Efficacy and Safety of Capecitabine Combined with Bevacizumab or Single Agent of Capecitabine as Maintenance Therapy for Advanced Colorectal Cancer
-
摘要:
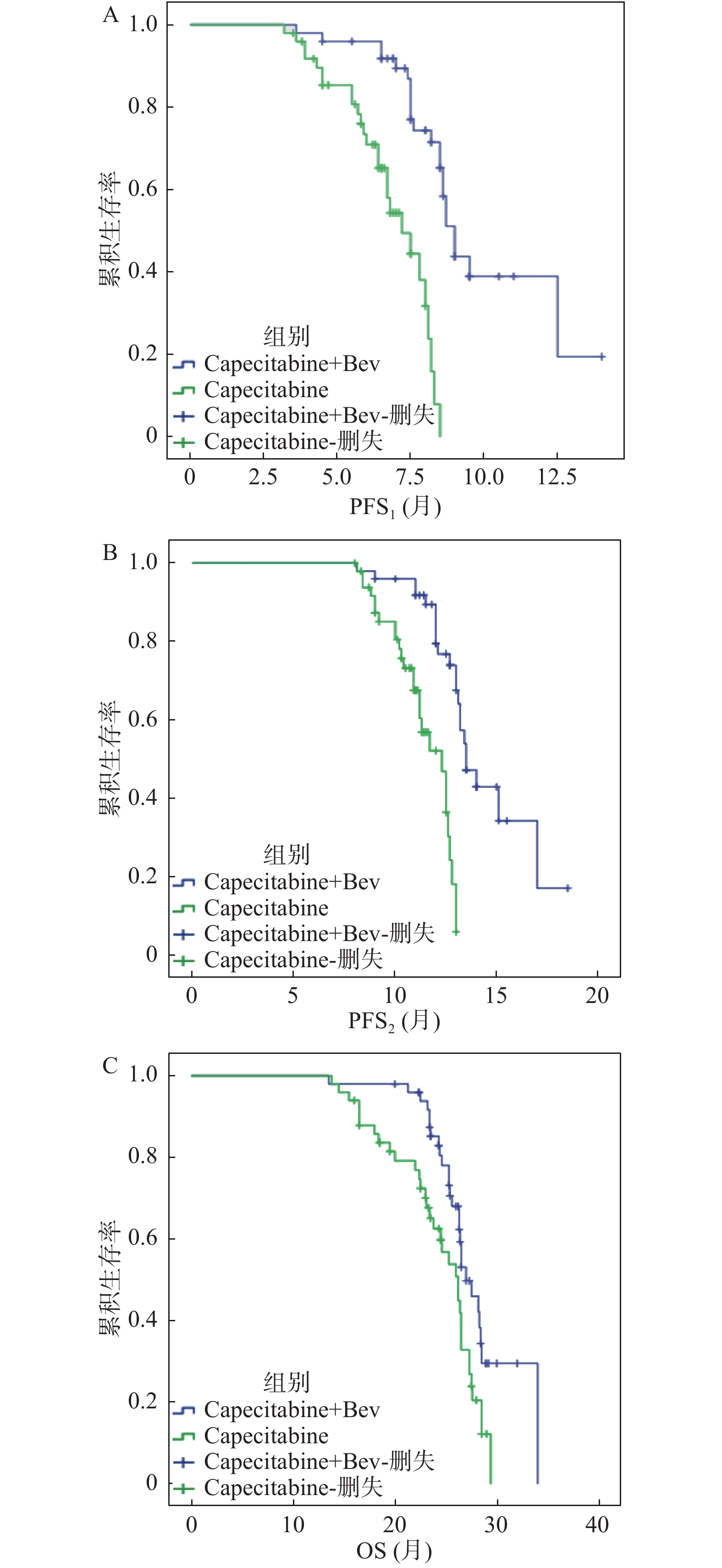
目的 探索XELOX方案联合贝伐珠单抗一线诱导治疗晚期结直肠癌序贯卡培他滨联合贝伐珠单抗或卡培他滨单药维持治疗的疗效与安全性。 方法 100例晚期结直肠癌患者,一线予以XELOX方案(奥沙利铂和卡培他滨)联合贝伐珠单抗诱导治疗6周期,疗效评价为完全缓解、部分缓解或疾病稳定,按1∶1分成卡培他滨联合贝伐珠单抗组(n = 50例)和卡培他滨单药组(n = 50例)维持治疗至首次疾病进展或毒副反应不可耐受。主要研究终点为维持治疗至疾病进展时间(PFS1),次要研究终点为诱导治疗开始至疾病进展时间(PFS2)、总生存时间(OS)和毒副反应。 结果 100例晚期结直肠癌患者进行维持治疗,2组基线临床病理学特征均衡,卡培他滨联合贝伐珠单抗维持治疗的中位PFS1、中位PFS2及中位OS均较卡培他滨单药组显著延长,分别为9.0(95%CI:8.52~9.47)比7.2(6.16~8.24,P < 0.0001)个月、13.5(12.55~14.45)比12.3(11.21~13.38,P < 0.0001)个月和27.0(25.31~28.69)比26.2(24.09~28.31,P = 0.011)个月。亚组分析显示,联合维持治疗显著延长同时性转移、RAS/BRAF突变亚组的PFS1及男性、肝转移亚组的OS。2组化疗相关毒副反应相似,最常见为中性粒细胞下降、粘膜炎、手足综合征和腹泻。联合组高血压(20% 比4%)及蛋白尿(14% 比 2%)的发生率明显高于单药组。 结论 卡培他滨联合贝伐珠单抗及卡培他滨单药均可作为安全有效的晚期结直肠癌一线经XELOX方案联合贝伐珠单抗诱导治疗获得疾病控制后维持治疗的选择。卡培他滨联合贝伐珠单抗维持治疗有更好的生存获益。 Abstract:Objective To investigate the efficacy and safety of capecitabine combined with bevacizumab or single agent of capecitabine as the maintenance therapy following the induction of XELOX regimen combined with bevacizumab in the first-line treatment of advanced colorectal cancer. Methods A total of 100 patients with the advanced colorectal cancer were treated with XELOX regimen (oxaliplatin plus capecitabine) combined with bevacizumab as first-line induction therapy for 6 cycles, whose efficacy were evaluated as the complete response, partial response or stable disease. These patients were divided in a 1∶1 ratio into the capecitabine combined with bevacizumab group (n = 50) and the single agent of capecitabine group (n = 50) for the maintenance therapy until the first disease progression or intolerable adverse events. The primary end point was progression-free survival from the initiation of maintenance therapy to the disease progression (PFS1). Secondary endpoints were progression-free survival from the initiation of induction therapy to the disease progression (PFS2), overall survival (OS) and adverse events. Results One-hundred patients were treated with capecitabine plus bevacizumab (n = 50) or single agent of capecitabine (n = 50) as the maintenance therapy. The median PFS1, median PFS2, and median OS of combination group were significantly longer than those of the monotherapy group, which were 9.0 (95% confidence interval (CI) 8.52-9.47) versus 7.2 (6.16-8.24, P < 0.0001) months, 13.5 (12.55-14.45) versus 12.3 (11.21-13.38, P < 0.0001) months and 27.0 (25.31-28.69) versus 26.2 (24.09-28.31, P = 0.011) months, respectively. Subgroup analysis showed that PFS1 of the combination group was significantly prolonged in the simultaneous metastasis and RAS/BRAF mutant subgroups. OS of the combination group was significantly prolonged in the male and liver metastatic subgroups. Similar treatment-related adverse events were observed in both groups. The most common toxicities both were neutropenia, mucositis, hand-foot syndrome and diarrhea. The incidence of hypertension (20% versus 4%) and proteinuria (14% versus 2%) were higher in the combination group than that in the monotherapy group. Conclusion Both capecitabine combined with the bevacizumab and the single agent of capecitabine can be considered as the appropriate options following the induction of XELOX regimen combined with bevacizumab as the first line treatment for advanced colorectal cancer with good efficacy and acceptable toxicities. Capecitabine combined with bevacizumab as maintenance therapy has a better survival benefit than single agent of capecitabine. -
Key words:
- Advanced colorectal cancer /
- Induction therapy /
- Maintenance therapy /
- Bevacizumab /
- Capecitabine
-
表 1 基线临床病理特征[n(%)]
Table 1. Baseline clinicopathological characteristics [n(%)]
基线特征 Cape/BEV(n = 50) Cape(n = 50) χ2 P n(%) n(%) 年龄 0.05 0.83 ≥65岁 16(32.0) 17(34.0) < 65岁 34(68.0) 33(66.0) 性别 0.18 0.67 男 32(64.0) 34(68.0) 女 18(36.0) 16(32.0) ECOG评分 0.22 0.64 0 13(26.0) 11(22.0) 1 37(74.0) 39(78.0) 原发部位 0.27 0.87 右半结肠 31(62.0) 30(60.0) 左半结肠 9(18.0) 11(22.0) 直肠 10(20.0) 9(18.0) 转移部位* 0.21 0.90 肝 26(52.0) 25(50.0) 肺 18(36.0) 17(34.0) 其他 11(22.0) 13(26.0) 转移时机 0.04 0.84 同时性转移 25(50.0) 24(48.0) 异时性转移 25(50.0) 26(52.0) 原发灶切除 0.16 0.69 是 28(56.0) 30(60.0) 否 22(44.0) 20(40.0) 诱导治疗疗效 0.30 0.86 CR 2(4.0) 3(6.0) PR 20(40.0) 21(42.0) SD 28(56.0) 26(52.0) RAS/BRAF 0.27 0.87 野生型 17(34.0) 16(32.0) 突变型 25(50.0) 24(48.0) 未知 8(16.0) 10(20.0) 备注:完全缓解(complete response,CR),部分缓解(partial response,PR),疾病稳定(stable disease,SD),ECOG 体力状况评分系统。*因患者可能存在多部位同时性转移,故总和超过100%。 表 2 维持治疗的毒副反应[n(%)]
Table 2. Adverse events of maintenance therapy [n(%)]
毒副反应 Cape/BEV(n = 50) Cape(n = 50) χ2 P n(%) n(%) 中性粒细胞下降 16(32.0) 15(30.0) 0.05 0.83 贫血 4(8.0) 3(6.0) 0.15 0.70 血小板下降 8(16.0) 7(14.0) 0.08 0.78 腹泻 11(22.0) 10(20.0) 0.06 0.81 粘膜炎 12(24.0) 13(26.0) 0.05 0.82 手足综合征 12(24.0) 10(20.0) 0.23 0.63 恶心/呕吐 4(8.0) 3(6.0) 0.15 0.70 高血压 10(20.0) 2(4.0) 6.06 0.01 蛋白尿 7(14.0) 1(2.0) 4.89 0.03 -
[1] Chen W,Zheng R,Baade P D,et al. Cancer statistics in China,2015[J]. CA Cancer J Clin,2016,66(2):115-132. doi: 10.3322/caac.21338 [2] Arnold M,Sierra M S,Laversanne M,et al. Global patterns and trends in colorectal cancer incidence and mortality[J]. Gut.,2017,66(4):683-691. doi: 10.1136/gutjnl-2015-310912 [3] Van Cutsem E,Nordlinger B,Adam R,et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases[J]. Eur J Cancer,2006,42(14):2212-2221. doi: 10.1016/j.ejca.2006.04.012 [4] Luo H Y,Li Y H,Wang W,et al. Single-agent capecitabine as maintenance therapy after induction of XELOX (or FOLFOX) in first-line treatment of metastatic colorectal cancer:Randomized clinical trial of efficacy and safety[J]. Ann Oncol,2016,27(6):1074-1081. doi: 10.1093/annonc/mdw101 [5] Geng R,Wang G,Qiu L,et al. Metronomic capecitabine as maintenance treatment after first line induction with XELOX for metastatic colorectal cancer patients[J]. Medicine (Baltimore),2020,99(51):e23719. [6] Simkens L H,van Tinteren H,May A,et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3):A phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group[J]. Lancet,2015,385(9980):1843-1852. doi: 10.1016/S0140-6736(14)62004-3 [7] Petrioli R,Francini E,Cherri S,et al. Capecitabine plus oxaliplatin and bevacizumab,followed by maintenance treatment with capecitabine and bevacizumab for patients aged > 75 years with metastatic colorectal cancer[J]. Clin Colorectal Cancer,2018,17(4):e663-e669. doi: 10.1016/j.clcc.2018.07.002 [8] Nakayama G,Ishigure K,Yokoyama H,et al. The efficacy and safety of CapeOX plus bevacizumab therapy followed by capecitabine plus bevacizumab maintenance therapy in patients with metastatic colorectal cancer:Amulti-center,single-arm,phase Ⅱ study (CCOG-0902)[J]. BMC Cancer,2017,17(1):243. doi: 10.1186/s12885-017-3245-1 [9] Tournigand C,Cervantes A,Figer A,et al. OPTIMOX1:A randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer-a GERCOR study[J]. J Clin Oncol,2006,24(3):394-400. doi: 10.1200/JCO.2005.03.0106 [10] Chibaudel B,Maindrault-Goebel F,Lledo G,et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 study[J]. J Clin Oncol,2009,27(34):5727-5733. doi: 10.1200/JCO.2009.23.4344 [11] D í az-Rubio E,G ó mez-España A,Massutí B,et al. Spanish cooperative group for the treatment of digestive tumors. First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer:The phase Ⅲ MACRO TTD study[J]. Oncologist,2012,17(1):15-25. doi: 10.1634/theoncologist.2011-0249 [12] Matter-Walstra K,Schwenkglenks M,Betticher D,et al. Bevacizumab continuation versus treatment holidays after first-line chemotherapy with bevacizumab in patients with metastatic colorectal cancer:A health economic analysis of a randomized phase 3 trial (SAKK 41/06)[J]. Clin Colorectal Cancer,2016,15(4):314-320.e2. doi: 10.1016/j.clcc.2016.03.002 [13] Aparicio T,Ghiringhelli F,Boige V,et al. Bevacizumab maintenance versus no maintenance during chemotherapy-free intervals in metastatic colorectal cancer:A randomized phase Ⅲ trial (PRODIGE 9)[J]. J Clin Oncol,2018,36(7):674-681. doi: 10.1200/JCO.2017.75.2931 [14] Noepel-Duennebacke S,Arnold D,Hertel J,et al. Impact of the localization of the primary tumor and RAS/BRAF mutational status on maintenance strategies after first-line oxaliplatin,fluoropyrimidine,and bevacizumab in metastatic colorectal cancer:Results from the AIO 0207 Trial[J]. Clin Colorectal Cancer,2018,17(4):e733-e739. doi: 10.1016/j.clcc.2018.07.007 [15] Aparicio T,Bennouna J,Le Malicot K,et al. Predictive factors for early progression during induction chemotherapy and chemotherapy-free interval:Analysis from PRODIGE 9 trial[J]. Br J Cancer,2020,122(7):957-962. doi: 10.1038/s41416-020-0735-8 [16] Loupakis F,Yang D,Yau L,et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer[J]. J Natl Cancer Inst,2015,107(3):dju427. [17] Manfredi S,Turpin A,Malka D,et al. Maintenance treatment with fluoropyrimidine plus bevacizumab versus fluoropyrimidine alone after induction chemotherapy for metastatic colorectal cancer:The bevamaint - prodige 71 -(FFCD 1710)phase Ⅲ study[J]. Dig Liver Dis,2020,52(10):1143-1147. doi: 10.1016/j.dld.2020.06.034 [18] Sherman S K,Lange J J,Dahdaleh F S,et al. Cost-effectiveness of maintenance capecitabine and bevacizumab for metastatic colorectal cancer[J]. JAMA Oncol,2019,5(2):236-242. doi: 10.1001/jamaoncol.2018.5070 [19] Geng R,Wang G,Qiu L,et al. Metronomic capecitabine as maintenance treatment after first line induction with XELOX for metastatic colorectal cancer patients[J]. Medicine(Baltimore),2020,99(51):e23719. -





 下载:
下载: