Application of Serum STAT3,HDAC2,and Del-1 Levels in the Evaluation of Disease Severity and Prognosis of Children with Respiratory Syncytial Virus Pneumonia
-
摘要:
目的 探究血清信号传导子及转录激活子3(signal transducer and activator of transcription 3,STAT3)、组蛋白去乙酰化酶2(histone deacetylase 2,HDAC2)、内皮发育调节基因-1(developmental endothelial locus-1,Del-1)水平在呼吸道合胞病毒感染性肺炎(respiratory syncytial virus pneumonia,RSVP)患儿病情及预后评估中的应用。 方法 选取佳木斯市中心医院2023年11月至2025年3月收治的109例RSVP患儿纳入RSVP组,根据病情将RSVP患儿分为低危组(31例)、中危组(42例)、高危组(36例)。根据治疗7 d后的预后情况,分为良好组(78例)、不良组(31例)。另选择同期在该院体检的109例健康儿童作为对照(NC组)。ELISA法检测血清STAT3、HDAC2、Del-1水平;Pearson相关分析血清STAT3、HDAC2、Del-1水平与肺功能指标的相关性;多因素Logistic回归分析影响RSVP患儿预后的因素;ROC曲线分析血清STAT3、HDAC2、Del-1水平对RSVP患儿预后的预测价值。 结果 与NC组相比,RSVP组血清STAT3、HDAC2水平升高,血清Del-1水平降低(P < 0.05);低危组、中危组、高危组血清STAT3、HDAC2水平依次升高,达峰时间比(time to peak tidal expiratory flow to total expiratory time ratio,TPTEF/TE)、达峰容积比(volume to peak expiratory flow to total expired volume ratio,VPEF/VE)、血清Del-1水平依次降低(P < 0.05);TPTEF/TE、VPEF/VE与血清STAT3、HDAC2水平呈显著负相关,与Del-1水平呈显著正相关(P < 0.05);与良好组相比,不良组初始血氧饱和度、血清Del-1水平降低,血清STAT3、HDAC2水平升高(P < 0.05);STAT3、HDAC2高表达是影响RSVP患儿预后的独立危险因素,Del-1高表达是独立保护因素(P < 0.05)。血清STAT3、HDAC2、Del-1水平单独预测RSVP患儿预后的AUC分别为0.824、0.818、0.842,联合预测的AUC为0.947,优于单独预测(ZSTAT3-三者联合 = 2.436、ZHDAC2-三者联合 = 2.570、ZDel-1-三者联合 = 2.611,P均 < 0.05)。 结论 RSVP患儿血清中STAT3、HDAC2表达上调,Del-1表达下调,三者与病情及肺功能指标密切相关,是影响RSVP患儿预后的重要因素,联合检测对预后评估有较高效能。 -
关键词:
- 呼吸道合胞病毒感染性肺炎 /
- 信号传导子及转录激活子3 /
- 组蛋白去乙酰化酶2 /
- 内皮发育调节基因-1 /
- 预后
Abstract:Objective To explore the application of serum levels signal transducer and activator of transcription 3 (STAT3), histone deacetylase 2 (HDAC2), and developmental endothelial locus-1 (Del-1) levels in the evaluation of disease severity and prognosis of children with respiratory syncytial virus pneumonia (RSVP). Methods A total of 109 children with RSVP admitted to Jiamusi Central Hospital from November 2023 to March 2025 were enrolled as the RSVP group. Based on disease severity, RSVP patients were categorized into low-risk group (31 cases), medium-risk group (42 cases), and high-risk group (36 cases). According to the prognosis after 7 days of treatment, they were divided into a favorable prognosis group (78 cases) and a poor prognosis group (31 cases). Additionally, 109 healthy children undergoing physical examination at the same hospital during the same period were selected as the normal control group (NC group). ELISA method was used to detect serum levels of STAT3, HDAC2, and Del-1. Pearson correlation analysis was used to explore the correlations between serum STAT3, HDAC2, Del-1 levels and pulmonary function indices. Multivariate logistic regression analysis was used to explore the factors affecting the prognosis of children with RSVP. Moreover, ROC curve was used to explore the predictive value of serum STAT3, HDAC2, and Del-1 levels for the prognosis of children with RSV. Results Compared with the NC group, the RSVP group had higher serum STAT3 and HDAC2 levels, and lower serum Del-1 levels(P < 0.05). Serum STAT3 and HDAC2 levels progressively increased from the low-risk group, medium risk group, to high-risk group, while the time to peak ratio (time to peak tidal expiratory flow to total expiratory time ratio, TPTEF/TE), peak volume ratio (volume to peak expiratory flow to total expired volume ratio, VPEF/VE), and serum Del-1 levels progressively decreased (P < 0.05). TPTEF/TE and VPEF/VE were prominently negatively correlated with serum STAT3 and HDAC2 levels, and prominently positively correlated with Del-1 levels (P < 0.05). Compared with the favorable prognosis group, the poor prognosis group had significantly lower initial blood oxygen saturation, serum Del-1 levels, and significantly higher serum STAT3 and HDAC2 levels (P < 0.05). High expression levels of STAT3 and HDAC2 were independent risk factors affecting the prognosis of children with RSVP, while high expression of Del-1 was an independent protective factor (P < 0.05). The AUC values of serum STAT3, HDAC2, and Del-1 levels alone in predicting the prognosis of children with RSV were 0.824, 0.818, and 0.842, respectively. The combined prediction AUC was 0.947, which was superior to individual predictions (ZSTAT3 - joint = 2.436, ZHDAC2 - joint = 2.570, ZDel-1 - joint = 2.611, all P < 0.05). Conclusion Serum expression of STAT3 and HDAC2 is upregulated, while the expression of Del-1 is down-regulated in children with RSVP. The three markers are closely related to the disease severity and pulmonary function indices, and are important factors affecting the prognosis of children with RSVP. Combined detection of these markers demonstrates high efficacy for prognosis evaluation. -
表 1 RSVP组、NC组血清STAT3、HDAC2、Del-1水平比较($\bar x \pm s$)
Table 1. Comparison of serum STAT3,HDAC2,and Del-1 levels between the RSVP group and the NC group ($\bar x \pm s$)
分组 n STAT3(ng/mL) HDAC2(ng/mL) Del-1(ng/mL) NC组 109 0.51 ± 0.09 4.17 ± 1.13 198.67 ± 33.52 RSVP组 109 0.76 ± 0.14 6.84 ± 1.65 162.93 ± 29.67 t − 15.682 13.939 8.335 P − < 0.001* < 0.001* < 0.001* *P < 0.05。 表 2 低危组、中危组、高危组肺功能指标及血清STAT3、HDAC2、Del-1水平比较($ \bar x \pm s$)
Table 2. Comparison of pulmonary function indices and serum STAT3,HDAC2,and Del-1 levels among low-risk,medium-risk,and high-risk groups ($ \bar x \pm s$)
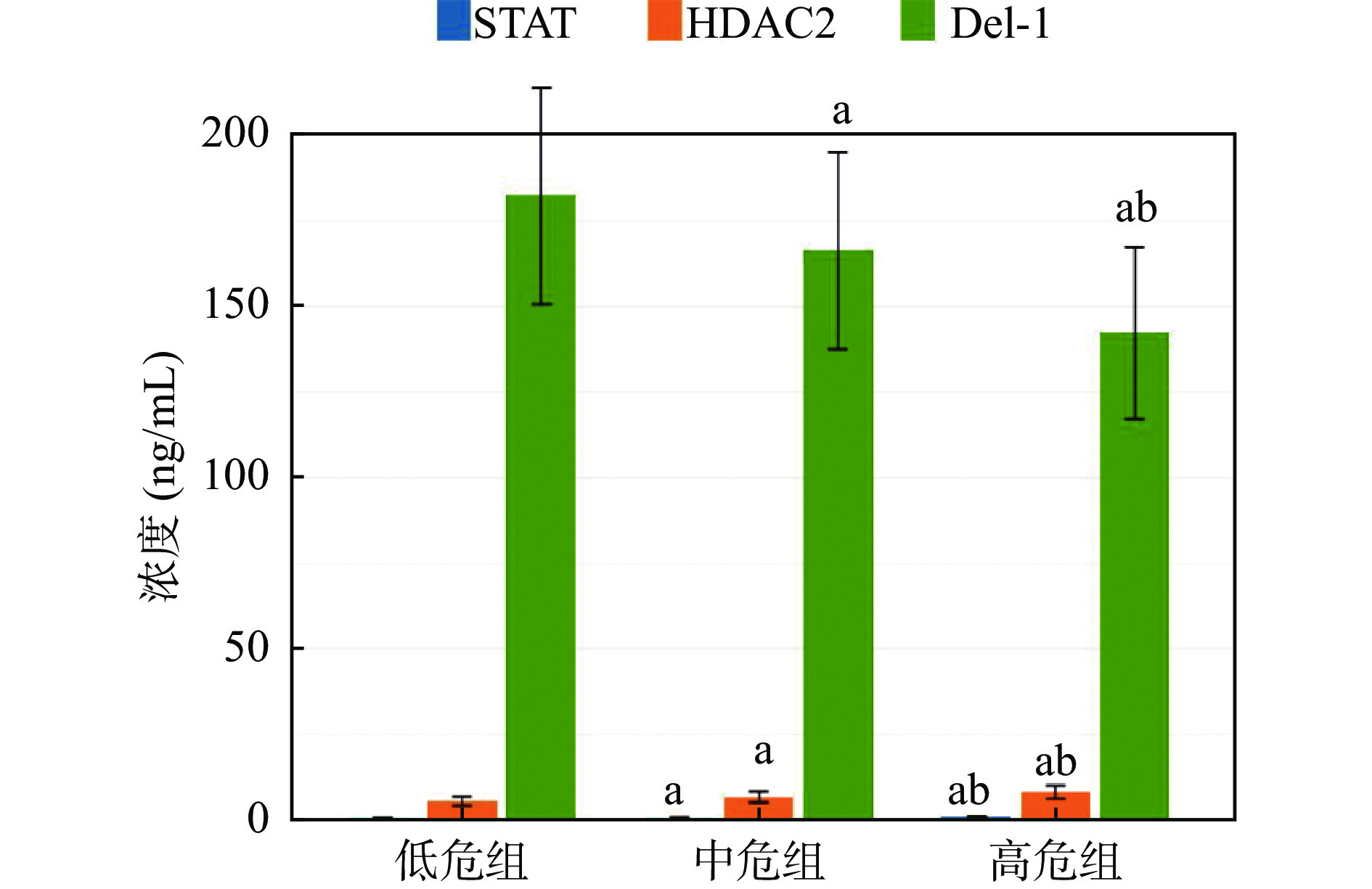
分组 n TPTEF/TE(%) VPEF/VE(%) STAT3(ng/mL) HDAC2(ng/mL) Del-1(ng/mL) 低危组 31 21.45 ± 3.09 28.82 ± 4.02 0.65 ± 0.11 5.51 ± 1.32 182.34 ± 31.65 中危组 42 19.24 ± 2.76a 24.68 ± 3.47a 0.72 ± 0.13a 6.74 ± 1.63a 166.33 ± 28.81a 高危组 36 14.18 ± 2.35ab 19.57 ± 2.87ab 0.91 ± 0.18ab 8.11 ± 1.97ab 142.25 ± 25.13ab F − 63.879 60.372 30.287 20.220 16.948 P − < 0.001* < 0.001* < 0.001* < 0.001* < 0.001* *P < 0.05;与低危组相比,aP < 0.05;与中危组比较,bP < 0.05。 表 3 RSVP患儿血清STAT3、HDAC2、Del-1水平与肺功能指标的相关性
Table 3. Correlation between serum STAT3,HDAC2,and Del-1 levels and pulmonary function Indices in children with RSV infection
指标 STAT3 HDAC2 Del-1 r P r P r P TPTEF/TE −0.567 0.002* −0.513 0.009* 0.601 < 0.001* VPEF/VE −0.497 0.015* −0.504 0.011* 0.583 0.001* *P < 0.05。 表 4 不同预后情况临床资料及血清STAT3、HDAC2、Del-1水平比较[($ \bar x \pm s$)/n(%)]
Table 4. Comparison of clinical data and serum STAT3,HDAC2,Del-1 levels by prognosis outcome [($\bar x \pm s$)/n(%)]
分组 n 性别 年龄(岁) 病程(d) 初始血氧饱和度(%) 临床特征 男 女 发热 咳嗽 呼吸困难 喘息 肺部啰音 良好组 78 34(43.59) 44(56.41) 3.15 ± 1.02 6.23 ± 1.68 94.15 ±
3.0255(70.51) 65(83.33) 41(52.56) 51(65.38) 57(73.08) 不良组 31 15(48.39) 19(51.61) 3.61 ± 1.34 6.79 ± 1.92 89.94 ±
3.4524(77.42) 28(90.32) 18(58.06) 23(74.19) 25(80.65) χ2/t − 0.206 1.936 1.507 6.302 0.530 0.865 0.270 0.790 0.682 P − 0.650 0.055 0.135 < 0.001* 0.466 0.352 0.603 0.374 0.409 分组 药物治疗 年龄分层 STAT3(ng/mL) HDAC2(ng/mL) Del-1(ng/mL) 糖皮质激素 支气管扩张剂 < 1岁 1-3岁 > 3岁 良好组 17(21.79) 23(29.49) 6(14.10) 45(57.69) 22(28.21) 0.71 ± 0.12 6.17 ± 1.52 172.64 ± 31.84 不良组 6(35.48) 14(45.19) 10(32.26) 14(45.19) 7(22.58) 0.90 ± 0.19 8.54 ± 2.06 138.49 ± 25.68 χ2/t 2.178 2.431 4.703 6.253 6.609 5.319 P 0.140 0.69 0.095 < 0.001* < 0.001* < 0.001* *P < 0.05。 表 5 多因素Logistic回归分析影响RSVP患儿预后的因素
Table 5. Multivariate logistic regression analysis of factors affecting the prognosis of children with RSVP
影响因素 β SE Wald χ2 OR 95%CI P 初始血氧饱和度 −0.182 0.096 3.575 0.834 0.691~1.001 0.059 STAT3 1.086 0.443 6.004 2.961 1.243~7.055 0.014* HDAC2 0.944 0.315 8.972 2.569 1.386~4.763 0.003* Del-1 −0.206 0.091 5.114 0.814 0.681~0.973 0.024* *P < 0.05。 表 6 血清STAT3、HDAC2、Del-1水平对RSVP患儿预后的预测价值
Table 6. Predictive value of serum STAT3,HDAC2,and Del-1 levels for the prognosis of children with RSVP
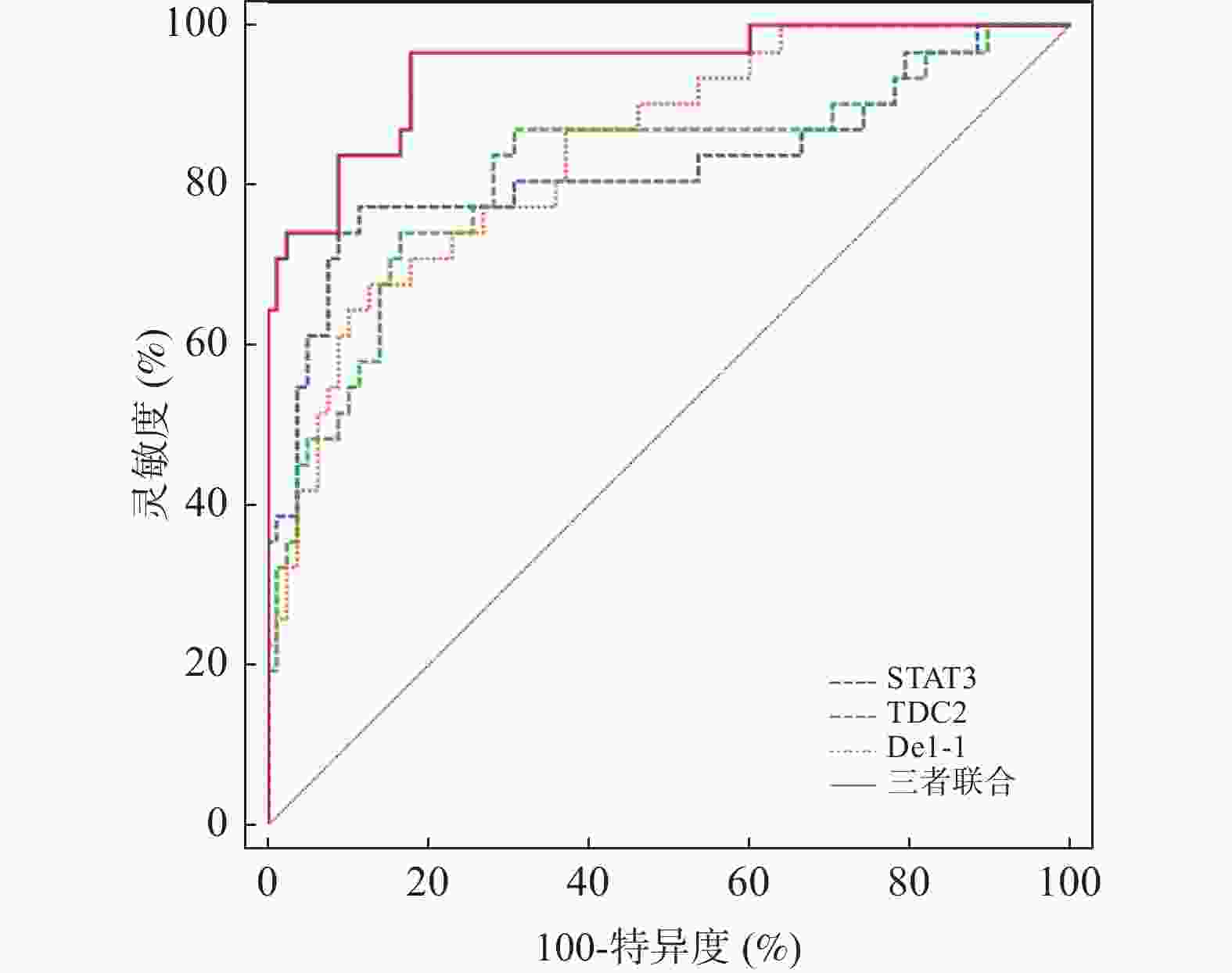
指标 截断值(ng/mL) 灵敏度(%) 特异度(%) Youden指数 AUC 95%CI P STAT3 0.79 77.42 88.46 0.659 0.824 0.739~0.890 < 0.001* HDAC2 7.34 74.19 83.33 0.575 0.818 0.733~0.886 < 0.001* Del-1 146.84 67.74 87.18 0.549 0.842 0.760~0.905 < 0.001* 三者联合 − 96.77 82.05 0.788 0.947 0.887~0.981 < 0.001* *P < 0.05。 -
[1] Agac A, Kolbe S M, Ludlow M, et al. Host responses to respiratory syncytial virus infection[J]. Viruses, 2023, 15(10): 1999. doi: 10.3390/v15101999 [2] Zhang H, Ge C, Fisher D, et al. Antiviral treatment for viral pneumonia: Current drugs and natural compounds[J]. Virol J, 2025, 22(1): 62. doi: 10.1186/s12985-025-02666-1 [3] Li Y, Wang X, Blau D M, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis[J]. Lancet, 2022, 399(10340): 2047-2064. doi: 10.1016/S0140-6736(22)00478-0 [4] Zhao C, Bai Y, Wang W, et al. Activation of STAT3-mediated ciliated cell survival protects against severe infection by respiratory syncytial virus[J]. J Clin Invest, 2024, 134(21): e183978. doi: 10.1172/JCI183978 [5] 刘爱丽, 段福明, 冯伟. 慢性阻塞性肺疾病患者血清TLR4、HDAC2水平与气道炎症反应及免疫功能的关系[J]. 医学临床研究, 2025, 42(3): 504-506. [6] Kwak N, Lee K H, Woo J, et al. Del-1 plays a protective role against COPD development by inhibiting inflammation and apoptosis[J]. Int J Mol Sci, 2024, 25(4): 1955. doi: 10.3390/ijms25041955 [7] Fine M J, Auble T E, Yealy D M, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia[J]. N Engl J Med, 1997, 336(4): 243-250. doi: 10.1056/NEJM199701233360402 [8] 胡亚美, 江载芳. 诸福棠实用儿科学[M]. 7版. 北京: 人民卫生出版社, 2002: 1177-1178. [9] 国家呼吸系统疾病临床医学研究中心, 中华医学会儿科学分会呼吸学组, 中国医师协会呼吸医师分会儿科呼吸工作委员会, 等. 儿童呼吸道合胞病毒感染诊断、治疗和预防专家共识[J]. 中华实用儿科临床杂志, 2020, 35(4): 241-250. [10] Kaler J, Hussain A, Patel K, et al. Respiratory syncytial virus: A comprehensive review of transmission, pathophysiology, and manifestation[J]. Cureus, 2023, 15(3): e36342. [11] Binns E, Tuckerman J, Licciardi P V, et al. Respiratory syncytial virus, recurrent wheeze and asthma: A narrative review of pathophysiology, prevention and future directions[J]. J Paediatr Child Health, 2022, 58(10): 1741-1746. doi: 10.1111/jpc.16197 [12] Colosia A, Costello J, McQuarrie K, et al. Systematic literature review of the signs and symptoms of respiratory syncytial virus[J]. Influenza Other Respir Viruses, 2023, 17(2): e13100. doi: 10.1111/irv.13100 [13] Davy K, Koskinas E, Watson C, et al. Respiratory syncytial virus-associated pneumonia in primary care in Malawi[J]. J Trop Pediatr, 2024, 70(4): fmae013. doi: 10.1093/tropej/fmae013 [14] Pandey P, Al Rumaih Z, Kels M J T, et al. Targeting ectromelia virus and TNF/NF-κB or STAT3 signaling for effective treatment of viral pneumonia[J]. Proc Natl Acad Sci USA, 2022, 119(8): e2112725119. doi: 10.1073/pnas.2112725119 [15] Fu L, Zhao J, Huang J, et al. A mitochondrial STAT3-methionine metabolism axis promotes ILC2-driven allergic lung inflammation[J]. J Allergy Clin Immunol, 2022, 149(6): 2091-2104. doi: 10.1016/j.jaci.2021.12.783 [16] 李华兵, 余少华, 李林. 血清NOX-4、STAT3在肺炎新生儿发生细菌性感染的早期诊断及治疗评价中的作用[J]. 检验医学与临床, 2025, 22(8): 1133-1137. [17] Zhang H, Chen J, Yu C, et al. Innate immune evasion of PRRSV nsp11 through degradation of the HDAC2 by its endoribonuclease activity[J]. Viruses, 2024, 16(5): 678. doi: 10.3390/v16050678 [18] 冯秋琴, 黄欢, 杨眉, 等. 组蛋白去乙酰化酶抑制剂对呼吸道合胞病毒感染诱发的气道炎性反应作用机制[J]. 吉林医学, 2023, 44(1): 6-9. [19] Saheb Sharif-Askari N, Mdkhana B, Hafezi S, et al. Calprotectin is regulated by IL-17A and induces steroid hyporesponsiveness in asthma[J]. Inflamm Res, 2024, 73(11): 1875-1888. doi: 10.1007/s00011-024-01937-x [20] Jia M, Fu H, Jiang X, et al. DEL-1, as an anti-neutrophil transepithelial migration molecule, inhibits airway neutrophilic inflammation in asthma[J]. Allergy, 2024, 79(5): 1180-1194. doi: 10.1111/all.15882 [21] Li R, Zeng J, Ren T. Expression of DEL-1 in alveolar epithelial cells prevents lipopolysaccharide-induced inflammation, oxidative stress, and eosinophil recruitment in acute lung injury[J]. Int Immunopharmacol, 2022, 110: 108961. doi: 10.1016/j.intimp.2022.108961 [22] 王君, 黄长明, 吴瑕, 等. 慢性阻塞性肺疾病患者血清内皮发育调节基因-1表达水平与炎症反应及疾病严重程度的相关性研究[J]. 现代检验医学杂志, 2025, 40(2): 160-163+168. [23] Leong J, Husain M. HDAC1 and HDAC2 are involved in influenza a virus-induced nuclear translocation of ectopically expressed STAT3-GFP[J]. Viruses, 2024, 17(1): 33. doi: 10.3390/v17010033 -





 下载:
下载: