Correlation of Serum miR-21 and miR-23a Levels with Cognitive Function and Inflammatory Response in Patients with Parkinson’ s Disease
-
摘要:
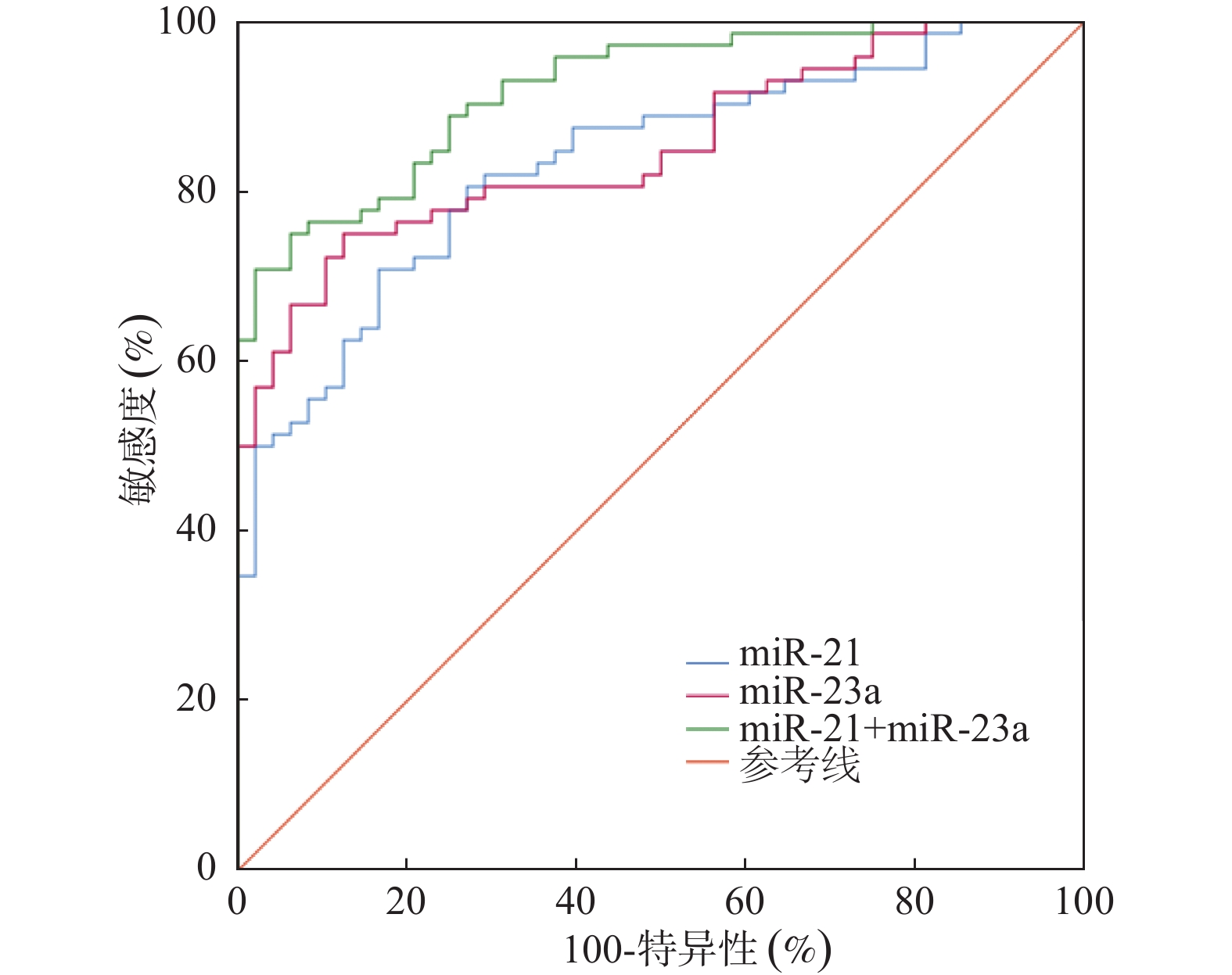
目的 探讨帕金森病(Parkinson's disease,PD)患者血清微小RNA-21(miR-21)和微小RNA-23a(miR-23a)的表达水平及其与认知功能和炎症反应的关系。 方法 选取2019年12月至2022年1月河北北方学院附属第二医院收治的PD患者120例及同期健康体检者115例为对照。采用实时荧光定量PCR检测血清miR-21、miR-23a表达,ELISA法测定血清IL-6、CRP、TNF-α的表达水平。依据简易精神状态量表(MMSE)将PD患者分为认知功能障碍组(MMSE<26,n = 72)和认知正常组(MMSE≥26,n = 48)。比较两组一般资料及相关指标水平,采用Spearman分析miR-21、miR-23a与MMSE的相关性,多因素Logistic回归分析认知功能障碍的影响因素,并以ROC曲线评估miR-21、miR-23a预测价值。 结果 与对照组相比,PD组血清miR-21、miR-23a、IL-6、CRP、TNF-α水平均显著升高(P < 0.01)。认知功能障碍组的miR-21、miR-23a、炎症因子水平均高于认知正常组(P < 0.01)。相关分析显示,miR-21、miR-23a与MMSE评分呈显著负相关(r = -0.472、-0.514,P < 0.001),与IL-6、CRP、TNF-α水平均呈正相关(P < 0.001)。多因素Logistic回归分析表明,miR-21、miR-23a高表达及UPDRS评分升高为PD患者认知功能障碍的独立危险因素(P < 0.05)。ROC曲线分析显示,miR-21与miR-23a联合预测认知功能障碍的AUC显著高于单一指标(P < 0.05)。 结论 PD患者血清miR-21和miR-23a表达上调,与认知功能和炎症反应相关,联合检测可较好预测认知功能障碍。 Abstract:Objective To investigate the expression levels of serum microRNA-21 (miR-21) and microRNA-23a (miR-23a) in patients with Parkinson’ s disease (PD) and their correlations with cognitive function and inflammatory responses. Methods A total of 120 PD patients admitted to the Second Affiliated Hospital of Hebei North University between December 2019 and January 2022 were enrolled, along with 115 healthy controls from the same period. Serum miR-21 and miR-23a levels were measured by quantitative real-time PCR, while serum levels of IL-6, CRP, and TNF-α were determined by ELISA. According to Mini-Mental State Examination (MMSE) scores, PD patients were classified into a cognitive impairment group (MMSE < 26, n = 72) and a normal cognition group (MMSE ≥ 26, n = 48). General characteristics in clinical and biochemical indicators levels were compared between the two groups. Spearman correlation analysis was used to assess the relationships of miRNAs and MMSE scores. Multivariate logistic regression analysis was employed to identify risk factors for cognitive impairment. The predictive value of miR-21 and miR-23a was evaluated using Receiver Operating Characteristic (ROC) curve analysis. Results Serum miR-21, miR-23a, IL-6, CRP, and TNF-α levels were significantly higher in the PD group than in the control group (P < 0.01). The cognitive impairment group showed higher levels of miR-21, miR-23a, and inflammatory factor than the cognitively normal group (P < 0.01). Correlation analysis revealed that miR-21 and miR-23a levels were negatively correlated with MMSE scores (r = -0.472, -0.514; P < 0.001) and positively correlated with IL-6, CRP, and TNF-α (P < 0.001). Multivariate Logistic regression analysis revealed that high expression of miR-21, miR-23a, and a higher UPDRS score, were independent risk factors for cognitive impairment in PD patients (P < 0.05). Combined detection of miR-21 and miR-23a showed higher predictive accuracy for cognitive impairment than either marker alone (P < 0.05). Conclusion Serum expression levels of miR-21 and miR-23a was upregulated in PD patients, which were associated with cognitive function and inflammatory response. Combined detection shows good predictive value for cognitive impairment.. -
Key words:
- Parkinson's disease /
- microRNA-21 /
- microRNA-23a /
- Cognitive function /
- Inflammatory response
-
表 1 血清miR-21、miR-23a、炎症因子水平比较($\bar x \pm s $)
Table 1. Comparison of serum miR-21,miR-23a and inflammatory factor levels($\bar x \pm s $)
组别 n miR-21 miR-23a IL-6(ng/L) CRP(mg/L) TNF-α(μg/L) PD组 120 1.48 ± 0.33 1.62 ± 0.41 22.45 ± 4.21 12.58 ± 3.75 14.32 ± 3.36 对照组 115 1.02 ± 0.04 1.03 ± 0.06 13.28 ± 3.05 6.39 ± 1.92 8.95 ± 2.87 t − 14.843 15.275 19.052 15.824 13.148 P − <0.001* <0.001* <0.001* <0.001* <0.001* *P < 0.05。 表 2 一般资料比较[($ \bar x \pm s $)/M(P25,P75)]
Table 2. Comparison of general characteristics [($ \bar x \pm s $)/M(P25,P75)]
项目 认知功能障碍组(n=72) 认知功能正常组(n=48) t/χ2/Z P 年龄(岁) 60.25 ± 8.26 59.75 ± 8.38 0.323 0.747 性别(男/女) 41/31 29/19 0.143 0.705 BMI(kg/m2) 23.75 ± 2.84 23.24 ± 2.90 0.956 0.341 吸烟史(有/无) 29/43 25/23 1.622 0.203 饮酒史(有/无) 31/41 22/26 0.090 0.764 高血压(有/无) 38/34 27/21 0.140 0.708 糖尿病(有/无) 22/50 14/34 0.026 0.871 受教育年限(/年) 7.50 ± 1.06 9.75 ± 1.21 10.760 <0.001* H-Y分级(<3级/≥3级) 30/42 31/17 6.052 0.014* MMSE评分(分) 21.25 ± 3.00 27.50 ± 1.03 13.882 <0.001* UPDRS评分(分) 38.75 ± 6.32 29.25 ± 5.01 8.739 <0.001** 平均病程(年) 9.00(6.00,12.00) 3.00(1.00,6.00) −9.313 <0.001* *P < 0.05。 表 3 血清miR-21、miR-23a、炎症因子水平比较($\bar x \pm s $)
Table 3. Comparison of serum miR-21,miR-23a and inflammatory factor levels ($\bar x \pm s $)
组别 n miR-21 miR-23a IL-6(ng/L) CRP(mg/L) TNF-α(μg/L) 认知功能障碍组 72 1.63 ± 0.40 1.84 ± 0.45 25.02 ± 4.52 14.68 ± 4.18 17.09 ± 3.88 认知功能正常组 48 1.26 ± 0.23 1.30 ± 0.36 18.60 ± 3.75 9.43 ± 3.11 10.17 ± 2.59 t − 5.797 6.958 8.145 7.434 10.843 P − <0.001* <0.001* <0.001* <0.001* <0.001* *P < 0.05。 表 4 PD组患者血清miR-21、miR-23a水平与炎症因子水平的相关性
Table 4. Correlation between serum miR-21 and miR-23a levels and inflammatory factors levels in PD patients
项目 miR-21 miR-23a r P r P MMSE评分(分) −0.472 <0.001* −0.514 <0.001* IL-6(ng/L) 0.451 <0.001* 0.399 <0.001* CRP(mg/L) 0.557 <0.001* 0.644 <0.001* TNF-α(μg/L) 0.625 <0.001* 0.516 <0.001* *P < 0.05。 表 5 影响PD患者认知功能障碍的危险因素
Table 5. Risk factors affecting cognitive impairment in PD patients
变量 B SE Waldχ2 OR 95%CI P 受教育年限 0.340 0.213 2.549 1.405 0.925~2.133 0.110 H-Y分级 0.173 0.162 1.142 1.189 0.866~1.633 0.285 MMSE评分 0.419 0.189 4.908 1.520 1.049~2.202 0.027* UPDRS评分 0.398 0.192 4.299 1.489 1.022~2.169 0.038* miR-21 0.520 0.195 7.111 1.682 1.148~2.465 0.008* miR-23a 0.462 0.206 5.026 1.587 1.060~2.376 0.025* 平均病程 −0.405 0.189 4.735 0.667 0.034~0.808 0.035* *P < 0.05。 -
[1] Reddy A P, Ravichandran J, Carkaci-Salli N. Neural regeneration therapies for Alzheimer’ s and Parkinson’ s disease-related disorders[J]. Biochim Biophys Acta Mol Basis Dis, 2020, 1866(4): 165506. doi: 10.1016/j.bbadis.2019.06.020 [2] Vijiaratnam N, Simuni T, Bandmann O, et al. Progress towards therapies for disease modification in Parkinson’ s disease[J]. Lancet Neurol, 2021, 20(7): 559-572. doi: 10.1016/S1474-4422(21)00061-2 [3] Jurado-Coronel J C, Cabezas R, Ávila Rodríguez M F, et al. Sex differences in Parkinson’ s disease: Features on clinical symptoms, treatment outcome, sexual hormones and genetics[J]. Front Neuroendocrinol, 2018, 50: 18-30. doi: 10.1016/j.yfrne.2017.09.002 [4] Fan T S, Liu S C, Wu R M. Alpha-synuclein and cognitive decline in parkinson disease[J]. Life (Basel), 2021, 11(11): 1239. doi: 10.3390/life11111239 [5] Li W W, Fan D Y, Shen Y Y, et al. Association of the polygenic risk score with the incidence risk of Parkinson’ s disease and cerebrospinal fluid α-synuclein in a Chinese cohort[J]. Neurotox Res, 2019, 36(3): 515-522. doi: 10.1007/s12640-019-00066-2 [6] Zhang P, Rasheed M, Liang J, et al. Emerging potential of exosomal non-coding RNA in Parkinson’ s disease: A review[J]. Front Aging Neurosci, 2022, 14: 819836. doi: 10.3389/fnagi.2022.819836 [7] He M, Zhang H N, Tang Z C, et al. Diagnostic and therapeutic potential of exosomal microRNAs for neurodegenerative diseases[J]. Neural Plast, 2021, 2021: 8884642. [8] Bai X, Bian Z. microRNA-21 is a versatile regulator and potential treatment target in central nervous system disorders[J]. Front Mol Neurosci, 2022, 15: 842288. doi: 10.3389/fnmol.2022.842288 [9] 中华医学会神经病学分会帕金森病及运动障碍学组, 中国医师协会神经内科医师分会帕金森病及运动障碍专业. 中国帕金森病的诊断标准(2016版)[J]. 中华神经科杂志, 2016, 49(4): 268-271. [10] Galea M, Woodward M. Mini-mental state examination (MMSE)[J]. Aust J Physiother, 2005, 51(3): 198. doi: 10.1016/S0004-9514(05)70034-9 [11] Postuma R B, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease[J]. Mov Disord, 2015, 30(12): 1591-1601. [12] Kashihara K, Kitayama M. Time taken for and causes of a decline to hoehn and yahr stage 5 in patients with Parkinson’ s disease[J]. Intern Med, 2023, 62(5): 711-716. doi: 10.2169/internalmedicine.8922-21 [13] Tansey M G, Wallings R L, Houser M C, et al. Inflammation and immune dysfunction in parkinson disease[J]. Nat Rev Immunol, 2022, 22(11): 657-673. doi: 10.1038/s41577-022-00684-6 [14] Mao H, Ding L. Downregulation of miR-21 suppresses 1-methyl-4-phenylpyridinium-induced neuronal damage in MES23.5 cells[J]. Exp Ther Med, 2019, 18(4): 2467-2474. [15] Zhao L, Wang Z. microRNAs: Game changers in the regulation of α-synuclein in Parkinson’ s disease[J]. Parkinsons Dis, 2019, 2019: 1743183. [16] Zhu X, Yao Y, Liu Y, et al. Regulation of ADAM10 by microRNA-23a contributes to epileptogenesis in pilocarpine-induced status epilepticus mice[J]. Front Cell Neurosci, 2019, 13: 180. [17] Nie C, Sun Y, Zhen H, et al. Differential expression of plasma exo-miRNA in neurodegenerative diseases by next-generation sequencing[J]. Front Neurosci, 2020, 14: 438. doi: 10.3389/fnins.2020.00438 [18] Barbagallo C, Mostile G, Baglieri G, et al. Specific signatures of serum miRNAs as potential biomarkers to discriminate clinically similar neurodegenerative and vascular-related diseases[J]. Cell Mol Neurobiol, 2020, 40(4): 531-546. doi: 10.1007/s10571-019-00751-y -





 下载:
下载: