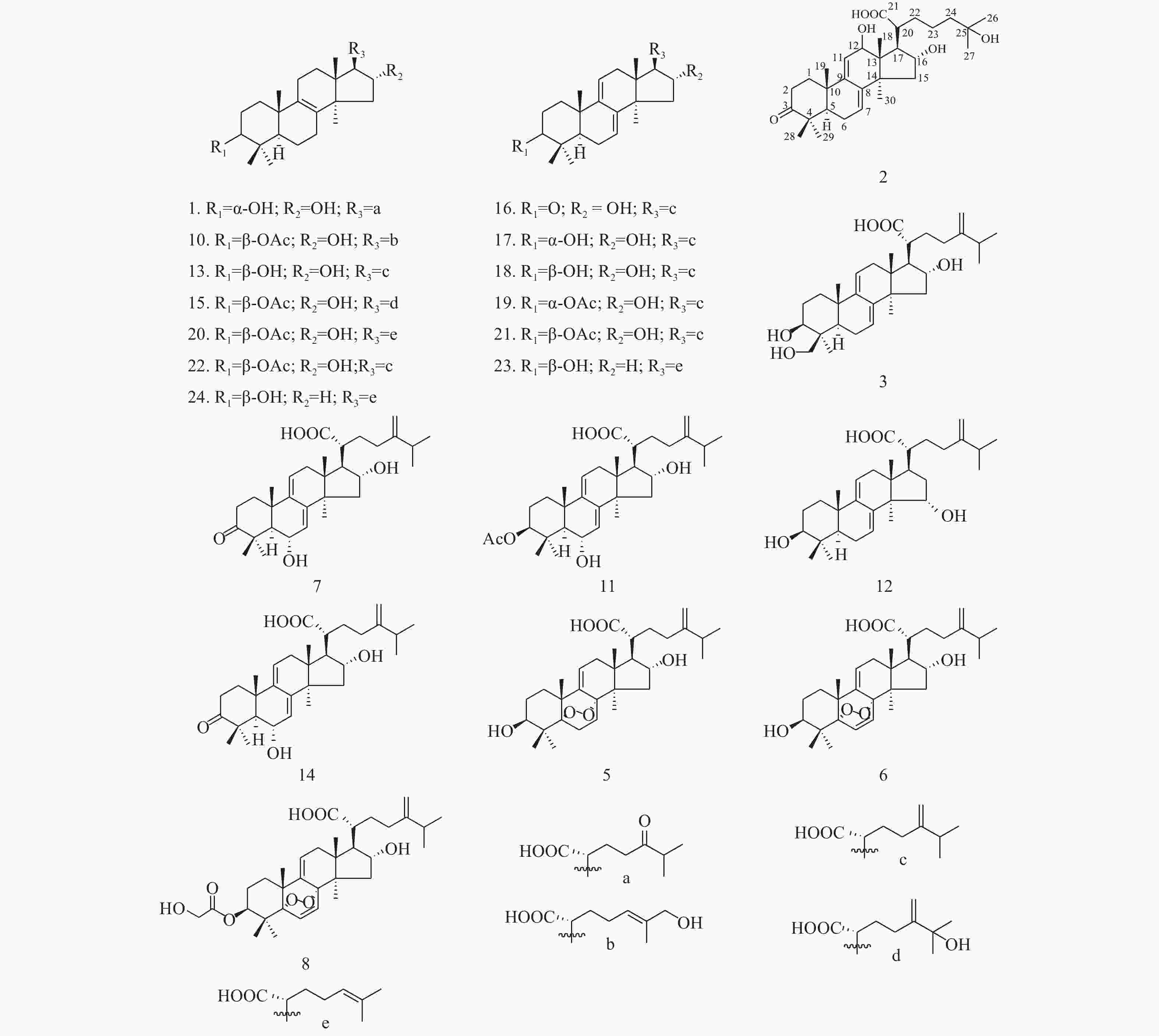
Analysis of Triterpene Chemical Constituents in Poria cocos 95% Ethanol Extract via UPLC-IT-TOF/MS
-
摘要:
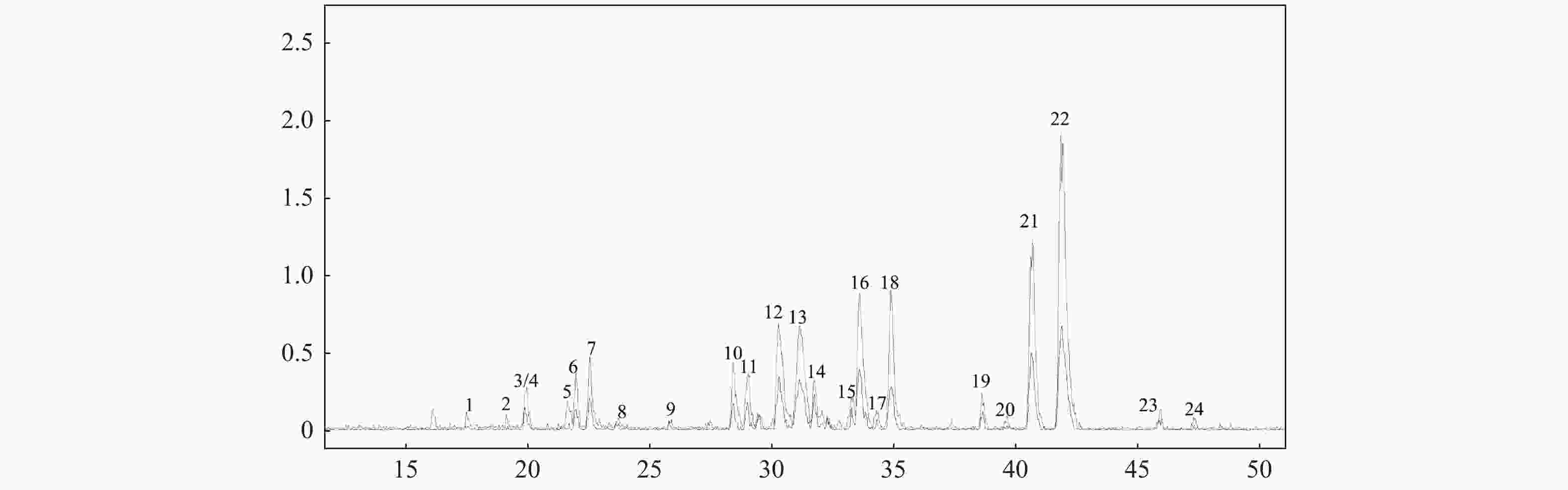
目的 采用超高效液相色谱串联离子阱飞行时间质谱(UPLC-IT-TOF/MS)技术定性分析茯苓95%乙醇提取物的三萜类化学成分。 方法 采用UHPLC XB-C18色谱柱,甲酸/水(0.05/100,v/v)-乙腈作为流动相,梯度洗脱,流速为0.20 mL/min;紫外检测波长范围为190~400 nm。使用ESI离子源,三氟乙酸钠校正,电喷雾正负离子同时检测,Shimadzu Composition Formula Predictor软件定性分析茯苓三萜类化合物。 结果 通过多级质谱数据分析,结合氮规则和Scifinder数据库检索,从茯苓醇提取物中鉴定了22个三萜类成分,结构类型包括羊毛甾-8-烯型三萜、羊毛甾-7,9(11)-二烯型三萜和其他羊毛甾三萜类型。 结论 通过LC-MS快速分析了茯苓醇提取物中三萜类成分,为其药效物质基础和质量控制提供了依据。 -
关键词:
- 茯苓 /
- 三萜 /
- UPLC-IT-TOF/MS
Abstract:Objective To evaluate the triterpene chemical constituents in Poria cocos 95% ethanol extract by ultra-performance liquid chromatography ion trap time-of-flight mass spectrometry (UPLC-IT-TOF/MS) method. Methods UPLC was performed with the UHPLC XB-C18 and methanoic acid/water (0.05/100, v/v)-acetonitrile solution (gradient elution) was employed with a flow rate of 0.2 mL·min-1. The detection wavelength range was 190~400 nm. Triterpene chemical compositions in Poria cocos were qualitatively analyzed using ESI ion with electrospray positive and negative ions simultaneous detection, sodium trifluoroacetace correction and Shimadzu Composition Formula Predictor software. Results Through the analysis of the multistage tandem mass spectrometry, combined with the application of nitrogen rule and the Scifinder database search, 22 triterpenes with lanostan-8-en, lanostan-7, 9(11)-diene type and other lanostane triterpene were identified. Conclusion This study quickly analyzed the triterpenes in the Poria cocos ethanol extract via LC-MS, which provided the basis for the pharmacodyamic material basis and quality control. -
Key words:
- Poria cocos /
- Triterpene /
- UPLC-IT-TOF/MS
-
表 1 茯苓中色谱峰的LC/ESI–MSn鉴定结果(1)
Table 1. Characterization of the peaks in LC/ESI–MSn chromatogram of Poria cocos (1)
色谱峰 保留时间 分子式 分子量 ESI+ (误差,mDa) ESI− (误差,mDa) 化合物名称 1 17.51 C30H48O5 488 [M+Na]+ 511.3328 (−6.6)
MSn: 511-493.3316 (C30H46O4, +2.8),
453.3319 (C30H44O3, −4.4)[M−H]−487.3402 (−2.7) Daedaleanic acid B[10] 2 19.15 C30H46O6 502 [M+Na]+ 525.3301 (+11.4) [M−H]−501.3164 (−5.8) Pinicolic acid F [9] 3 19.90 C31H48O5 500 MSn: 483.3468 (C31H46O4, −0.1), 465.3319 (C31H44O3, −4.4) [M−H]−499.3427 (−0.2) 29α-羟基去氢土莫酸
29α-Hydroxydehy-dropachymic acid[14]4 19.90 C30H48O6 504 [M+Na]+ 527.3331 (−1.2)
MSn: 527- 495.3077 (C29H44O5, +6.3), 437.2886 (C27H42O3, −14.0)[M−H]−503.3373 (−0.5) - 5 21.69 C31H48O6 516 [M+Na]+539.3047 (+6.8)
MSn: 539- 521.2809 (C31H46O5, −6.5), 507.2633 (C30H44O5, −4.6)[M−H]−515.3056 (+4.2) 5,8α-Dioxy-3β,16α-dihydroxylanosta-7,
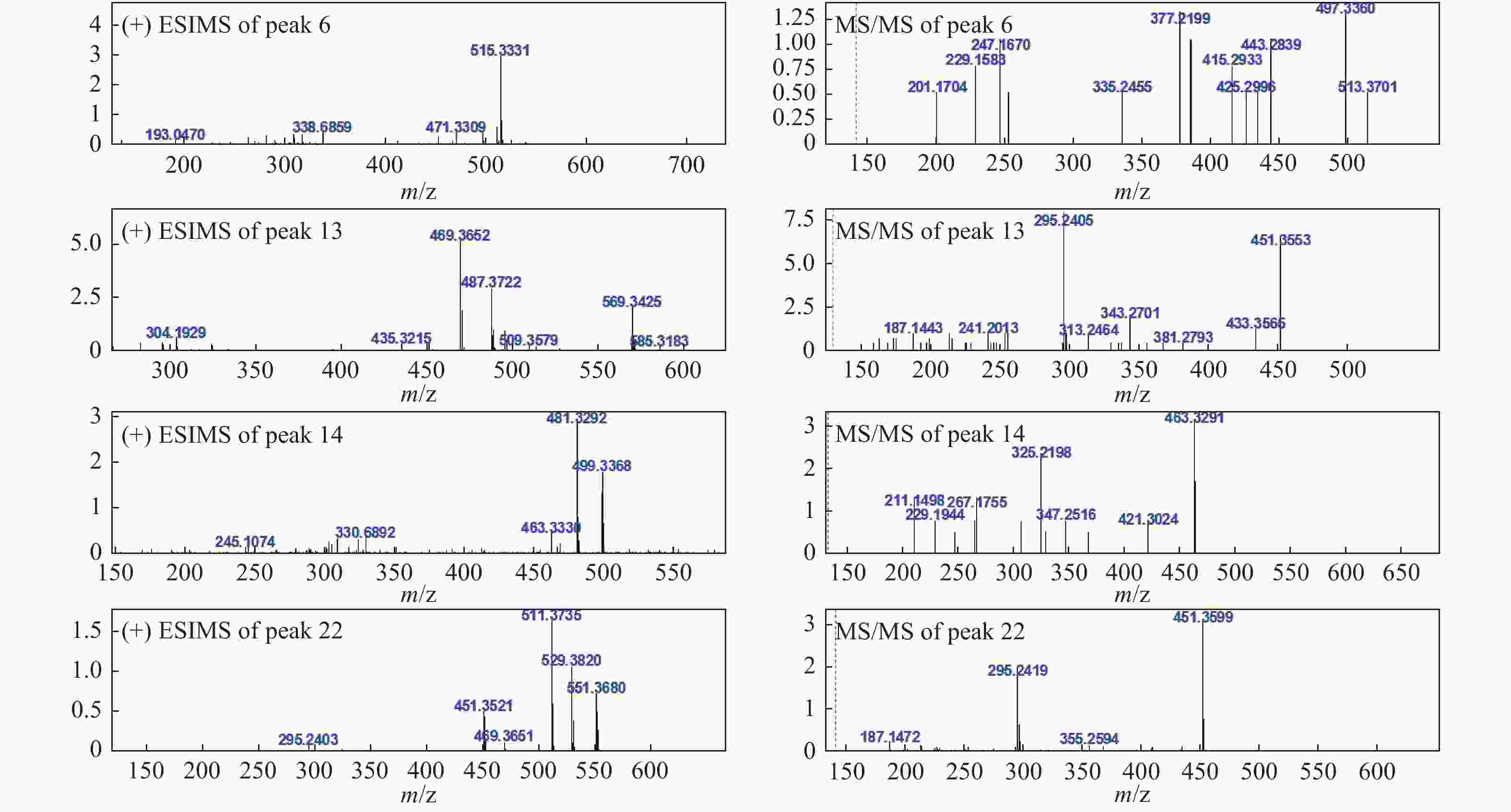
24- dien-21-oic acid [18]6 21.97 C31H46O6 514 [M+H]+515.3331 (−3.6)
MSn: 515- 497.3360 (C31H44O5, +9.8), 443.2839
(C31H38O2, −10.6), 433.3267 (C30H40O2, +16.6), 425.2996
(C28H40O3, −5.4), 415.2933 (C30H38O, −6.2), 385.2397
(C24H32O4, +2.4), 377.2199 (C25H28O3, +8.8), 335.2455
(C24H30O, +8.6), 253.1655 (C18H20O, +6.8), 247.1670
(C16H22O2, −2.3), 229.1583 (C16H20O, −0.4), 201.1704 (C15H20, +6.6)[M−H]−513.3151 (−7.1)
MSn: 513- 483.3167 (C31H46O4, −5.6)5α,8α-过氧化去氢土莫酸
5α,8α-Peroxydehydrotumulosic acid[19]7 22.54 C31H46O5 498 [M+H]+ 499.3287 (−13.1)
MSn: 481.3308 ( (C31H44O4, −0.4), 463.3147 (C31H42O3, −6.0)[M−H]−497.3193 (−7.9)
MSn: 497- 403.2418 (C24H26O5, −7.2), 389.2435 (C27H34O2, −5.1),
371.2336(C27H32O, −4.4), 355.2131 (C26H28O, +6.4)6α-羟基猪苓酸C
6α-Hydroxypolyporenic acid C[11]8 23.69 C33H48O8 572 [M+H]+573.3312 (−11.1) [M−H]−571.3213 (−6.3) 3-(2-羟基乙酰氧基)- 5α,8α-过氧化去氢土莫酸
3-(2-hydroxyacetoxy)- 5α,
8α-peroxydehydrotumulosic acid [20]9 25.78 C31H46O5 498 [M+H]+ 499.3433 (+1.5)
MSn: 481.3408 ( (C31H44O4, +9.6)- 451.3147 (C30H42O3, −1.4),
435.2955 (C29H38O3, +6.2), 325.2132 (C22H28O2, −3.0),
299.2112 (C20H26O2, +10.6)[M−H]−497.3236 (−3.6) - 表 1 茯苓中色谱峰的LC/ESI–MSn鉴定结果(2)
Table 1. Characterization of the peaks in LC/ESI–MSn chromatogram of Poria cocos (2)
色谱峰 保留时间 分子式 分子量 ESI+ (误差,mDa) ESI- (误差,mDa) 化合物名称 10 28.42 C32H50O6 530 [M+Na]+553.3443 (−5.7)
MSn: 553- 535.3422 (C32H48O5, +2.8), 495.3437
(C30H48O4, −0.8), 477.3274 (C30H46O3, −6.5), 435.3364
(C28H44O2,+13.0), 353.2548 (C22H34O2, +9.7)[M−H]−529.3461 (−7.4) 3-乙酰氧基-16α,26-二羟基-羊毛甾-8,24-二烯-21-酸
3- Acetoxy-16α,26-dihydroxy-lanosta-8,24-dien-21-oic acid[10]11 29.01 C33H50O6 542 MSn: 525.3513 (C33H48O5, −6.2)- 507.3459
(C33H46O4, −1.0), 465.3365 (C31H44O3, +0.2),
447.3544 (C32H46O, −7.7)[M−H]−541.3460 (−7.5) 6α-羟基去氢茯苓酸
6α-Hydroxy-dehydropachymic acid[13]12 31.15 C31H48O4 484 [M+Na]+485.3592 (−3.3)
MSn: 485- 467.3492 (C31H46O3, −2.9), 449.3408 (C31H44O2, −0.6),
311.2388 (C22H30O, +1.9), 293.2259 (C22H28, −0.5)[M−H]−483.3413 (−6.7)
MSn: 483- 437.3393 (C30H46O2, −3.2), 421.2978 (C29H42O2, −13.4)3β,15α-二羟基-羊毛甾-7,9(11),24-三烯-21-酸
3β,15α-dihydroxylanosta-7,9(11),24-trien-21-oic acid[15]13 30.27 C31H50O4 486 [M+H]+ 487.3722 (−6.0)
MSn: 487- 469.3719 (C31H48O3, +4.3),
451.3553 (C31H46O2, −1.8), 343.2701
(C23H34O2, +6.9), 313.2464 (C22H32O, −6.2),
295.2405 (C22H30, −1.5)[M−H]−485.3570 (−6.6)
MSn: 483- 423.3371 (C29H44O2,+10.2)土莫酸
Tumulosic acid[10-11]14 31.74 C31H46O5 498 [M+H]+ 499.3368 (−5.0)
MSn: 499- 481.3292 (C31H44O4, −2.0), 463.3291 (C31H42O3, +8.4),
421.3024 (C29H40O2, −7.7), 325.2198 (C22H28O2, +3.8), 307.2102 (C22H26O, +4.6)[M−H]−497.3199 (−7.3)
MSn: 497- 425.2942 (C31H38O, +9.2)29-羟基猪苓酸 C
29-Hydroxypolyporenic acid C[16]15 33.26 C33H52O6 544 [M+H]+ 545.3733 (−10.4)
MSn: 545- 527.3696 (C33H50O5, −3.5), 451.3097 (C31H46O2, +2.6), 433.3267 (C31H44O, −19.8), 295.2433 (C22H30,+2.3)[M−H]−543.3623 (−6.8)
MSn: 543- 467.3227 (C31H48O3, +9.6)25-羟基茯苓酸
25-Hydroxypachimic acid[11]16 33.57 C31H46O4 482 [M+H]+ 483.3420 (−4.9)
MSn: 483- 465.3327 (C31H44O3, −3.6), 447.3246 (C31H42O2, −1.2), 309.2214 (C22H28O, +0.1)[M−H]−481.3266 (−5.7)
MSn: 481- 311.1817 (C21H28O2, −14.0)猪苓酸 C
Polyporenic acid C[11]17 34.28 C31H48O4 484 [M+H]+ 485.33637 (+1.2)
MSn: 483- 467.3486 (C31H46O3, −3.4), 449.3532 (C31H44O2, +11.8), 311.2343 (C22H30O, −2.6), 293.2330 (C22H28,+6.6)[M−H]−483.3378 (−10.2)
MSn:3-表去氢土莫酸
3-epi-Dehydrotumulosic acid acid[11]表 1 茯苓中色谱峰的LC/ESI–MSn鉴定结果(3)
Table 1. Characterization of the peaks in LC/ESI–MSn chromatogram of Poria cocos (3)
色谱峰 保留时间 分子式 分子量 ESI+ (误差,mDa) ESI− (误差,mDa) 化合物名称 18 34.89 C31H48O4 484 [M+H]+ 485.3589 (−3.6)
MSn: 483- 467.3526 (C31H46O3, +0.6), 449.3377 (C31H44O2, −3.7), 311.2408 (C22H30O, +3.9), 293.2265 (C22H28, 0.1)[M−H]−483.3406 (−7.4)
MSn: 481- 337.2465 (C24H34O, −7.2)去氢土莫酸
Dehydrotumulosic acid[17]19 38.63 C33H50O5 526 [M+H]+ 527.3671(−6.0)
MSn: 527- 509.3564 (C33H48O4, −6.1), 449.3398 (C31H44O2, −1.6), 293.2240 (C22H28, −2.4)[M−H]−525.3493 (−9.2)
MSn:3-表去氢茯苓酸
3-epi-Dehydropachymic acid[14]20 39.64 C32H50O5 514 [M+H]+ 515.3708(−2.3)
MSn: 515- 497.3555 (C32H46O4, −7.0), 437.3398 (C30H44O2, −1.6), 295.2422 (C22H30,+0.2)[M−H]−513.3532 (−5.3) 3-O-乙酰基-16α-羟基松苓酸
3-O-Acetyl-16α-hydroxytrametenolic acid[12]21 40.64 C33H50O5 526 [M+H]+ 527.3697 (−3.4)
MSn: 527- 509.3594 (C33H48O4, −3.1), 449.3446 (C31H44O2, +3.2), 353.2501 (C24H32O2, +2.6),
293.2254 (C22H28, −1.0)[M−H]−525.3511 (−7.4)
MSn: 525- 479.3471 (C32H48O3, −6.0), 465.3351 (C31H46O3, −2.3), 355.2295 (C23H32O3, +1.6),去氢茯苓酸dehydropachymic acid[11] 22 41.87 C33H52O5 528 [M+H]+ 529.3820 (−6.8)
MSn: 529- 511.3735 (C33H50O4, −4.7), 451.3599 (C31H46O2, −3.0), 295.2419 (C22H30, −0.1)[M−H]−527.3666 (−7.6)
MSn: 527- 465.3330 (C31H46O3, −4.4)茯苓酸
Pachymic acid[10-11]23 45.90 C30H46O3 454 [M+H]+ 455.3532 (+1.2)
MSn: 455- 437.3457 (C30H44O2, +4.3), 311.2370 (C22H30O, +0.1), 295.2381 (C22H30, −3.9)[M−H]−453.3342 (−3.2) 3-羟基-羊毛甾-7,9(11),24-三烯-21酸
Dehydrotrametenolic acid[12]24 47.30 C30H48O3 456 [M+H]+ 457.3663 (−1.3)
MSn: 457- 439.3502 (C30H46O2, −6.9), 313.2445 (C22H32O, −8.1), 295.2463 (C22H30, +4.3)[M−H]−455.3471 (−6.0) 3-氢化松苓酸
trametenolic acid[13] -
[1] 王萌,张毅,李金田. 从《神农本草经》论茯苓在经方中的应用[J]. 中国中医基础医学杂志,2017,23(8):1149-1151. [2] 邓桃妹,彭代银,俞年军,等. 茯苓化学成分和药理作用研究进展及质量标志物的预测分析[J]. 中草药,2020,51(10):2703-2717. doi: 10.7501/j.issn.0253-2670.2020.10.013 [3] 田婷,陈华,殷璐,等. 茯苓和茯苓皮水和乙醇提取物的利尿作用及其活性成分的分离鉴定[J]. 中国药理学和毒理学杂志,2014,28(1):57-62. [4] Xu H,Wang Y C,Jurutka P W,et al. 16α-Hydroxytrametenolic acid from Poria cocos improves intestinal barrier function through the glucocorticoid receptor-mediated PI3K/Akt/NF-kappaB pathway[J]. J Agric Food Chem,2019,67(39):10871-10879. doi: 10.1021/acs.jafc.9b04613 [5] Gao Y Q,Yan H,Jin R R,et al. Antiepileptic activity of total triterpenes isolated from Poria cocos is mediated suppression of aspartic and glutamic acids in the brain[J]. Pharm Biol,2016,54(11):2528-2535. doi: 10.3109/13880209.2016.1168853 [6] 董建设,赵俊峰,张林超,等. 茯苓酸通过Wnt信号通路对肾癌细胞生物学特性的影响[J]. 中国老年学杂志,2019,39(9):2241-2244. doi: 10.3969/j.issn.1005-9202.2019.09.061 [7] 樊燕青,孙兰池,李大鹏. 茯苓酸对舌鳞状细胞癌细胞CAL-27 增殖、凋亡及细胞周期的影响[J]. 中成药,2021,43(7):1909-1914. doi: 10.3969/j.issn.1001-1528.2021.07.043 [8] Lee S R,Lee S,Moon E,et al. Bioactivity-guided isolation of anti-inflammatory triterpenoids from the sclerotia of Poria cocos using LPS-stimulated Raw264.7 cells[J]. Bioorg Chem,2017,70:94-99. doi: 10.1016/j.bioorg.2016.11.012 [9] Chen B S,Zhang J J,Han J J,et al. Lanostane triterpenoids with glucose-uptake-stimulatory activity from peels of the cultivated edible mushroom Wolfiporia cocos[J]. J Agric Food Chem,2019,67(26):7348-7364. doi: 10.1021/acs.jafc.9b02606 [10] 邹叶挺. 茯苓化学组分表征及其抗顺铂肠损伤作用初步研究[D]. 南京: 南京中医药大学硕士论文. 2019: 10–45. [11] 郑艳,杨秀伟. 中药材规范化种植茯苓化学成分研究[J]. 中国现代中药,2017,19(1):44-50. [12] 李慧,黄帅,单连海,等. 茯苓皮中三萜酸类成分的研究[J]. 华西药学杂志,2016,31(1):6-10. [13] Nukaya H, Yamashiro H, Fukazawa H, et al, Isolation of inhibitors of TPA-induced mouse ear edema from Hoelen, Poria cocos[J]. Chem Pharm Bull, 1996, 44(4): 847–849. [14] 王坤凤. 茯苓化学成分及质量控制方法研究[D]. 北京: 北京中医药大学硕士论文. 2014: 62–85. [15] Zhang H F,Feng B,Liu K,et al. Isolation and purification of two triterpenoids from the Chinese medicinal plant Fomes officinalis ames[J]. Asian J Chem,2013,25(11):6130-6132. doi: 10.14233/ajchem.2013.14285 [16] Zheng Y,Yang X W. Two new lanostane triterpenoids from Poria cocos[J]. J Asian Nat Prod Res,2008,10(4):289-292. doi: 10.1080/10286020701782742 [17] 邹叶挺,徐金娣,龙芳,等. 整合UPLC-QTOF-MS/MS全扫描和模拟MRM方法综合评价茯苓乙醇提取物与后续乙酸乙酯萃取物三萜酸类组分化学一致性[J]. 药学学报,2019,54(1):130-137. [18] Lin H C, Chang T C, Chang W L, et al. Pharmaceutical composition and extract of Poria for enhancing uptake of nutrients: The United States of America, US 9757392 B2 [P]. 2017.09. 12. [19] Akihisa T,Nakamura Y,Tokuda H,et al. Triterpene acids from Poria cocos and their anti-tumor-promoting effects[J]. J Nat Prod,2007,70(6):948-953. doi: 10.1021/np0780001 [20] Li S,Wang Z,Gu R,et al. A new epidioxy-tetracyclic triterpenoid from Poria cocos Wolf[J]. Nat Prod Res,2016,30(15):1712-1717. doi: 10.1080/14786419.2015.1136909 -





 下载:
下载: