Effect of PTEN Gene Expression on Apoptosis of Thyroid Cancer Cells BCPAP and FTC133 and Expression of ERK and AKT
-
摘要:
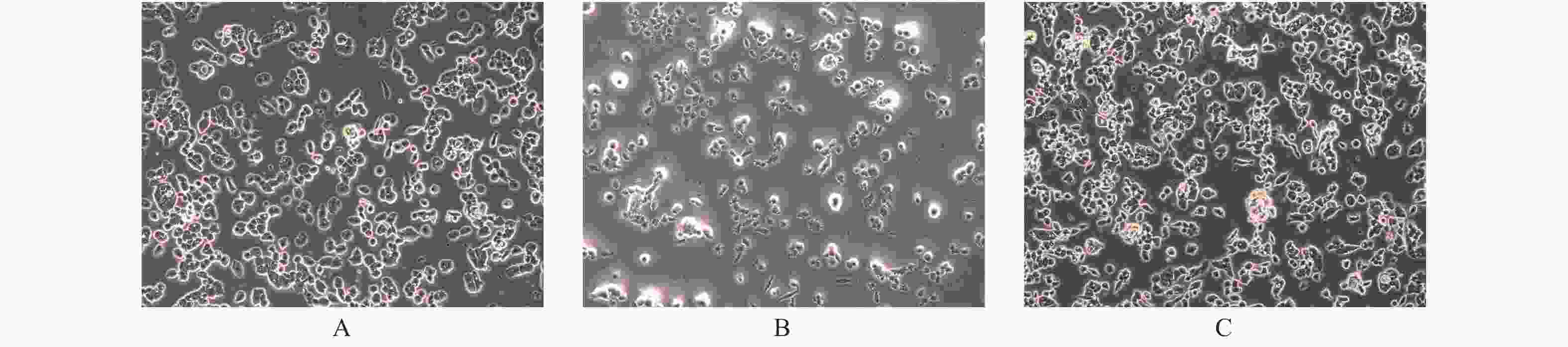
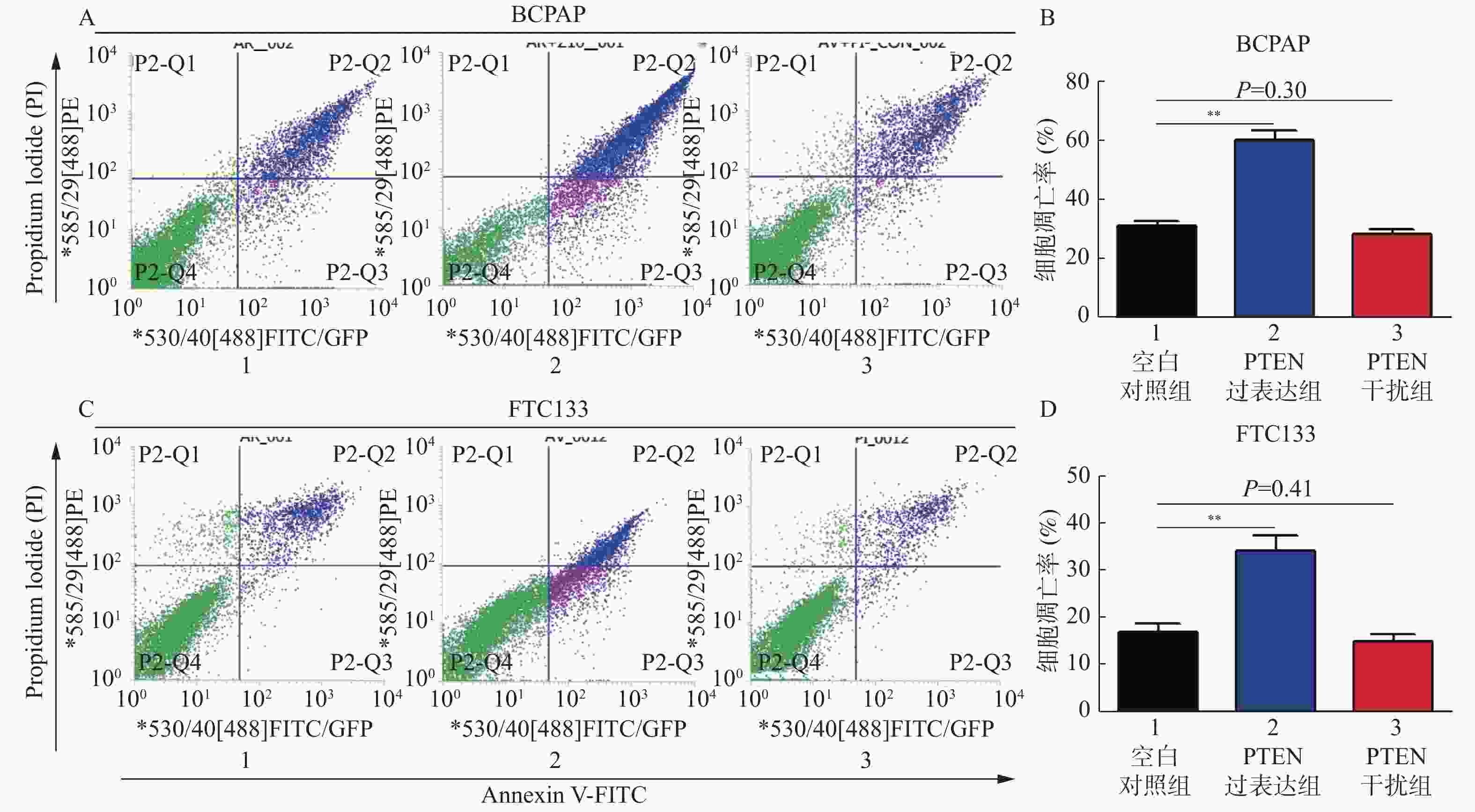
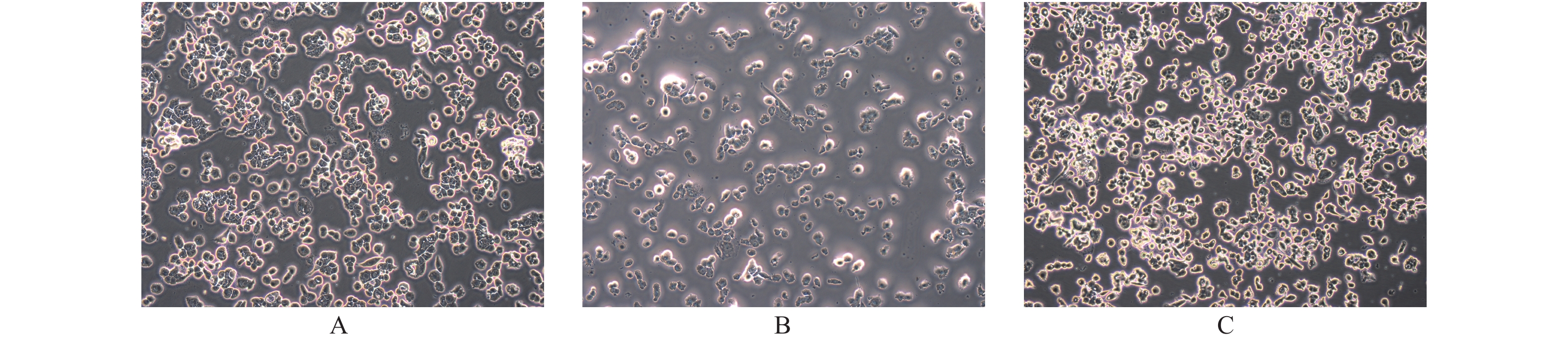
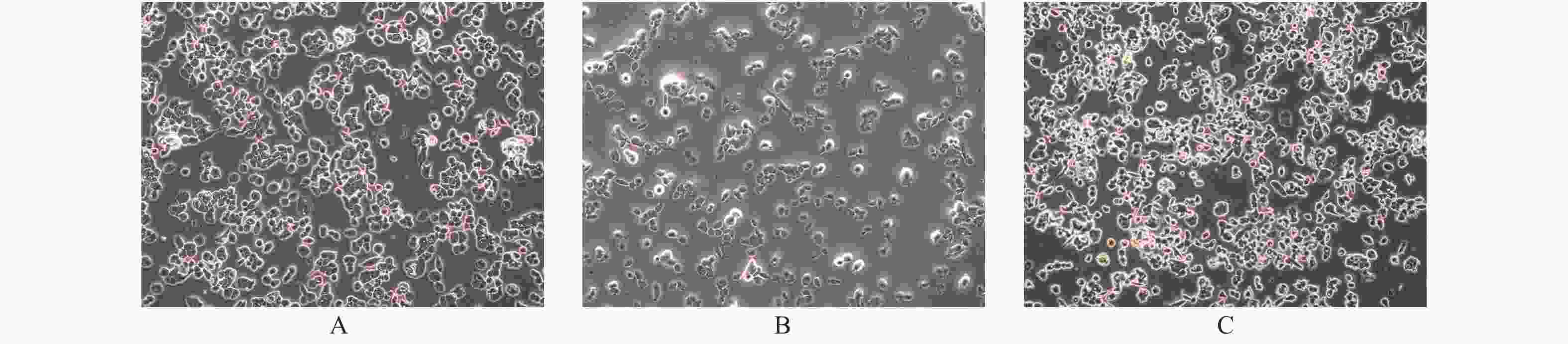
目的 探讨PTEN基因表达对甲状腺癌BCPAP细胞和FTC133细胞凋亡以及信号通路蛋白ERK和AKT表达的影响。 方法 BCPAP细胞和FTC133细胞经PTEN-pCMV6转染和si-PTEN RNA干扰后,光镜下观察细胞形态变化,MTT检测细胞活力,Annexin V-FITC和PI双染色流式细胞仪检测细胞凋亡率,Western blot检测Caspase 8、9、12、3、Bax、Bcl-2、ERK和AKT的表达水平,分析PTEN基因的不同表达与甲状腺癌BCPAP和FTC133细胞凋亡以及信号通路蛋白表达的关系。 结果 与对照组相比较,甲状腺癌BCPAP和FTC133细胞在PTEN基因过表达时,光镜下均出现细胞变形、体积缩小、核固缩等凋亡改变,细胞活力降低(P < 0.01),细胞凋亡率增加(P < 0.01),凋亡蛋白Caspase 8、9、12、3以及促凋亡蛋白Bax表达量增加(P < 0.01),抗凋亡蛋白Bcl-2表达量减低(P < 0.01),ERK和AKT表达量均较对照组减低(P < 0.05),ERK的相对表达量在FTC133细胞的减低程度显著高于BCPAP细胞(P < 0.01),AKT的相对表达量减低程度在BCPAP和FTC133,差异无统计学意义(P > 0.05);PTEN基因干扰表达减低时,光镜下BCPAP和FTC133细胞数目、细胞形态、体积及细胞核形态变化、细胞活力和细胞凋亡率变化,差异无统计学意义(P > 0.05);凋亡蛋白Caspase 8、9、12、3以及促凋亡蛋白Bax表达量减低(P < 0.01),抗凋亡蛋白Bcl-2表达量增加(P < 0.01),FTC133细胞ERK和AKT表达量分别较空白对照组均为增高(P < 0.01),BCPAP细胞ERK表达量较对照组增高(P < 0.01)。而AKT表达量与对照组比较,差异无统计学意义(P > 0.05),ERK的相对表达量增高程度在BCPAP和FTC133,差异无统计学意义(P > 0.05),AKT的相对表达量在FTC133细胞的增高程度显著高于BCPAP细胞(P < 0.05)。 结论 PTEN基因可促进BCPAP和FTC133细胞凋亡,线粒体途径、死亡受体途径和内质网激活通路均参与BCPAP和FTC133细胞凋亡的过程,ERK在PTEN基因调控BCPAP细胞凋亡发挥重要作用,而AKT和ERK均参与PTEN基因促进FTC133凋亡的过程。 Abstract:Objective To investigate the effect of PTEN gene expression on the apoptosis of BCPAP cells and FTC133 thyroid cancer cells and on the expression of signal pathway proteins ERK and AKT. Methods BCPAP cells and FTC133 cells were transfected with PTEN-pCMV6 and silenced si-PTEN RNA, before the cell microscopic morphology changes were observed. Cell viability was measured by MTT, and apoptosis rate was measured by Annexin V-FITC and PI double staining flow cytometer, respectively. Furthermore, the protein expression levels of ERK, AKT, Bax, Bcl-2, caspase 8, 9, 12 and 3 were detected by Western blot. The association between different expression of PTEN gene and the apoptosis of BCPAP and FTC133 thyroid cancer cells and the expression of signal pathway proteins were analyzed. Results Compared with control group, when the PTEN gene was overexpressed, BCPAP and FTC133 thyroid cancer cells showed microscopic apoptotic changes such as deformation, volume reduction and nuclear pyknosis. The cell viability was decreased (P < 0.01), and the cell apoptosis rate was increased (P < 0.01). The expression levels of the apoptotic proteins caspase 8, 9, 12, 3, and proapoptotic protein Bax were significantly increased (P < 0.01), and the expression of anti-apoptotic protein Bcl-2 was significantly decreased (P < 0.01), the expression of ERK and AKT were decreased (P < 0.05). The reduction degree of ERK relative expression in FTC133 cells was significantly higher than that in BCPAP cells (P < 0.01), while the reduction degree of AKT relative expression in BCPAP and FTC133 cells was not statistically significant (P > 0.05). After PTEN gene silencing, compared with the control group, the number, cell morphology, volume and nuclear morphology of BCPAP and FTC133 were not significantly different (P > 0.05). The change of cell viability and apoptosis rate were not significantly different (P > 0.05). The expression levels of apoptotic proteins caspase 8, 9, 12, 3, and proapoptotic protein Bax was significantly decreased (P < 0.01), and the expression level of anti-apoptotic protein Bcl-2 were significantly increased (P < 0.01). The expression levels of ERK and AKT in FTC133 cells were higher than those in the control group (P < 0.01), and the expression levels of ERK in BCPAP cells were higher than those in the control group (P < 0.01). Compared with the control group, there was no statistically significant difference in the expression level of AKT (P > 0.05). The relative expression level of ERK increased in BCPAP and FTC133, but there was no statistically significant difference (P > 0.05). The relative expression level of AKT increased in FTC133 cells than that in BCPAP cells (P < 0.05). Conclusion PTEN gene promotes the apoptosis of BCPAP and FTC133 cells. Mitochondrial pathway, death receptor pathway and endoplasmic reticulum activation pathway are all involved in the process of BCPAP and FTC133 cell apoptosis. ERK plays an important role in PTEN gene regulation of BCPAP cell apoptosis, while AKT and ERK both are involved in the process of PTEN gene promoting FTC133 apoptosis. -
Key words:
- PTEN /
- Thyroid cancer /
- Apoptosis /
- ERK /
- AKT
-
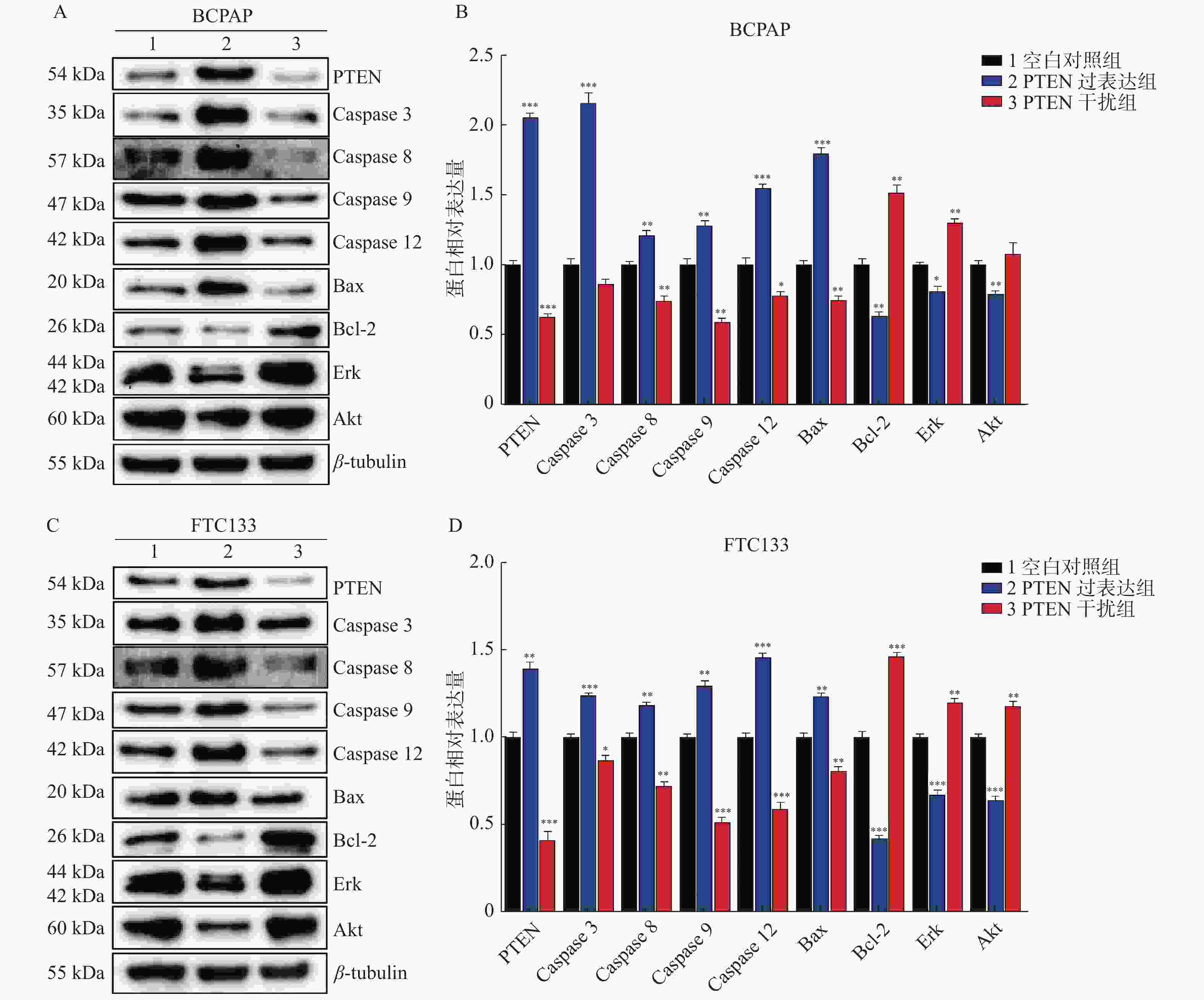
图 4 PTEN基因表达变化Western blot检测BCPAP和FTC133细胞相关蛋白表达
A:BCPAP细胞各相关因子Western印迹蛋白表达;B:BCPAP细胞各相关因子相对表达水平;C:FTC133细胞各相关因子Western印迹蛋白表达;D:FTC133细胞各相关因子相对表达水平。 与对照组比较,*P < 0.05,**P < 0.01,***P < 0.001。
Figure 4. The protein’s expression of BCPAP and FTC133 by Western blot with different PTEN expression levels
表 1 PTEN基因表达变化MTT检测BCPAP和FTC133细胞OD值及细胞活力(
$\bar x \pm s$ )Table 1. The OD and cell viability of BCPAP and FTC133 detected by MTT with different PTEN expression levels (
$\bar x \pm s$ )组别 BCPAP FTC133 OD 细胞活力(%) OD 细胞活力(%) 空白对照组 1.506 ± 0.165 100.00 ± 10.97 0.813 ± 0.123 100.00 ± 15.20 PTEN过表达组 0.345 ± 0.117* 22.92 ± 7.76* 0.040 ± 0.008* 4.91 ± 0.95* P < 0.001 < 0.001 < 0.001 < 0.001 PTEN干扰组 1.804 ± 0.086* 109.75 ± 8.17 0.943 ± 0.050* 112.66 ± 7.49 P < 0.001 0.082 0.032 0.051 与对照组比较,*P < 0.05。 表 2 PTEN基因表达变化Western blot检测BCPAP和FTC133细胞ERK和AKT表达(
$\bar x \pm s $ )Table 2. The expression of ERK and AKT of BCPAP and FTC133 by Western blot with different PTEN expression levels (
$\bar x \pm s $ )分组 BCPAP FTC133 ERK AKT ERK AKT 对照组 115.90 ± 4.41 140.04 ± 8.45 148.38 ± 5.39 126.76 ± 4.24 PTEN过表达组 95.02 ± 7.19* 112.45 ± 5.44* 101.43 ± 8.00* 82.76 ± 5.17* P 0.009 0.002 < 0.001 < 0.001 PTEN干扰组 156.47 ± 7.87* 147.49 ± 5.05 179.55 ± 6.59* 150.91 ± 6.63* P < 0.001 0.210 0.001 0.002 与对照组比较,*P < 0.05。 -
[1] Siegel R L,Miller K D,Jemal A. Cancer statistics,2017[J]. CA Cancer J Clin,2017,67(1):7-30. doi: 10.3322/caac.21387 [2] 刘志春,赵亮. PTEN和Ki-67在甲状腺癌组织中的表达及其临床意义[J]. 中国普外基础与临床杂志,2016,23(11):1348-1352. doi: 10.7507/1007-9424.20160345 [3] 陈超,张伟丽,冯长松. IncRNA PCAT19靶向miR-143-3p通过信号通路PI3K/Akt对甲状腺癌细胞增殖和凋亡的影响及机制[J]. 中国老年学杂志,2020,40(8):1712-1717. doi: 10.3969/j.issn.1005-9202.2020.08.043 [4] Seong Keun Yoo,Young Shin Song,Eun Kyung Lee,et al. Integrative analysis of genomic and transcriptomic characteristics associated with progression of aggressive thyroid cancer[J]. Nat Commun,2019,10(1):2764-2776. doi: 10.1038/s41467-019-10680-5 [5] 孙健玮. PTEN基因与肿瘤相关性的研究进展[J]. 中国当代医药,2019,26(32):20-23. doi: 10.3969/j.issn.1674-4721.2019.32.007 [6] Hanzhang Zhu,Qiaoyu Liu,Junwei Tang,et al. Alpha1-ACT functions as a tumour suppressor in hepatocellular carcinoma by inhibiting the PI3K/AKT/mTOR signalling pathway via activation of PTEN[J]. Cell Physiol Biochem,2017,41(6):2289-2306. doi: 10.1159/000475648 [7] 李金国,宋西成. 甲状腺癌相关信号传导通路的研究进展[J]. 肿瘤学杂志,2018,24(4):293-296. doi: 10.11735/j.issn.1671-170X.2018.04.B001 [8] Jahanbani I,Al Abdallah A,Ali R H,et al. Discriminatory miRNAs for the management of papillary thyroid carcinoma and noninvasive follicular thyroid neoplasms with papillary-like nuclear features[J]. Thyroid,2018,28(3):319-327. doi: 10.1089/thy.2017.0127 [9] 黄敏,倪庆峰. Beclin-1、PTEN蛋白在甲状腺癌中的表达及其意义[J]. 中国普通外科杂志,2017,26(12):1642-1646. doi: 10.3978/j.issn.1005-6947.2017.12.022 [10] 江泽友,徐灿,葛一漫. shRNA干扰IRS-1基因通过PI3K/AKT通路对人乳头状甲状腺癌细胞TPC-1增殖和转移能力的调控作用[J]. 中国免疫学杂志,2018,34(11):1674-1678. doi: 10.3969/j.issn.1000-484X.2018.11.015 [11] Rodgers S J,Ferguson D T,Mitchell C A,et al. Regulation of PI3K effector signalling in cancer by the phosphoinositide phosphatases[J]. Biosci Rep,2017,37(1):1-18. [12] 翟明慧,袁殿宝,赵峻峰,等. 过表达PTEN基因对甲状腺髓样癌细胞生长及细胞周期的影响[J]. 临床与病理杂志,2019,39(3):464-469. doi: 10.3978/j.issn.2095-6959.2019.03.002 [13] Li Za,Qiu Rb,Qiu Xc,et al. EYA2 promotes lung cancer cell proliferation by downregulating the expression of PTEN[J]. Oncotarget,2017,8(67):110837-110848. doi: 10.18632/oncotarget.22860 [14] 孙健玮,郑立民. 抑癌基因PTEN的研究进展[J]. 中国当代医药,2019,26(13):19-22. doi: 10.3969/j.issn.1674-4721.2019.13.006 [15] 陈政华. 甲状腺乳头状癌组织中血管内皮生长因子-C和PTEN蛋白的表达及其与临床病理参数的关系[J]. 中国老年杂志,2016,36(5):1124-1125. [16] Beg S,Siraj A K,Jehan Z,et al. PTEN loss is associated with follicular variant of middle eastern papillary thyroid carcinoma[J]. Br J Cancer,2015,112(12):1938-1943. doi: 10.1038/bjc.2015.169 [17] 王振,汪静宇,郭志琴,等. 同源磷酸酶-张力蛋白基因在分化型甲状腺癌组织中的表达及意义[J]. 中国基层医药,2017,24(4):536-539. doi: 10.3760/cma.j.issn.1008-6706.2017.04.014 [18] 于丰铭,徐扬. Caspase-3的研究进展[J]. 中国细胞生物学学报,2020,42(11):2072-2078. [19] 马汉宁,姬艳燕,陈伟,等. 甘草次酸通过抑制Caspase 3/Bax/Bcl-2凋亡信号通路保护心脏骤停心肺复苏大鼠心脏功能[J]. 中药药理与临床,2019,35(4):28-33. [20] Aral Kübra1,Aral Cüneyt Asım,Kapila Yvonne. The role of caspase-8,caspase-9,and apoptosis inducing factor in periodontal disease[J]. J Periodontol,2019,90(3):288-294. doi: 10.1002/JPER.17-0716 [21] Anderson S L,Townsend HGG,Singh B. Role of toll-like receptor 4 and caspase-3,-8,and-9 in lipopolysaccharide-induced delay of apoptosis in equine neutrophils.[J]. Am J Vet Res,2018,79(4):424-432. doi: 10.2460/ajvr.79.4.424 [22] Karimi A S,Tafvizi F,Tajabadi E M. Heat-killed probiotic bacteria induce apoptosis of HT-29 human colon adenocarcinoma cell line via the regulation of Bax/Bcl2 and caspases pathway[J]. Hum& Exp Toxicology,2019,38(9):1069-1081. [23] 王旭同,张永辉. 微小RNA-1269a靶向调控PTEN对肝癌细胞迁移、侵袭和PI3K/Akt信号通路的影响[J]. 临床肿瘤学杂志,2021,26(3):193-199. doi: 10.3969/j.issn.1009-0460.2021.03.001 [24] G F Zhang,J M Zhong,L Lin. MiR-19 enhances pancreatic cancer progression by targeting PTEN through PI3K/AKT signaling pathway[J]. Eur Rev Med Pharmacol Sci,2020,24(3):1098-1107. [25] Jiang Li,Qiao Yanguo,Wang Zhenghui,et al. Inhibition of microRNA-103 attenuates inflammation and endoplasmic reticulum stress in atherosclerosis through disrupting the PTEN-mediated MAPK signaling[J]. J Cell Physiol,2020,235(1):380-393. doi: 10.1002/jcp.28979 [26] 孙健玮,王剑松,刘子超,等. PTEN基因调控Raf1磷酸化对前列腺癌PC3细胞凋亡的影响[J]. 昆明医科大学学报,2020,41(1):18-25. doi: 10.3969/j.issn.1003-4706.2020.01.003 [27] LeBoeuf B,Anderson B,Young M,et al. Evaluating the effectiveness of MAPK,AKT,and mTOR inhibitors in reducing proliferation in cellular models of papillary and follicular thyroid cancer[J]. Cancer Res,2018,78(13):2887-2888. [28] 张春英,阴广维,尤鸣达,等. siRNA 靶向沉默TAK1基因对甲状腺癌细胞增殖、迁移和p38 MAPK 信号通路的抑制作用[J]. 吉林大学学报(医学版),2021,47(1):110-117. -





 下载:
下载: