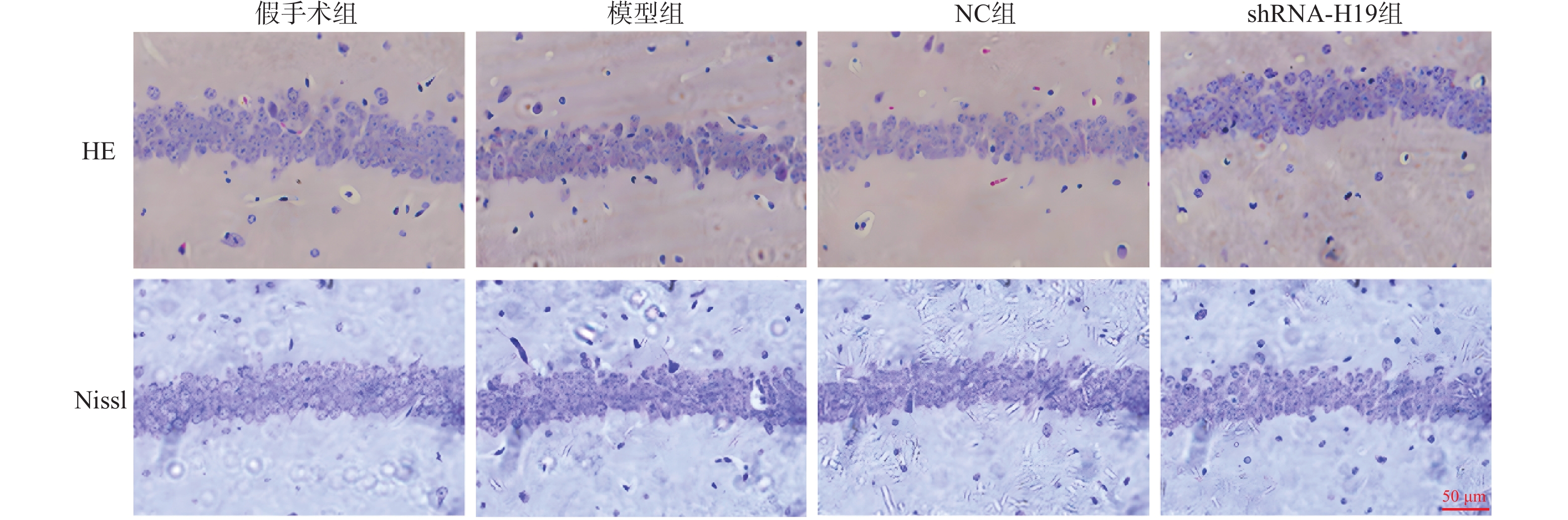
目的 探讨长非编码RNA(long non-coding RNA,lncRNA)H19(简称H19)对血管性痴呆(vascular dementia,VD)模型神经炎症及认知功能的作用。 方法 采用双侧颈总动脉闭塞构建VD小鼠模型,48只小鼠随机均分为假手术组、模型组、NC组和shRNA-H19组,其中假手术组不结扎。造模成功后,NC组和shRNA-H19组小鼠脑内分别注射AAV2/1-shRNA-空载、AAV2/1-shRNA-H19。水迷宫法观察小鼠认知水平;HE和Nissl染色观察海马CA1区病理及神经元损伤情况;TUNEL染色检测神经元凋亡;酶联免疫吸附测定(enzyme-linked immunosorbent assay,ELISA)法检测海马组织肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、白细胞介素(interleukin,IL)-1β、IL-6水平;免疫荧光染色检测海马CA1区离子钙结合衔接分子1(ionized cacium-binding adapter molecule-1,Iba-1)蛋白表达;实时荧光定量聚合酶链式反应(quantitative real-time polymerase chain reaction,qRT-PCR)和(或)Western blot法检测海马组织H19、B细胞淋巴瘤-2(Bcl-2)、活化的胱天蛋白酶-3(cleaved Caspase-3)、Toll样受体4(toll-like receptor 4,TLR4)、核因子-κB(nuclear factor-kappa B,NF-κB)蛋白和(或)mRNA表达。 结果 与模型组比较,shRNA-H19组小鼠认知能力升高(P < 0.05),海马CA1区病理及神经元损伤减轻,海马组织Bcl-2水平升高,神经元凋亡率、H19、TNF-α、IL-1β、IL-6、Iba-1、cleaved Caspase-3、TLR4、NF-κB p-p65/NF-κB p65水平均降低(P < 0.05);而NC组上述指标均无显著性差异(P > 0.05)。 结论 抑制海马组织H19表达可改善VD小鼠神经炎症和认知功能,抑制海马CA1区神经元凋亡及小胶质细胞浸润,其机制可能与下调TLR4/NF-κB p65通路相关。 -
关键词:
- 血管性痴呆 /
- 长非编码RNA H19 /
- 神经元凋亡 /
- 神经炎症 /
- TLR4/NF-κB p65通路 /
- 小鼠
Abstract:Objective To explore the effects of long non-coding RNA (lncRNA) H19 on neuroinflammation and cognitive function in a vascular dementia (VD) model. Methods A VD mouse model was established by bilateral common carotid artery occlusion. Forty-eight mice were randomly and equally divided into four groups: sham surgery group, model group, NC group, and shRNA-H19 group. The sham surgery group underwent no ligation. Following successful model establishment, AAV2/1-shRNA-vehicle and AAV2/1-shRNA-H19 were injected into the brain of mice in the NC group and shRNA-H19 group, respectively. Cognitive levels was assessed useing Morris water maze test. Pathological changes in the hippocampal CA1 region and neuronal injury were observed by HE and Nissl staining. Neuronal apoptosis was detected by TUNEL staining. Levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 in hippocampal tissue were measured by enzyme-linked immunosorbent assay (ELISA). Expression of ionized calcium-binding adapter molecule-1 (Iba-1) protein in the hippocampal CA1 region was detected by immunofluorescence staining. Expression of H19, B-cell lymphoma-2 (Bcl-2), cleaved caspase-3, toll-like receptor 4 (TLR4), and nuclear factor-kappa B (NF-κB) protein and/or mRNA in hippocampal tissue were detected by quantitative real-time polymerase chain reaction (qRT-PCR) and/or Western blot. Results Compared with the model group, shRNA-H19 mice exhibited improved cognitive function (P < 0.05), reduced pathological changes and neuronal injury in the hippocampal CA1 region, elevated Bcl-2 levels in hippocampal tissue, and decreased neuronal apoptosis rate, H19, TNF-α, IL-1β, IL-6, Iba-1, cleaved caspase-3, TLR4, and NF-κB p-p65/NF-κB p65 levels (P < 0.05). The NC group showed no significant differences in the above indicators (P > 0.05). Conclusion Inhibition of H19 expression in hippocampal tissue ameliorates neuroinflammation and improve cognitive function in VD mice, inhibits neuronal apoptosis and microglial infiltration in the hippocampal CA1 region. The underlying mechanism may be related to downregulation of the TLR4/NF-κB p65 signaling pathway. -
Key words:
- Vascular dementia /
- Long non-coding RNA H19 /
- Neuronal apoptosis /
- Neuroinflammation /
- TLR4/NF-ΚB P65 pathway /
- Mice
-
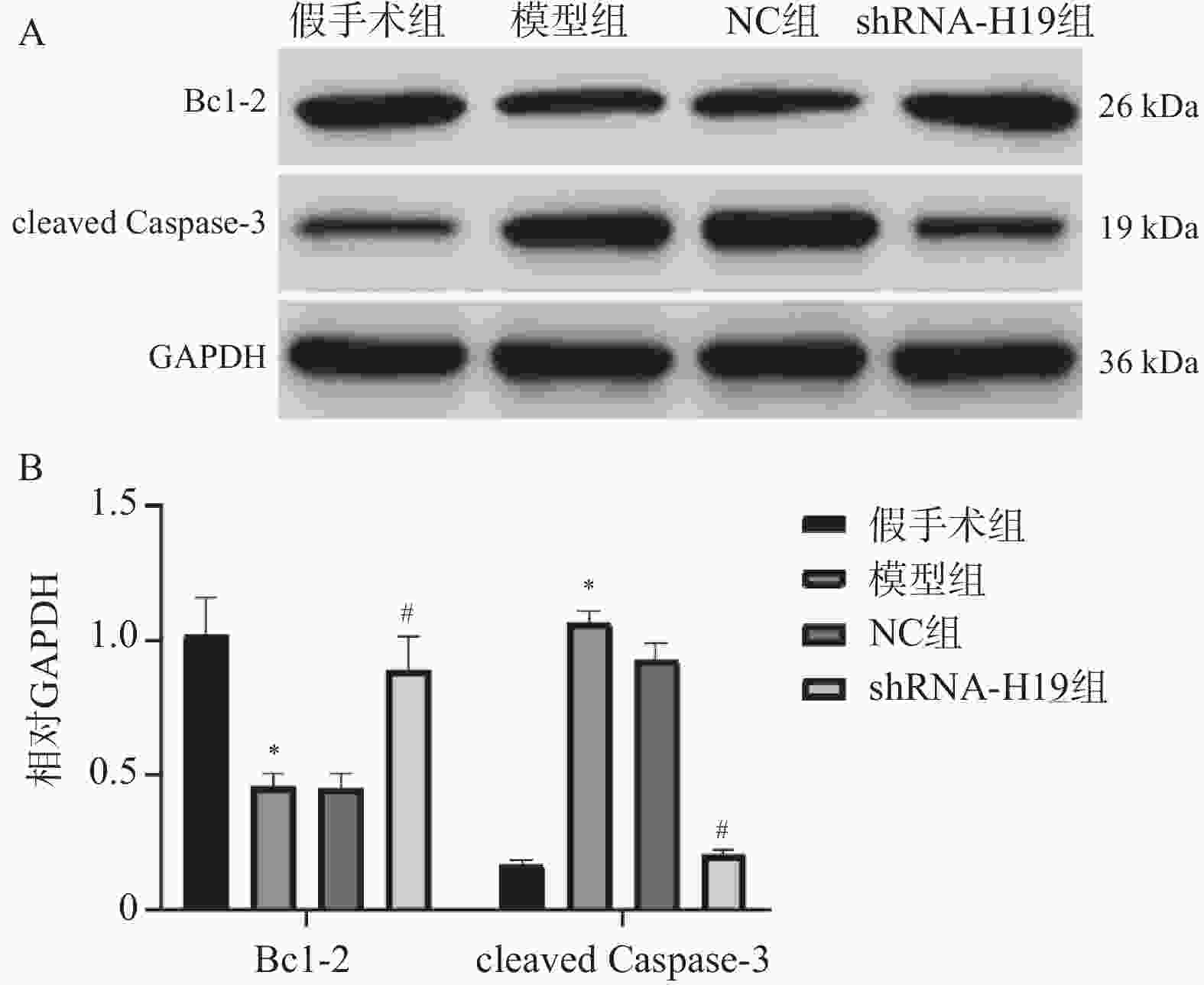
图 3 各组VD小鼠海马组织Bcl-2、cleaved Caspase-3蛋白表达水平变化($ \bar x \pm s $,n = 6)
A:各组Bcl-2、cleaved Caspase-3蛋白电泳图;B:各Bcl-2、cleaved Caspase-3蛋白统计学分析;与假手术组比较,*P < 0.05;与NC组比较,#P < 0.05。
Figure 3. Changes in protein expression levels of Bcl-2 and cleaved Caspase-3 in hippocampal tissue of VD mice in each group ($ \bar x \pm s $,n = 6)
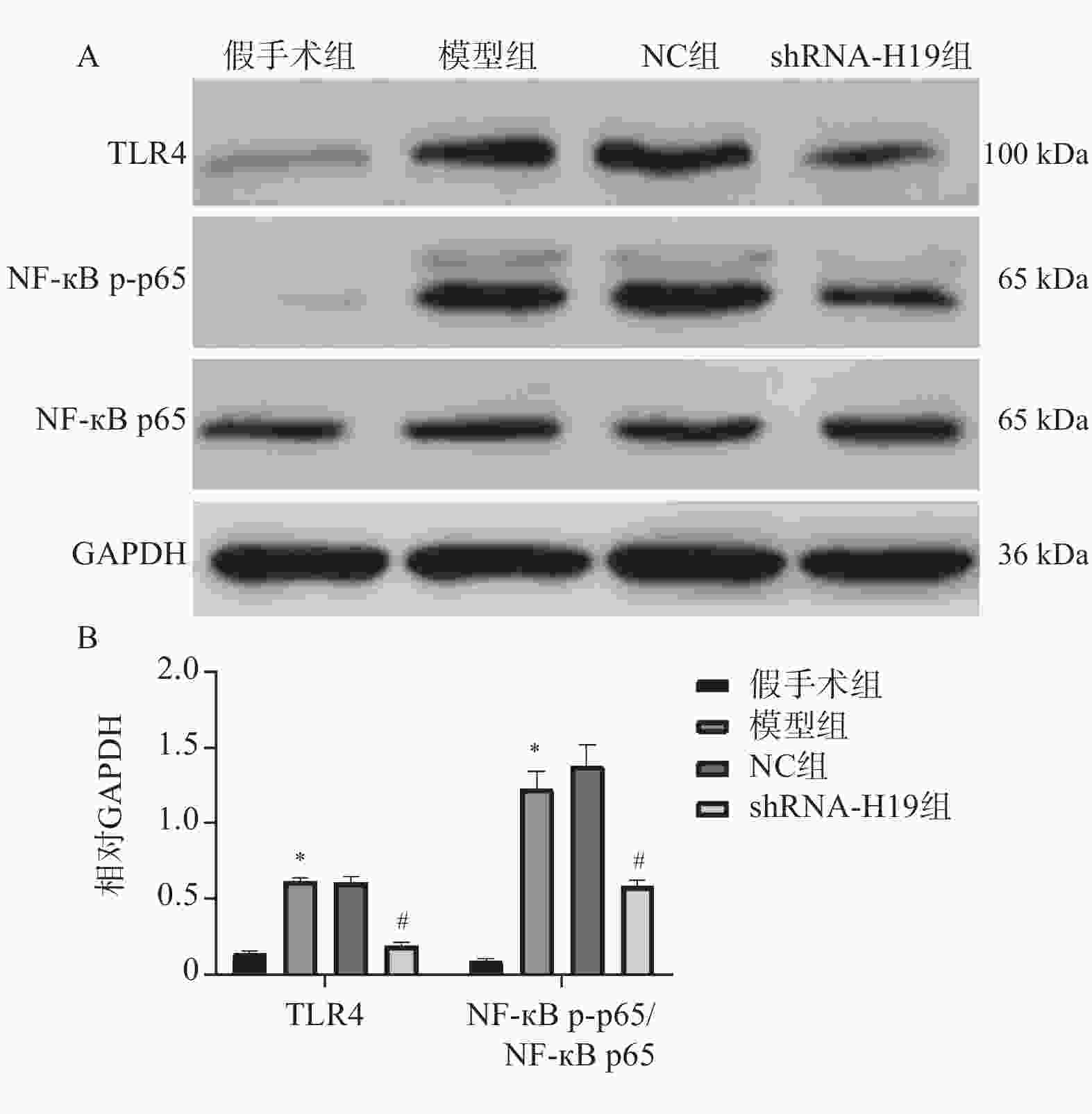
图 5 各组VD小鼠海马组织TLR4、NF-κB p-p65、NF-κB p65蛋白表达水平变化($ \bar x \pm s $,n = 6)
A:各组TLR4、NF-κB p-p65、NF-κB p65蛋白电泳图;B:各TLR4、NF-κB p-p65/NF-κB p65蛋白统计学分析。与假手术组比较,*P < 0.05;与NC组比较,#P < 0.05。
Figure 5. Changes in protein expression levels of TLR4,NF-κB p-p65 and NF-κB p65 in hippocampal tissue of VD mice in each group ($ \bar x \pm s $,n = 6)
表 1 qRT-PCR引物序列
Table 1. qRT-PCR primer sequences
基因 引物序列(5'-3') H19 F: TCCCAGAACCCACAACATGAA R: TTCACCTTCCAGAGCCGATTC TLR4 F: CCGCTCTGGCATCATCTTCA R: CCCACTCGAGGTAGGTGTTTCTG NF-κB p65 F: TGACGGGAGGGGAAGAAATC R: TGAACAAACACGGAAGCTGG GAPDH F: ACAGCAACAGGGTGGTGGAC R: TTTGAGGGTGCAGCGAACTT 表 2 各组VD小鼠海马组织H19 mRNA水平比较($ \bar x \pm s $)
Table 2. Comparison of H19 mRNA levels in hippocampaltissue of VD mice among groups ($ \bar x \pm s $)
组别 n H19(/GAPDH) 假手术组 6 1.09 ± 0.12 模型组 6 1.82 ± 0.09▲ NC组 6 1.95 ± 0.06 shRNA-H19组 6 1.27 ± 0.15# F 86.12 P <0.001* 与假手术组比较,▲P < 0.05;与NC组比较,#P < 0.05;*P < 0.05。 表 3 各组VD小鼠水迷宫测试的认知能力比较($ \bar x \pm s $)
Table 3. Comparison of cognitive function in the Morris Water Maze Test among VD mice groups ($ \bar x \pm s $)
组别 n 逃避潜伏期(s) 穿越平台次数(次) 假手术组 12 20.17 ± 2.08 8.33 ± 0.78 模型组 12 47.67 ± 5.47▲ 0.92 ± 0.67▲ NC组 12 49.08 ± 6.16 0.83 ± 0.58 shRNA-H19组 12 28.08 ± 3.60# 6.00 ± 0.95# F 116.6 294.9 P <0.001* <0.001* 与假手术组比较,▲P < 0.05;与NC组比较,#P < 0.05;*P < 0.05。 表 4 各组VD小鼠海马CA1区神经元凋亡率比较($ \bar x \pm s $)
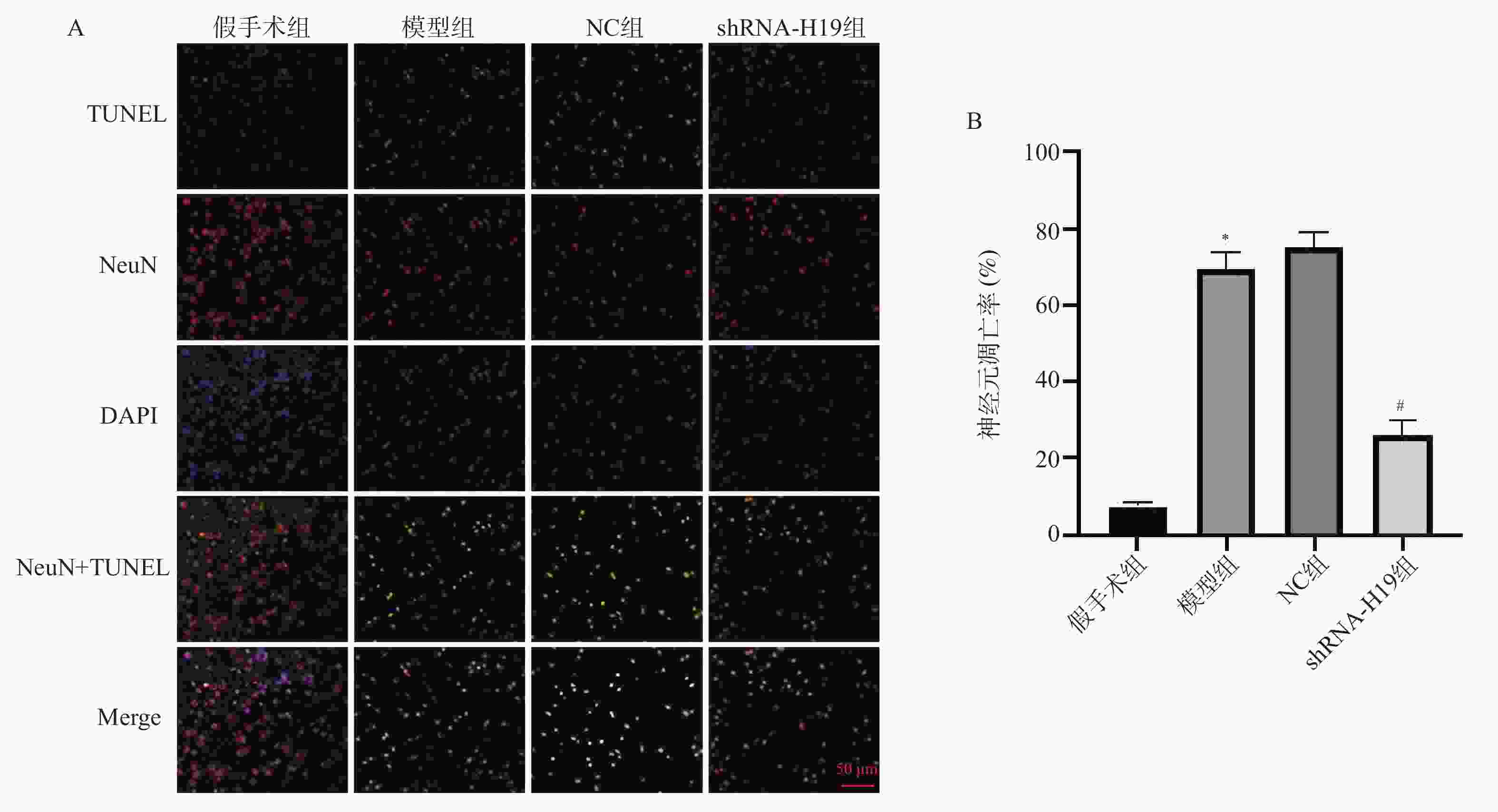
Table 4. Comparison of neuronal apoptosis rates in the hippocampal CA1 region of VD mice among groups ($ \bar x \pm s $)
组别 n 神经元凋亡率(%) 假手术组 6 7.36 ± 1.31 模型组 6 69.28 ± 4.33▲ NC组 6 74.91 ± 4.24 shRNA-H19组 6 26.13 ± 3.59# F 507.5 P <0.001* 与假手术组比较,▲P < 0.05;与NC组比较,#P < 0.05;*P < 0.05。 表 5 各组VD小鼠海马组织TNF-α、IL-1β、IL-6、Iba-1水平比较($ \bar x \pm s $)
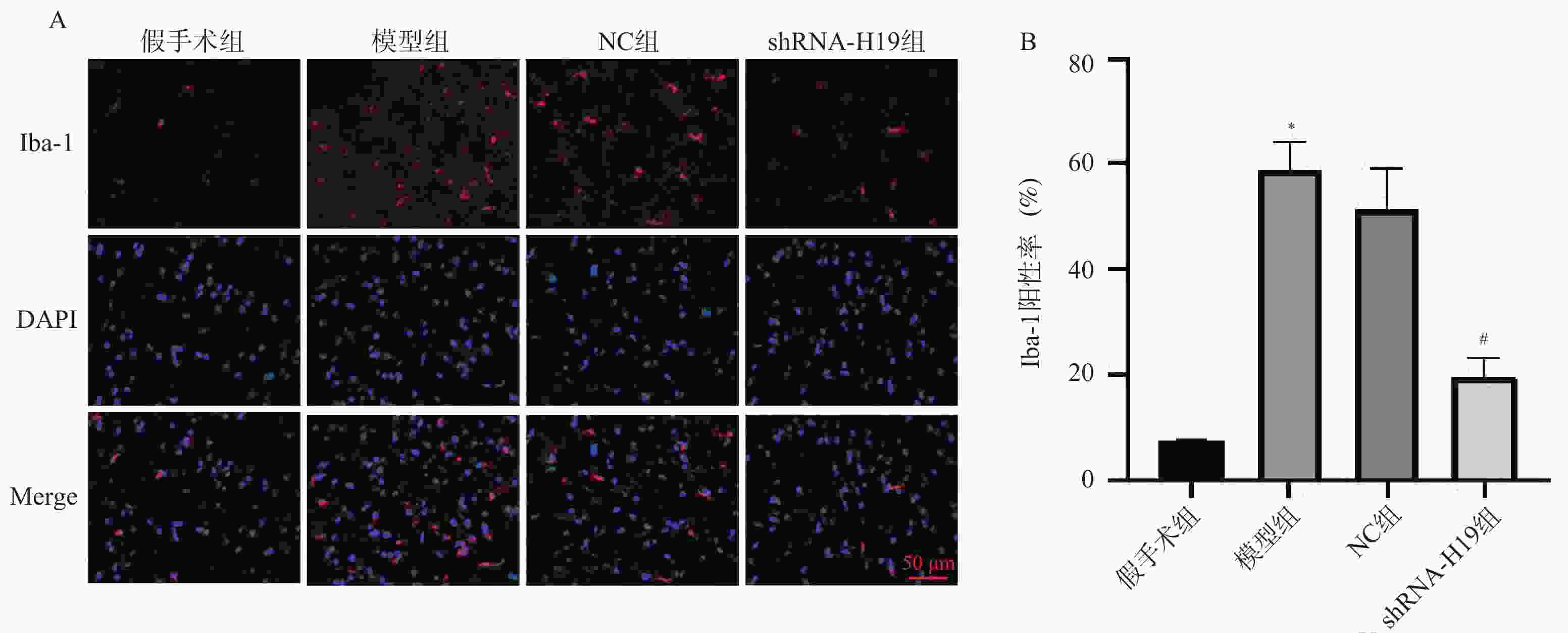
Table 5. Comparison of TNF-α,IL-1β,IL-6,and Iba-1 levels in hippocampal tissue of VD mice among groups ($ \bar x \pm s $)
组别 n TNF-α(pg/mL) IL-1β(pg/mL) IL-6(pg/mL) Iba-1(%) 假手术组 6 32.83 ± 1.42 52.80 ± 6.67 63.55 ± 10.18 7.29 ± 0.62 模型组 6 87.17 ± 6.26▲ 141.19 ± 10.22▲ 120.71 ± 8.34▲ 58.78 ± 5.50▲ NC组 6 80.61 ± 12.13 152.10 ± 9.92 135.81 ± 6.54 51.38 ± 7.59 shRNA-H19组 6 46.75 ± 5.37# 92.77 ± 7.53# 103.26 ± 13.08# 19.63 ± 3.43# F 76.03 166.0 60.27 146.7 P <0.001* <0.001* <0.001* <0.001* 与假手术组比较,▲P < 0.05;与NC组比较,#P < 0.05;*P < 0.05。 表 6 各组VD小鼠海马组织TLR4、NF-κB p65 mRNA水平比较($ \bar x \pm s $)
Table 6. Comparison of TLR4 and NF-κB p65 mRNA levels in hippocampal tissue among groups ($ \bar x \pm s $)
组别 n TLR4(/GAPDH) NF-κB p65(/GAPDH) 假手术组 6 1.15 ± 0.12 1.07 ± 0.14 模型组 6 1.94 ± 0.11▲ 1.35 ± 0.13▲ NC组 6 1.93 ± 0.11 1.38 ± 0.16 shRNA-H19组 6 1.19 ± 0.15# 1.12 ± 0.10# F 74.62 8.598 P <0.001* <0.001* 与假手术组比较,▲P < 0.05;与NC组比较,#P < 0.05;*P < 0.05。 -
[1] 于骄洋, 王鑫淼, 王明阳, 等. 血管性痴呆发病机制与中西医治疗研究概况[J]. 辽宁中医药大学学报, 2022, 24(2): 71-75. doi: 10.13194/j.issn.1673-842x.2022.02.017 [2] 程雪琪, 赵琳娜, 郭玉莹, 等. 小胶质细胞死亡与缺血性脑卒中后神经炎症关系的研究进展[J]. 中华老年心脑血管病杂志, 2024, 26(5): 593-596. [3] 曾辉, 王云玲, 刘莹, 等. 血管性痴呆与阿尔茨海默病患者海马区MRI影像学特征及与认知功能的关系[J]. 国际神经病学神经外科学杂志, 2025, 52(1): 38-43. doi: 10.16636/j.cnki.jinn.1673-2642.2025.01.007 [4] Bai Y, Ren H, Bian L, et al. Regulation of glial function by noncoding RNA in central nervous system disease[J]. Neurosci Bull, 2023, 39(3): 440-452. doi: 10.1007/s12264-022-00950-6 [5] Gu E, Pan W, Chen K, et al. LncRNA H19 regulates lipopolysaccharide (LPS)-induced apoptosis and inflammation of BV2 microglia cells through targeting miR-325-3p/NEUROD4 axis[J]. J Mol Neurosci, 2021, 71(6): 1256-1265. doi: 10.1007/s12031-020-01751-0 [6] 张汝希, 易亚乔, 刘涛阳, 等. 阿尔茨海默病神经炎症损伤中miRNA介导TLR4信号通路的作用机制及其中西医防治的研究进展[J]. 神经疾病与精神卫生, 2025, 25(2): 144-152. [7] 廖玲, 郑文, 张元亚, 等. 基于Nrf2/HO-1信号通路介导的炎症反应探讨天智颗粒对血管性痴呆大鼠神经炎症的影响[J]. 中国免疫学杂志, 2025, 41(4): 815-821, 827. [8] Zhong L, Liu P, Fan J, et al. Long non-coding RNA H19: Physiological functions and involvements in central nervous system disorders[J]. Neurochem Int, 2021, 148: 105072. doi: 10.1016/j.neuint.2021.105072 [9] 贺程成, 谢晶美, 郭宝琴, 等. 长新冠患者纵向海马亚结构体积变化与认知的关系[J]. 中国CT和MRI杂志, 2025, 23(2): 46-49. [10] Visser K, Koggel M, Blaauw J, et al. Blood-based biomarkers of inflammation in mild traumatic brain injury: A systematic review[J]. Neurosci Biobehav Rev, 2022, 132: 154-168. doi: 10.1016/j.neubiorev.2021.11.036 [11] Mizuno D, Kawahara M, Konoha-Mizuno K, et al. The role of zinc in the development of vascular dementia and Parkinson’s disease and the potential of carnosine as their therapeutic agent[J]. Biomedicines, 2024, 12(6): 1296. doi: 10.3390/biomedicines12061296 [12] Chen Q, Wu B, Shi Z, et al. LncRNA H19 knockdown promotes neuropathologic and functional recovery via the Nrf2/HO-1 axis after traumatic brain injury[J]. CNS Neurosci Ther, 2024, 30(7): e14870. doi: 10.1111/cns.14870 [13] Wan P, Su W, Zhang Y, et al. LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury[J]. Cell Death Differ, 2020, 27(1): 176-191. doi: 10.1038/s41418-019-0351-4 [14] Zhong G, Wang X, Zhang Q, et al. Exploring the therapeutic implications of natural compounds modulating apoptosis in vascular dementia[J]. Phytother Res, 2024, 38(11): 5270-5289. doi: 10.1002/ptr.8316 [15] Gan L, Liao S, Tong Y, et al. Long noncoding RNA H19 mediates neural stem/progenitor cells proliferation, differentiation and apoptosis through the p53 signaling pathway after ischemic stroke[J]. Biochem Biophys Res Commun, 2022, 597: 8-15. doi: 10.1016/j.bbrc.2022.01.095 [16] Wang L, Xu B, Sun S, et al. Overexpression of long non-coding RNA H19 relieves hypoxia-induced injury by down-regulating microRNA-107 in neural stem cells[J]. Neurosci Lett, 2021, 753: 135855. doi: 10.1016/j.neulet.2021.135855 [17] Hou P, Yang Y, Li Z, et al. TAK-3 inhibits lipopolysaccharide-induced neuroinflammation in traumatic brain injury rats through the TLR-4/NF-κB pathway[J]. J Inflamm Res, 2024, 17: 2147-2158. doi: 10.2147/JIR.S454099 [18] 宋晓雨, 张杰, 王旭, 等. 认知障碍疾病神经炎症相关生物标记物研究进展[J]. 中国药理学通报, 2024, 40(12): 2218-2223. [19] 张浛芮, 吕鹤群, 曾春利, 等. 基于lncRNA H19的m6A甲基化调控S1PR2/TLR4/NLRP3通路探讨电针血清对缺血性脑卒中后血脑屏障的作用机制[J]. 世界科学技术-中医药现代化, 2024, 26(10): 2716-2725. -





 下载:
下载: