Evaluative Value of Serum TNFSF14,TLR7,and sCD14-ST Levels in Assessing Disease Severity and Prognosis of Respiratory Syncytial Virus Infection-related Pneumonia in Children
-
摘要:
目的 探讨呼吸道合胞病毒感染性肺炎患儿血清肿瘤坏死因子超家族成员14(tumor necrosis factor superfamily member 14,TNFSF14)、Toll样受体7(toll-like receptor 7,TLR7)、可溶性白细胞分化抗原14亚型(soluble cluster of differentiation 14 subtype,sCD14-ST)水平对病情和预后的评估价值。 方法 选取2024年7月至2025年7月收治的154例呼吸道合胞病毒感染性肺炎患儿为患病组,将病情严重程度分为轻度组52例和重度组102例。同期在本院体检健康的儿童154例为对照组。采用ELISA法检查血清TNFSF14、TLR7、sCD14-ST水平。根据预后情况分为预后不良组35例和预后良好组119例。ROC曲线分析血清中TNFSF14、TLR7、sCD14-ST水平在评估呼吸道合胞病毒感染性肺炎患儿病情及预后方面的价值。多元Logistic回归分析筛选影响预后不良的独立因素。 结果 患病组血清TNFSF14、TLR7、sCD14-ST水平均显著高于对照组(P < 0.05)。与轻度组相比,重度组患儿血清TNFSF14、TLR7、sCD14-ST水平均显著升高(P < 0.05)。Spearman秩相关分析显示,血清TNFSF14、TLR7、sCD14-ST水平与患儿入院时CXR病灶范围分级均呈显著正相关(P均<0.001),其中TNFSF14相关性最强(rs = 0.484),其次为TLR7(rs = 0.421)和sCD14-ST(rs = 0.349)。分层分析显示,重度组中三者与病灶范围分级的相关性(TNFSF14:rs = 0.525,TLR7:rs = 0.493,sCD14-ST:rs = 0.424;P < 0.001)均显著强于轻度组(TNFSF14:rs = 0.31,TLR7:rs = 0.27,sCD14-ST:rs = 0.24;P < 0.05)。与单独检测相比,血清TNFSF14(Z = 4.884,P = <0.001)、TLR7(Z = 2.792,P = 0.003)、sCD14-ST(Z = 4.803,P < 0.001)水平联合检测对呼吸道合胞病毒感染性肺炎患儿病情的评估价值较高。预后不良组和预后良好组病灶范围分级、病变类型、有无并发症比较具有显著性差异(P < 0.05)。与预后良好组相比,预后不良组血清TNFSF14、TLR7、sCD14-ST水平均显著升高(P < 0.05)。与单独检测相比,血清TNFSF14(Z = 3.902,P < 0.001)、TLR7(Z = 3.434,P < 0.001)、sCD14-ST(Z = 2.394,P = 0.017)水平联合检测对呼吸道合胞病毒感染性肺炎患儿预后的评估价值较高。多元Logistic回归分析结果显示,血清TNFSF14(OR = 4.351)、TLR7(OR = 2.635)、sCD14-ST(OR = 1.695)、弥漫性浸润(OR = 4.121)和存在并发症(OR = 3.570)水平为影响呼吸道合胞病毒感染性肺炎患儿预后不良的因素(P < 0.05)。 结论 呼吸道合胞病毒感染性肺炎患儿血清TNFSF14、TLR7、sCD14-ST水平均显著升高,三者联合检测能够提高对病情及预后的评估价值。 -
关键词:
- 呼吸道合胞病毒感染性肺炎 /
- 肿瘤坏死因子超家族成员14 /
- Toll样受体7 /
- 可溶性白细胞分化抗原14亚型
Abstract:Objective To investigate the significance of serum tumor necrosis factor superfamily member 14 (TNFSF14), toll-like receptor 7 (TLR7), and soluble cluster of differentiation 14 subtype (sCD14-ST) levels in evaluating disease severity and prognosis in children with pneumonia associated with respiratory syncytial virus (RSV) infection. Methods A total of 154 children with RSV infection-related pneumonia admitted between July 2024 and July 2025 were enrolled as the disease group, classified into mild group (n = 52) and severe group (n = 102) based on disease severity. Simultaneously, 154 healthy children who underwent routine physical examinations at our hospital served as the control group. Serum levels of TNFSF14, TLR7, and sCD14-ST were measured using ELISA. According to prognosis, patients were divided into poor prognosis group (n = 35) and good prognosis group (n = 119). ROC curve analysis was performed to evaluate the value of serum TNFSF14, TLR7, and sCD14-ST levels in assessing disease severity and prognosis in children with RSV infection-related pneumonia. Multivariate Logistic regression analysis was used to identify independent factors affecting poor prognosis. Results Serum levels of TNFSF14, TLR7, and sCD14-ST in the disease group were significantly higher than in the control group (P < 0.05). Compared with the mild group, children in the severe group showed significantly elevated serum levels of TNFSF14, TLR7, and sCD14-ST (P < 0.05). Spearman rank correlation analysis demonstrated that serum levels of TNFSF14, TLR7, and sCD14-ST showed significant positive correlations with chest X-ray (CXR) lesion extent grade at admission (all P <0.001), with TNFSF14 showing the strongest correlation (rs = 0.484) followed by TLR7 (rs = 0.421) and sCD14-ST (rs = 0.349). Stratified analysis showed that in the severe group, the correlations between all three markers and lesion extent grade (TNFSF14: rs = 0.525, TLR7: rs = 0.493, sCD14-ST: rs = 0.424; All P <0.001) were significantly stronger than in the mild group (TNFSF14: rs = 0.31, TLR7: rs = 0.27, sCD14-ST: rs = 0.24; all P <0.05). Compared with single marker detection, combined measurement of serum TNFSF14 (Z = 4.884, P < 0.001), TLR7 (Z = 2.792, P = 0.003), and sCD14-ST (Z = 4.803, P < 0.001) levels had a higher evaluation value for disease severity in children with RSV infection-related pneumonia. Significant differences were observed between the poor and good prognosis groups in lesion extent grade, lesion type, and presence of complications (P < 0.05). Compared with the good prognosis group, the poor prognosis group showed significantly elevated serum levels of TNFSF14, TLR7, and sCD14-ST (P < 0.05). Compared with individual detection, the combined detection of serum TNFSF14 (Z = 3.902, P < 0.001), TLR7 (Z = 3.434, P < 0.001), and sCD14-ST (Z = 2.394, P = 0.017) levels had a higher prognostic value for children with RSV pneumonia. Multivariate logistic regression analysis revealed that serum levels of TNFSF14 (OR = 4.351), TLR7 (OR = 2.635), sCD14-ST (OR = 1.695), diffuse infiltration (OR = 4.121), and presence of complications (OR = 3.570) were independent factors affecting poor prognosis in children with RSV infection-related pneumonia (P < 0.05). Conclusion Serum levels of TNFSF14, TLR7, and sCD14-ST are significantly elevated in children with RSV infection-related pneumonia. Combined detection of these three markers enhances the evaluation value for assessing disease severity and prognosis. -
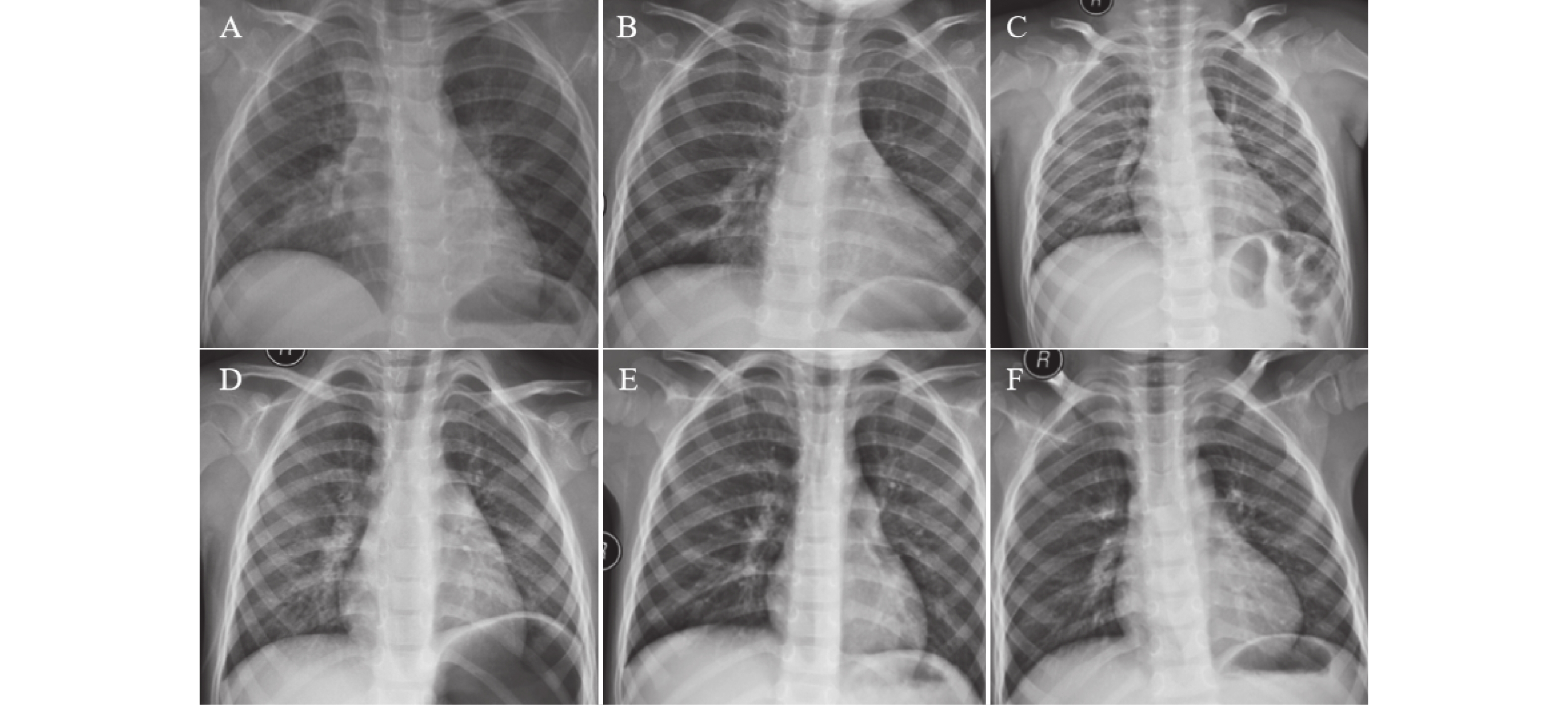
图 1 不同病情下影像图
A:入院时双肺弥漫性磨玻璃影、大片状高密度模糊影,累及多个肺叶,部分区域可见肺实变及支气管充气征,伴少量胸腔积液,肺容积略减小;B:住院期间部分区域炎症阴影较入院时有所吸收,但双肺仍可见弥漫性病变,实变范围未完全消退,胸腔积液量无明显增加或略有减少;C:出院时双肺炎症阴影大部分吸收,残留少量条索状纤维灶,无胸腔积液及其他并发症,肺功能逐步恢复;D:入院时双肺纹理轻度增粗、紊乱,肺野内可见散在、局限于肺门周围或肺叶下段的小斑片状模糊影,无肺实变、胸腔积液及纵隔移位;E:住院期间原散在小斑片状影较入院时明显吸收、变淡,肺纹理紊乱程度减轻,肺野清晰度改善;F:出院时双肺纹理基本恢复正常,原斑片状阴影完全吸收,肺野清晰,无残留病灶及并发症征象。
Figure 1. Chest X-rays of varying disease severity
表 1 两组一般资料比较[($ \bar x \pm s $)/n]
Table 1. Comparison of general data between two groups[($ \bar x \pm s $)/n]
组别 n 年龄(岁) 性别(男/女) 平均住院时间(d) 发热时间(d) 临床症状(发热/喘息/湿啰音/气促/喘鸣音) 患病组 154 3.22 ± 0.41 80/74 5.23 ± 0.58 4.14 ± 0.50 92/55/85/78/65 对照组 154 3.20 ± 0.44 75/79 - - - t/χ2 - 0.413 0.325 - - - P - 0.680 0.569 - - - 表 2 两组血清TNFSF14、TLR7、sCD14-ST水平比较[($ \bar x \pm s $)/n]
Table 2. Comparison of serum TNFSF14,TLR7,and sCD14-ST levels between the two groups[($ \bar x \pm s $)/n]
组别 n TNFSF14
(ng/mL)TLR7
(ng/mL)sCD14-ST
(ng/mL)患病组 154 24.07 ± 2.90 16.66 ± 1.76 30.93 ± 3.25 对照组 154 20.10 ± 2.63 13.15 ± 1.85 27.16 ± 3.10 t 12.584 17.058 10.417 P <0.001* <0.001* <0.001* *P < 0.05。 表 3 不同病情下血清TNFSF14、TLR7、sCD14-ST水平比较[($ \bar x \pm s $)/n]
Table 3. Comparison of serum TNFSF14,TLR7,and sCD14-ST levels across different severities[($ \bar x \pm s $)/n]
组别 n TNFSF14(ng/mL) TLR7(ng/mL) sCD14-ST(ng/mL) 重度组 52 25.02 ± 2.64 17.94 ± 1.95 32.52 ± 3.42 轻度组 102 23.58 ± 2.51 16.01 ± 1.72 30.12 ± 3.10 t 3.308 6.291 4.387 P 0.001* <0.001* <0.001* *P < 0.05。 表 4 血清TNFSF14、TLR7、sCD14-ST水平对呼吸道合胞病毒感染性肺炎患儿病情的评估效能(%)
Table 4. Diagnostic and prognostic value of serum TNFSF14,TLR7,and sCD14-ST levels in assessing disease severity in children with respiratory syncytial virus-induced pneumonia (%)
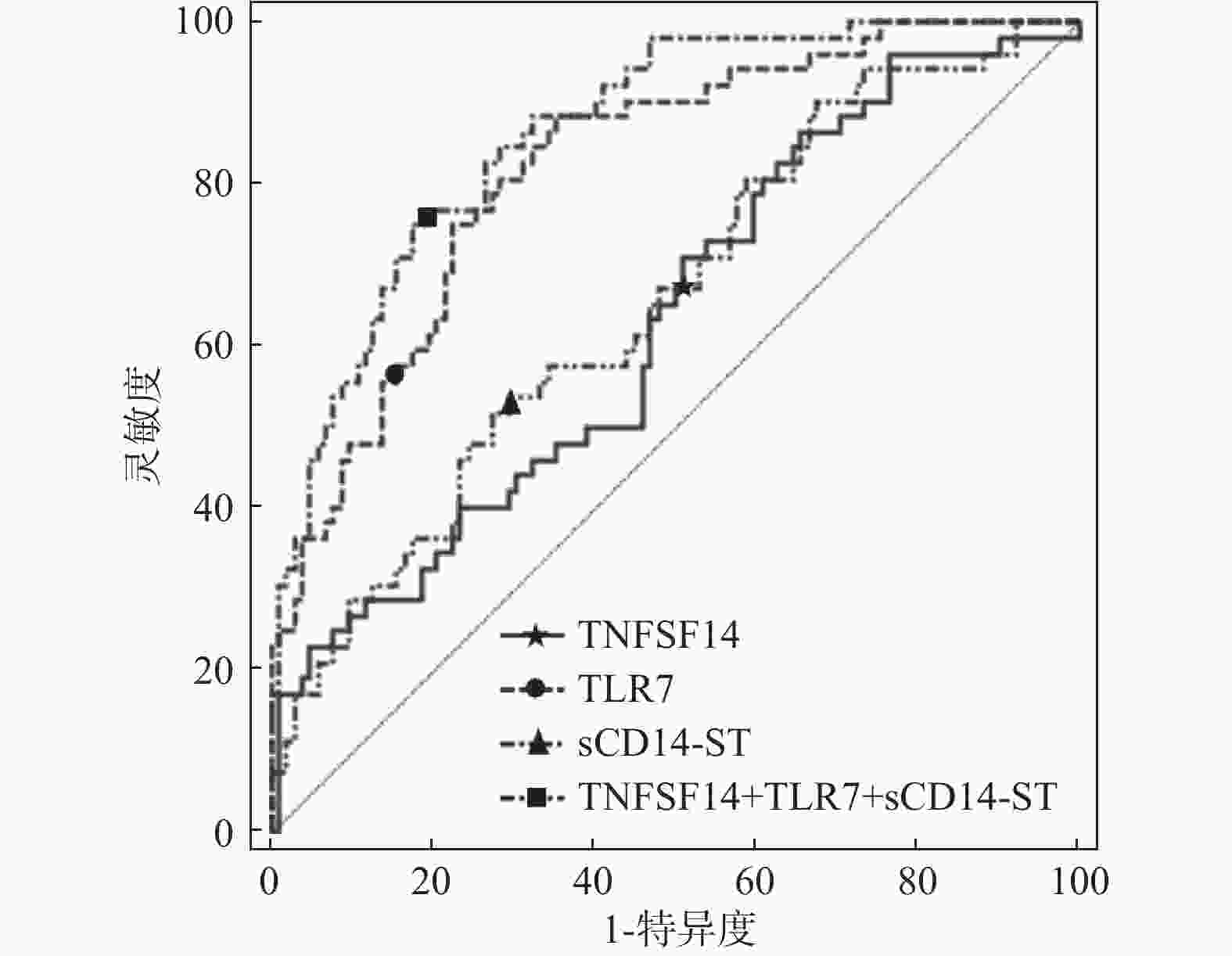
指标 AUC 95%CI 敏感性 特异性 约登指数 截断值 TNFSF14 0.630 0.549~0.707 65.40 50.00 0.154 24.565ng/mL TLR7 0.823 0.754~0.880 58.50 80.70 0.232 16.378ng/mL sCD14-ST 0.655 0.574~0.730 51.90 72.50 0.244 31.703ng/mL TNFSF14+TLR7+sCD14-ST 0.865 0.801~0.915 75.00 82.40 0.574 - 表 5 不同预后状况下一般资料比较[($\bar x \pm s$)/n(%)]
Table 5. Comparison of general Data across different severities[($ \bar x \pm s$)/n(%)]
项目 预后不良组(n = 35) 预后良好组(n = 119) t/χ2 P 年龄(岁) 3.20 ± 0.46 3.22 ± 0.40 0.251 0.802 性别 男 20(57.14) 60(50.42) 0.490 0.484 女 15(42.86) 59(49.58) 住院时间(d) 5.32 ± 0.60 5.20 ± 0.58 1.068 0.287 发热时间(d) 4.28 ± 0.51 4.10 ± 0.50 1.864 0.064 临床症状 发热 22(62.86) 70(58.82) 0.183 0.669 喘息 10(28.57) 45(37.82) 1.007 0.316 湿啰音 20(57.14) 65(54.62) 0.070 0.792 气促 18(51.43) 60(50.42) 0.011 0.916 喘鸣音 15(42.86) 50(42.02) 0.008 0.929 白细胞计数(×109/L) 9.25 ± 1.26 8.85 ± 1.06 1.878 0.062 C-反应蛋白(mg/L) 20.35 ± 3.15 19.54 ± 2.47 1.597 0.112 表 6 影像学资料[n(%)]
Table 6. Imaging data[n(%)]
影像学特征 预后良好组(n = 119) 预后不良组(n = 35) χ2 P 病灶范围分级 1 级 58(48.74) 3(8.58) 24.996 <0.001* 2 级 45(37.81) 16(47.06) 3 级 16(13.45) 16(47.06) 病变类型 间质性浸润 76(63.87) 12(34.29) 10.121 0.006* 肺泡实变 22(18.49) 10(28.57) 混合性病变 21(17.64) 13(37.14) 并发症 有 12(10.08) 12(34.29) 12.041 <0.001* 无 107(89.92) 23(65.71) *P < 0.05。 表 7 不同预后状况下血清TNFSF14、TLR7、sCD14-ST水平比较($ \bar x \pm s$/n)
Table 7. Comparison of serum TNFSF14,TLR7,and sCD14-ST levels under different prognosis($\bar x \pm s$/n)
组别 n TNFSF14(ng/mL) TLR7(ng/mL) sCD14-ST(ng/mL) 预后不良组 35 26.32 ± 3.10 18.11 ± 2.10 33.61 ± 3.59 预后良好组 119 23.41 ± 2.84 16.23 ± 1.66 30.14 ± 3.15 t 5.218 5.530 5.546 P <0.001* <0.001* <0.001* *P < 0.05。 表 8 呼吸道合胞病毒感染性肺炎患儿血清TNFSF14,TLR7和sCD14-ST水平对预后的评估(%)
Table 8. Evaluation efficacy of serum TNFSF14,TLR7,and sCD14-ST levels on prognosis in children with respiratory syncytial virus infectious pneumonia(%)
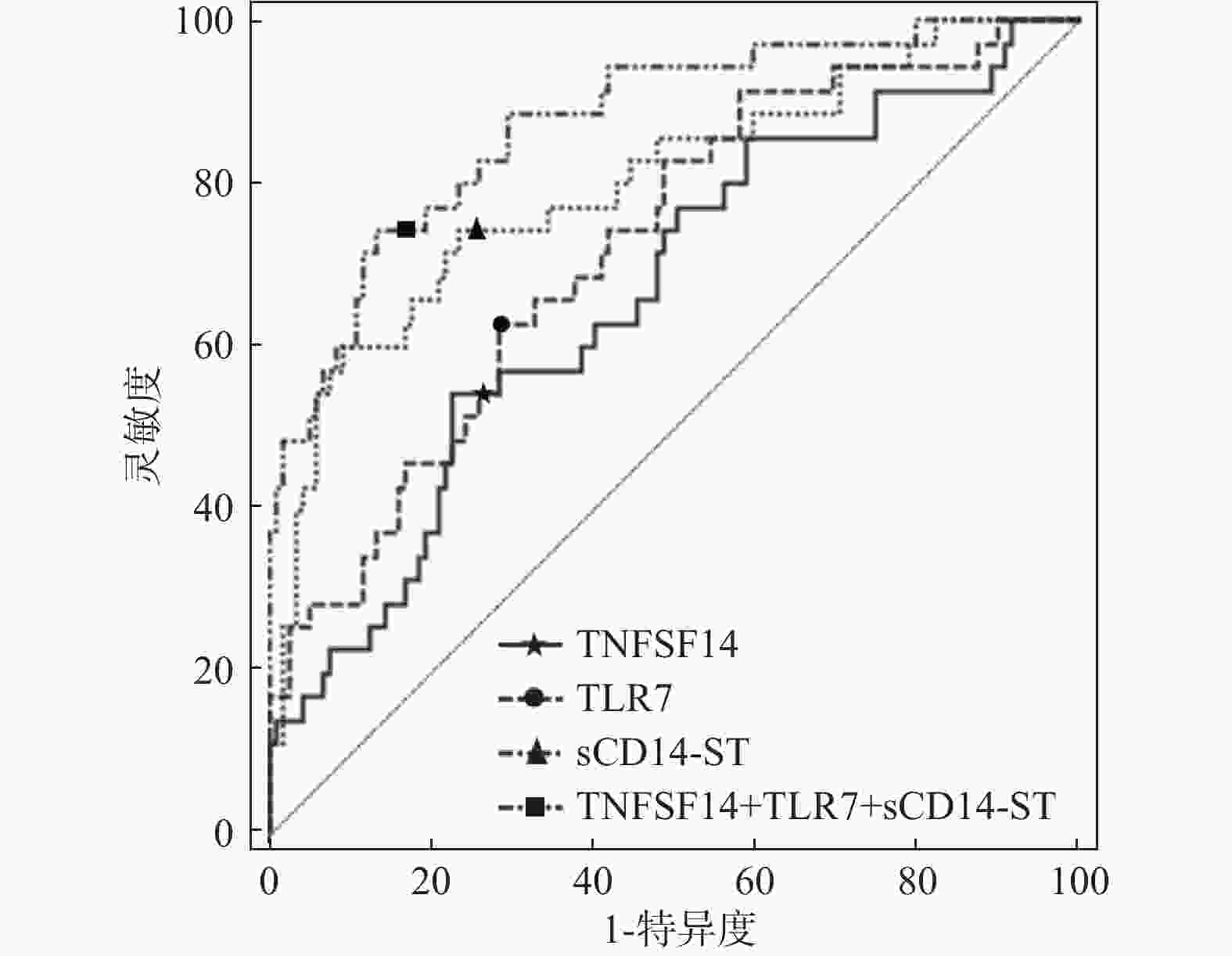
指标 AUC 95%CI 敏感性 特异性 约登指数 截断值 TNFSF14 0.664 0.584~0.738 54.30 77.30 0.316 25.591ng/mL TLR7 0.722 0.644~0.791 62.90 71.40 0.343 17.460ng/mL sCD14-ST 0.799 0.727~0.859 72.30 76.50 0.488 32.061ng/mL TNFSF14+TLR7+sCD14-ST 0.874 0.811~0.922 74.30 86.60 0.609 - 表 9 多元Logistic回归分析结果
Table 9. Results of multivariate logistic regression analysis
变量 B SE Waldχ2 P OR 95%CI 最小值 最大值 TNFSF14 1.470 0.152 93.581 <0.001* 4.351 3.230 5.861 TLR7 0.969 0.114 72.233 <0.001* 2.635 2.107 3.295 sCD14-ST 0.528 0.136 15.055 <0.001* 1.695 1.298 2.213 弥漫性浸润 1.416 0.185 58.592 <0.001* 4.121 2.868 5.922 存在并发症 1.273 0.215 35.033 <0.001* 3.570 2.342 5.441 *P < 0.05。 -
[1] 艾军, 宫文浩, 汪受传, 等. 小儿呼吸道合胞病毒肺炎中医证候与人体免疫指标相关性分析[J]. 中国中西医结合杂志, 2021, 41(10): 1251-1254. doi: 10.7661/j.cjim.20210128.031 [2] 王雪儿. 儿童呼吸道合胞病毒感染前后细菌性肺炎发病风险增加[J]. 国际儿科学杂志, 2025, 52(1): 21. doi: 10.3760/cma.j.issn.1673-4408.2025.01.102 [3] 刘小会, 徐保平, 尚云晓, 等. 雾化吸入重组人干扰素α1b治疗小儿呼吸道合胞病毒下呼吸道感染的有效性和安全性多中心、随机、双盲、安慰剂对照Ⅲ期临床研究[J]. 中华实用儿科临床杂志, 2025, 40(3): 180-186. doi: 10.3760/cma.j.cn101070-20241028-00694 [4] 宫化芬, 于学军, 郭芳, 等. 病毒感染后咳嗽患儿外周血EOS、血清IgE、血浆SP水平及联合用药治疗效果分析[J]. 中国防痨杂志, 2024, 46(S2): 262-264. [5] Fan H, Lu B, Cao C, et al. Plasma TNFSF13B and TNFSF14 function as inflammatory indicators of severe adenovirus pneumonia in pediatric patients[J]. Front Immunol, 2021, 11: 614781. doi: 10.3389/fimmu.2020.614781 [6] Rosenberg-Hasson Y, Holmes T H, Diray-Arce J, et al. Relationship of heterologous virus responses and outcomes in hospitalized COVID-19 patients[J]. J Immunol, 2023, 211(8): 1224-1231. doi: 10.4049/jimmunol.2300391 [7] Huang Y, Liu D, Chen M, et al. TLR7 promotes skin inflammation via activating NFκB-mTORC1 axis in Rosacea[J]. Peer J, 2023, 11: e15976. doi: 10.7717/peerj.15976 [8] Li H, Huang Y, Yang Q, et al. Pharmacological activation of TLR7 exerts inhibition on the replication of EV-D68 in respiratory cells[J]. J Virol, 2024, 98(6): e00434. [9] Shozushima T. Presepsin (sCD14-ST) as a new diagnostic biomarker of sepsis: Development of diagnostic tools using the whole blood[J]. Crit Care, 2011, 15(3): P3. [10] 中华人民共和国国家健康委员会, 国家中医药局. 儿童社区获得性肺炎诊疗规范(2019年版)[J]. 中华临床感染病杂志, 2019, 12(1): 6-13. [11] 江载芳, 申昆玲, 沈颖. 诸福棠实用儿科学[M]. 8版. 北京: 人民卫生出版社, 2015: 1258-1259. [12] 中国医师协会儿科医师分会, 中国儿童体检专家共识小组. 中国儿童健康体检专家共识[J]. 中国实用儿科杂志, 2022, 37(8): 865-870. doi: 10.19538/j.ek2022080601 [13] 屈春燕, 张凡, 袁晓华, 等. 呼吸道合胞病毒感染性肺炎患儿血清 miR-146a和 miR-145与Th17/Treg水平及临床意义[J]. 中华预防医学杂志, 2024, 58(11): 1733-1738. doi: 10.3760/cma.j.cn112150-20240617-00478 [14] Ware C F, Sedý J R. TNF Superfamily Networks: Bidirectional and interference pathways of the herpesvirus entry mediator (TNFSF14)[J]. Curr Opin Immunol, 2011, 23(5): 627-631. doi: 10.1016/j.coi.2011.08.008 [15] Shui J W, Kronenberg M. HVEM: An unusual TNF receptor family member important for mucosal innate immune responses to microbes[J]. Gut Microbes, 2013, 4(2): 146-151. doi: 10.4161/gmic.23443 [16] Xu W, Xu Z, Huang L, et al. Transcriptome sequencing identifies novel immune response genes highly related to the severity of human adenovirus type 55 infection[J]. Front Microbiol, 2019, 10: 130. doi: 10.3389/fmicb.2019.00130 [17] Hastie A T, Moore W C, Meyers D A, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes[J]. J Allergy Clin Immunol, 2010, 125(5): 1028-1036. e13. [18] Miles M A, Liong S, Liong F, et al. TLR7 promotes chronic airway disease in RSV-infected mice[J]. Front Immunol, 2023, 14: 1240552. doi: 10.3389/fimmu.2023.1240552 [19] Ye J, Wang Y, Liu X, et al. TLR7 signaling regulates Th17 cells and autoimmunity: Novel potential for autoimmune therapy[J]. J Immunol, 2017, 199(3): 941-954. doi: 10.4049/jimmunol.1601890 [20] Galliera E, Massaccesi L, Yu L, et al. SCD14-ST and new generation inflammatory biomarkers in the prediction of COVID-19 outcome[J]. Biomolecules, 2022, 12(6): 826. doi: 10.3390/biom12060826 [21] Zhang R, Sun G, Xing Z, et al. Evaluating the roles of sCD14 and sCD14-ST in diagnosing COPD and predicting an acute exacerbation of COPD[J]. Intensive Care Res, 2022, 2(1-2): 26-33. doi: 10.1007/s44231-022-00004-5 [22] Zhou W, Rao H, Ding Q, et al. Soluble CD14 subtype in peripheral blood is a biomarker for early diagnosis of sepsis[J]. Lab Med, 2020, 51(6): 614-619. doi: 10.1093/labmed/lmaa015 -





 下载:
下载: