Clinical Value of Fecal SDC2 Gene Methylation Detection in Early Screening of Colorectal Cancer
-
摘要:
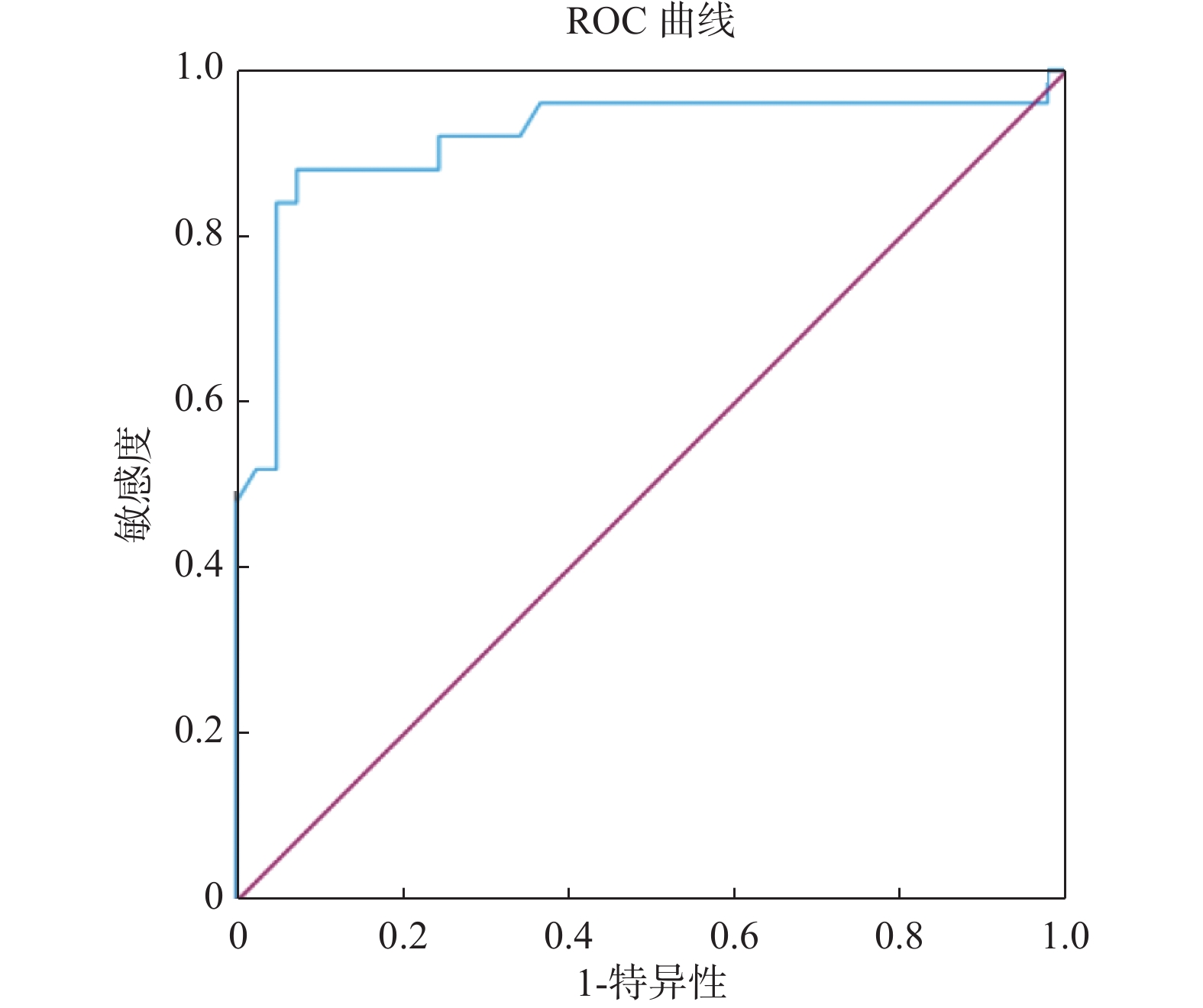
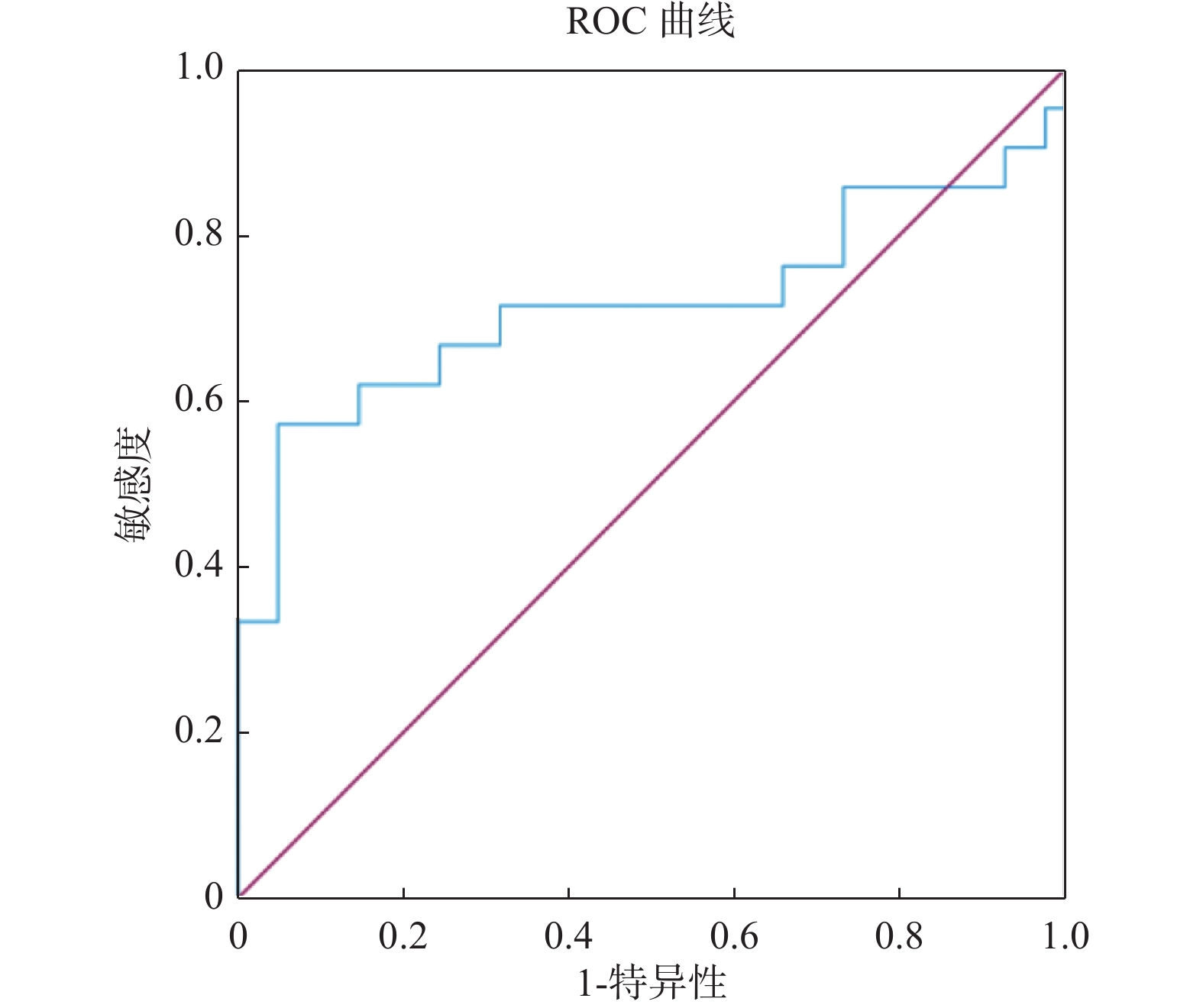
目的 探究粪便重组人黏结蛋白聚糖2(syndecan-2,SDC2)基因甲基化检测在结直肠癌早期筛查中的临床价值。 方法 选择2021年3月至2022年10月昆明医科大学第三附属医院收治的87例受检者作为研究对象,其中结直肠正常者41例,结直肠腺瘤患者21例,结直肠癌患者25例。研究将提取研究对象的粪便DNA用于进行粪便SDC2基因甲基化检测,根据试剂盒设定的Cutoff值,确定被测标本的阴性和阳性结果,对比SDC2基因甲基化检测结果与结直肠镜诊断结果,对受检者一般资料进行卡方检验分析,对SDC2基因甲基化循环阈值(cycle threshold,Ct)进行方差分析用于评估SDC2基因甲基化在结直肠肿瘤中的表达程度,并做受试者工作特征曲线(receiver operating characteristic curve,ROC curve)计算曲线下面积(area under the curve,AUC)用于评估SDC2基因甲基化在结直肠肿瘤中的诊断价值,分析SDC2基因甲基化在结直肠肿瘤诊断中的灵敏度和特异度。 结果 在87例入组人群中检测出SDC2基因甲基化阳性35例,阴性52例,其在结直肠腺瘤患者和结直肠癌患者粪便中的阳性率分别为57.14%(12/21)、84.00%(21/25),差异具有统计学意义(P = 0.044)。ROC曲线下分析结果显示,SDC基因甲基化检测诊断结直肠腺瘤和结直肠癌的曲线下面积(AUC)分别为0.715、0.918,灵敏度分别为57.14%、88.00%,特异度分别为95.12%,92.68%,差异有统计学意义(P = 0.0107,P < 0.0001)。 结论 粪便SDC2基因甲基化对于检测结直肠癌及其癌前病变有着较高的特异度,并且随着癌变进程检测灵敏度不断升高,故在结直肠癌早期筛查中具有重要临床价值。 Abstract:Objective This study aims to investigate the clinical application of fecal recombinant human adhesin syndecan 2 (SDC2) gene methylation test in the early screening of colorectal tumors. Methods Eighty-seven subjects admitted to the hospital from March 2021 to October 2022 were selected as the study subjects, including 41 cases with normal colorectum, 21 with colorectal adenoma and 25 with colorectal cancer. In this study, the fecal DNA of the subjects was extracted for the fecal SDC2 gene methylation test, and the negative and positive results of the tested specimens were determined according to the Cutoff value set by the kit. The effects of the SDC2 gene methylation test were compared with the results of colonoscopy diagnosis. The analysis of variance on SDC2 gene methylation cycle thresholds (Ct) was performed to assess the expression of SDC2 methylation in colorectal tumors. The subject operating characteristic curve (ROC) was used to calculate the area under the curve (AUC) to evaluate the diagnostic value of SDC2 gene methylation in colorectal tumors and to analyze the sensitivity and specificity of SDC2 gene methylation in colorectal tumor diagnosis. Results Among the 87 enrolled cases, 35 were positive for SDC2 gene methylationand 52 were negative. The positive rate in the stool of colorectal adenoma patients and colorectal cancer patients was 57.14% (12/21) and 84.00% (21/25), respectively and there were statistically significant differences (P = 0.044). The results of the analysis under the ROC curve showed that the area of SDC2 gene methylation under the curve (AUC) of the test for the diagnosis of colorectal adenoma and colorectal cancer was 0.715 and 0.918, respectively, with a sensitivity of 57.14% and 88.00% and a specificity of 95.12% and 92.68%, respectively and there were statistically significant differences (P = 0.0107, P < 0.0001). Conclusion Fecal SDC2 gene methylation has a high specificity for detecting colorectal cancer and its precancerous lesions, and the sensitivity of detection increases as the cancer progresses, so it is of great clinical value in early screening of colorectal cancer. -
Key words:
- SDC2 gene /
- Colorectal cancer /
- Clinical value
-
老鹳草为我国传统中药,来源于牻牛儿苗科植物牻牛儿苗Erodium stephanianum Willd.、老鹳草Geranium wilfordii Maxim.或野老鹳草Geranium carolinianum L.的干燥地上部分,收载于2020年版《中国药典》(一部),前者习称“长嘴老鹳草”,后两者习称“短嘴老鹳草”[1]。其性辛,味辛、苦、平、归肝、肾、脾经、祛风湿、通经络、主治筋骨酸痛、泄泻痢疾等。对牻牛儿苗,野老鹳草的化学成分研究表明其提取物具有较好抗氧化和抗炎镇痛的作用[2]。老鹳草不但具有祛风燥湿、活血通络,抗病原维生素和止泻的作用;而且它还具有抗病毒作用、抗氧化作用、对肠道和肝肾的影响、抗炎和镇痛作用以及降糖的作用等广泛的药理作用[3-4]。柯里拉京、鞣花酸、没食子酸、老鹳草素、原儿茶酸、金丝桃苷等为其中6种有效成分[5-8]。
超声提取法具备时间短、能耗低、省时省力,操作简单、容易掌握等优点,被广泛用于提取中药中的有效成分[9]。
本实验采用超声提取法[10],改变溶剂的种类,溶剂的体积分数,提取时间,料液比等条件,采用L9(34)正交实验优化老鹳草中6种有效成分的提取方法,选出同时提取老鹳草中6种成分的最佳提取方法工艺,使其对于老鹳草的有效成分提取更方便、更快捷、更实用。
1. 材料与方法
1.1 实验仪器
日本岛津公司 LC-2010A 高效液相色谱仪,岛津 LCsolution 色谱工作站,色谱柱:ZORBAX SB-C18(4.6×250 mm,5 μm),电子天平(BS224S):北京赛多利斯科学仪器有效公司,超声清洗仪(SK3200H):上海科导超声仪器有效公司,0.22 μm尼龙微孔滤头:天津市津腾试验设备有限公司,移液枪:Eppendorf Research plus。
1.2 实验样品及试剂
甲醇、乙腈(色谱纯,江苏汉邦科技有限公司),超纯水,磷酸(成都化夏化学试剂有限公司),老鹳草(购于国家药品检定研究院),老鹳草素对照品、没食子酸对照品、柯里拉京对照品、金丝桃苷对照品、鞣花酸对照品、原儿茶酸对照品(购于成都德思特生物技术有限公司)。
1.3 对照品及供试品溶液的制备
1.3.1 对照品溶液的配制
取一定量老鹳草素对照品,没食子酸对照品,金丝桃苷对照品,柯里拉京对照品,鞣花酸对照品,原儿茶酸对照品并准确称重,置于容量瓶,加入甲醇,溶解并定容,使其配制成1.0 g/mL的对照品溶液,待用。
1.3.2 对照品溶液的配制
取一定量老鹳草粉,约1 g,精密称定,置于150 mL具塞锥形瓶中,加入一定量溶剂,按L9(34)正交实验设计表中各条件配制,待用。
1.4 色谱条件
色谱柱:ZORBAX SB-C18(4.6×250 mm,5 μm);流速:1.0 ml/min;检测波长:280 nm;柱温:25 ℃;进样量:10 μL;以乙腈-0.1%磷酸为流动相进行梯度洗脱,梯度洗脱程序见表1。
表 1 HPLC梯度洗脱程序表、Table 1. The HPLC gradient elution procedures时间(min) 0.1%磷酸(Phosphoric) 乙腈(Acetonitrile) 0 96 4 10 95 5 15 88 12 20 86 14 30 85 15 40 79 21 50 79 21 60 55 45 70 55 45 1.5 单因素实验
1.5.1 料液比
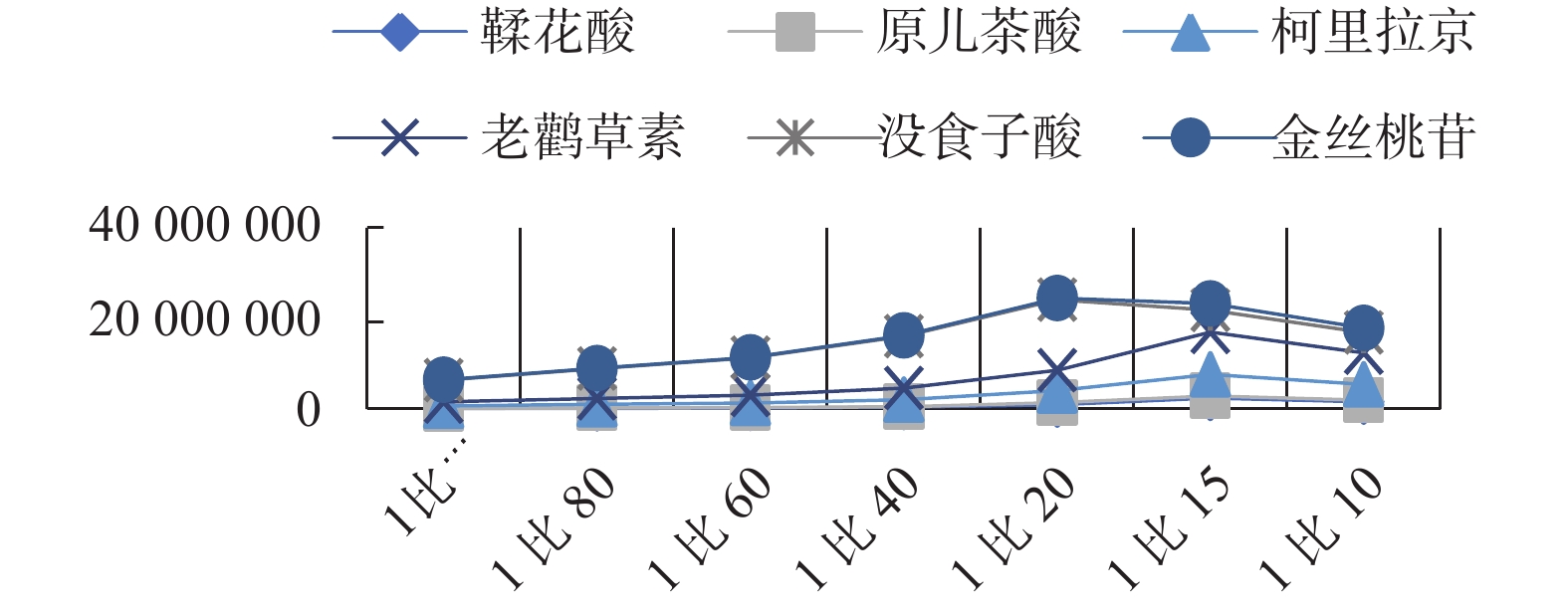
取7份老鹳草样品,每份约0.5 g,精密称定,分别置于7个具塞锥形瓶,分别加入甲醇5 mL、7.5 mL、10 mL、20 mL、30 mL、40 mL、50 mL,配制成1∶10、1∶15、1∶20、1∶40、1∶60、1∶80、1∶100(g/ mL)料液比的溶液,称定重量,超声提取30 min,取出放冷,用无水甲醇补足重量,尼龙微孔滤头过滤,取续滤液进样分析。
1.5.2 提取时间
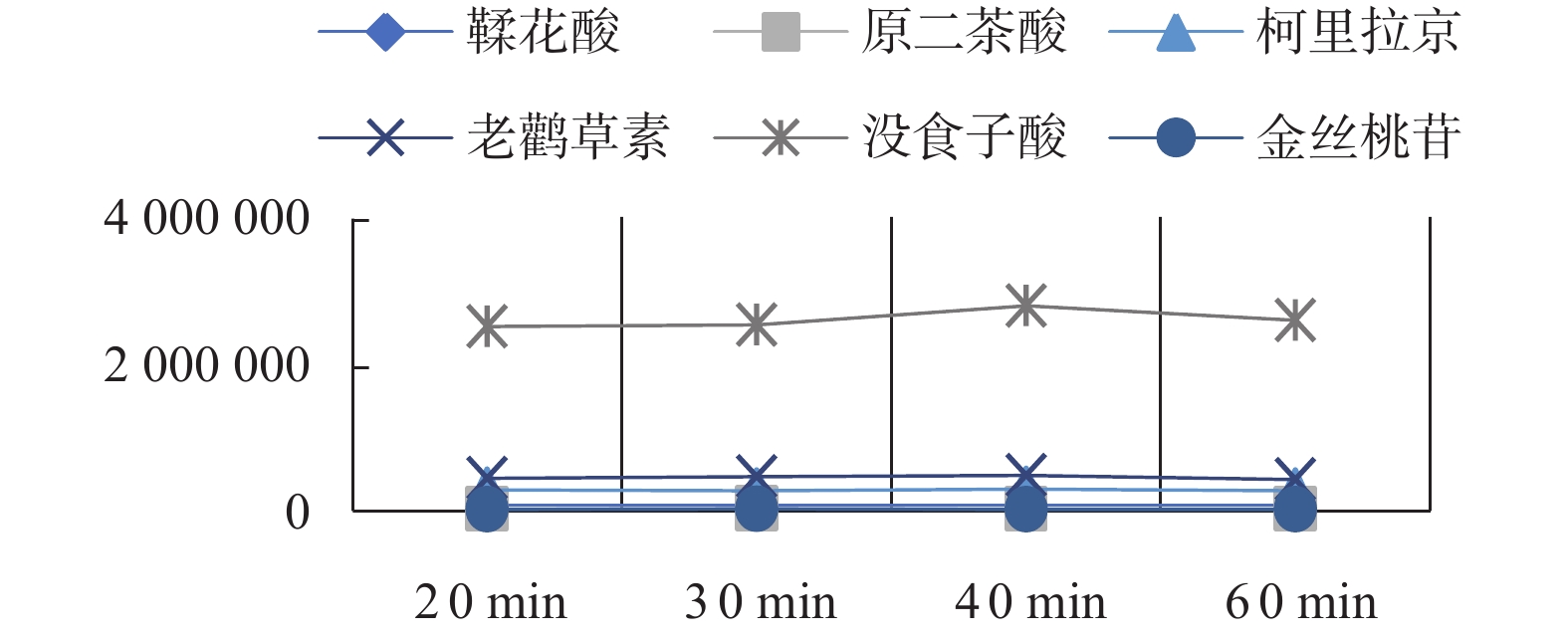
取4份老鹳草样品,每份约0.5 g,精密称定,分别置于4个具塞锥形瓶,分别加入60%的甲醇50 ml(料液比1∶10 g/mL),称重,分别超声20 min、30 min、40 min、60 min后,取出放冷,用甲醇补足减失重量,0.22 μm尼龙微孔滤头过滤,取续滤液进样分析,通过高效液相色谱仪记录老鹳草素、没食子酸、柯里拉京、金丝桃苷、鞣花酸、原儿茶酸的峰面积。
1.5.3 溶剂体积分数
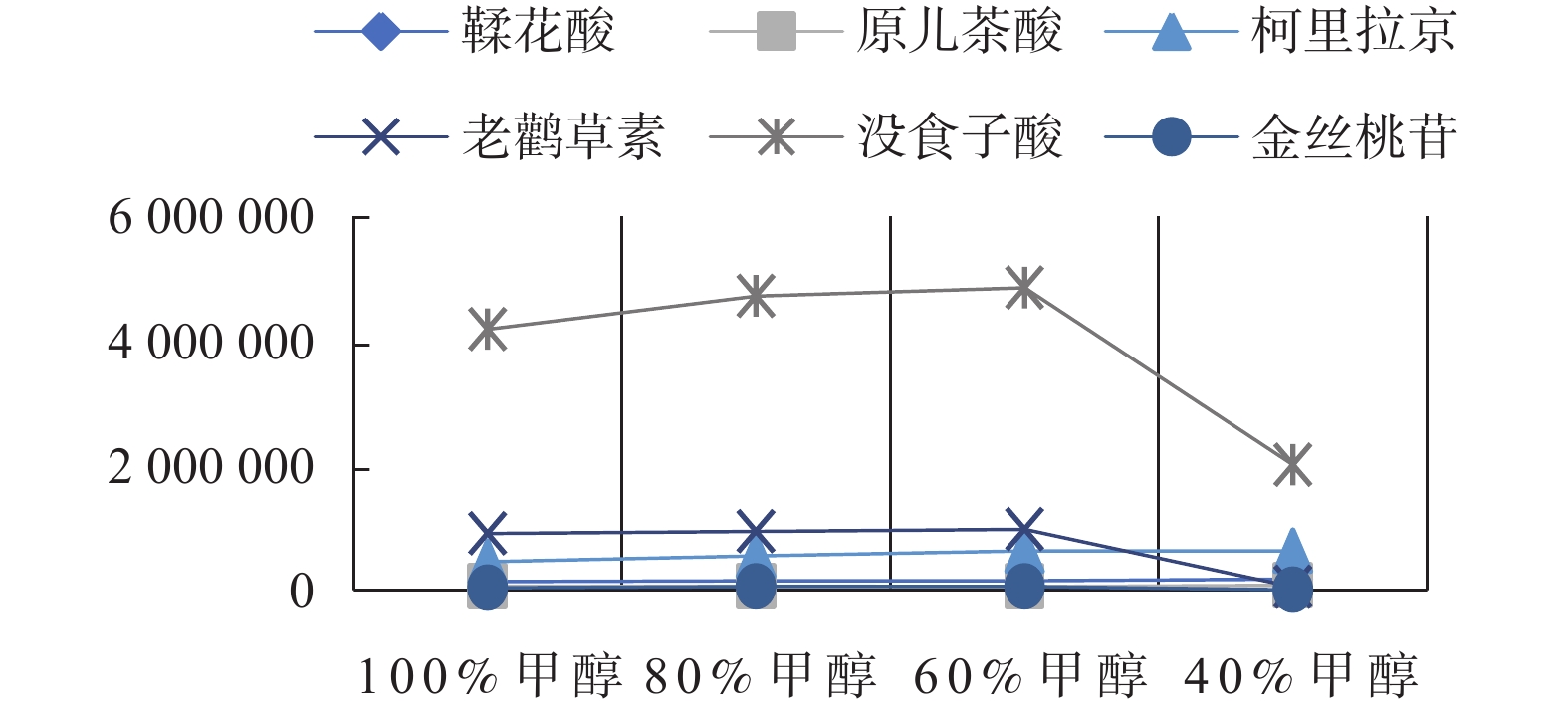
取5份老鹳草样品,每份约0.5 g,精密称定,分别置于5个具塞锥形瓶,分别加入体积分数为40%、60%、80%、80%、100%的甲醇,称重,超声30 min后,取出放冷用各自对应浓度甲醇补足减失重量,0.22 μm尼龙微孔滤头过滤,取续滤液进样分析,通过高效液相色谱仪记录老鹳草素、没食子酸、柯里拉京、金丝桃苷、鞣花酸、原儿茶酸的峰面积。
1.6 正交实验
依据单因素实验结果,以提取时间为A因素、甲醇体积分数为B因素、料液比为C因素,选取对没食子酸、老鹳草素、柯里拉京、金丝桃苷、鞣花酸、原儿茶酸的6种有效成分提取效果有意义的水平做正交实验,采用L9(34)正交表,得到老鹳草中6种有效成分的最佳提取工艺。
2. 结果
2.1 高效液相色谱
按照1.4项下操作,老鹳草供试品溶液各目标峰分离良好,其高效液相色谱图见图1,各对照品溶液保留时间及高效液相色谱图见图2~图7。
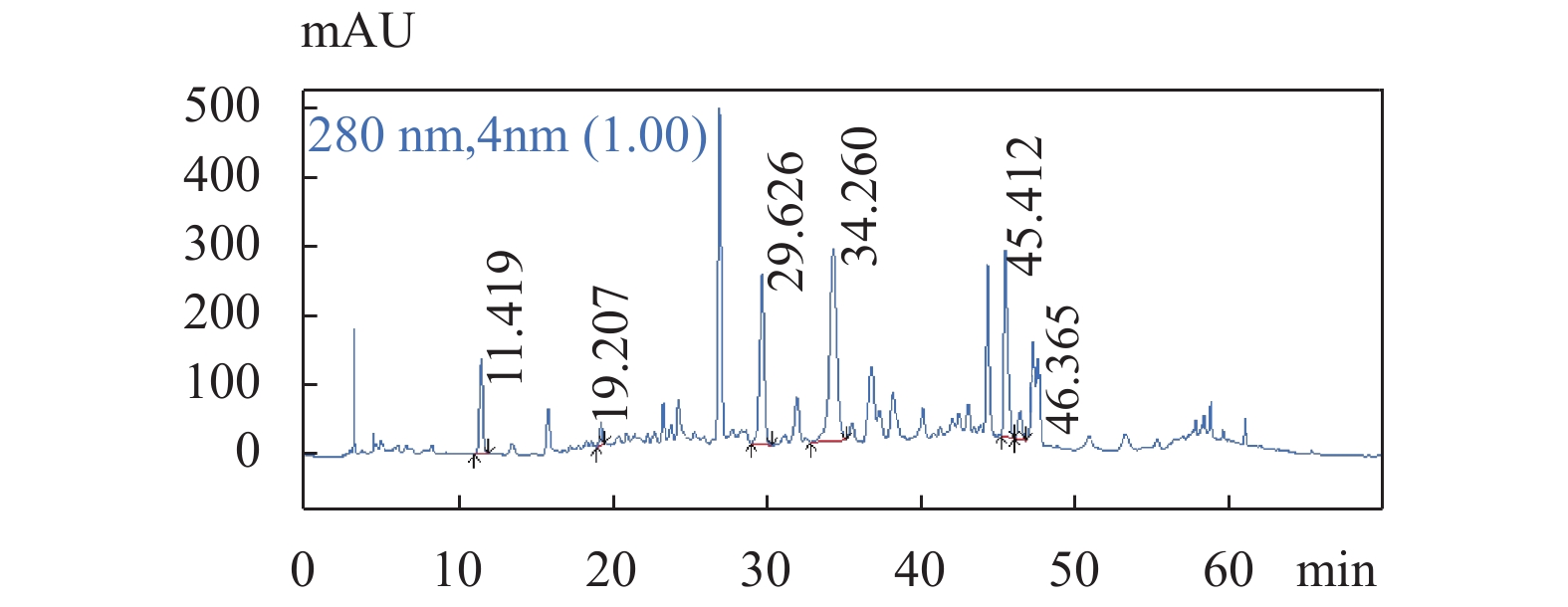 图 1 供试品溶液色谱图色谱条件:流速:1.0 mL/min;柱温:25 ℃;检测波长:280 nm;梯度洗脱程序见表1。Figure 1. The chromatogram of Geranium wilfordii Maxim
图 1 供试品溶液色谱图色谱条件:流速:1.0 mL/min;柱温:25 ℃;检测波长:280 nm;梯度洗脱程序见表1。Figure 1. The chromatogram of Geranium wilfordii Maxim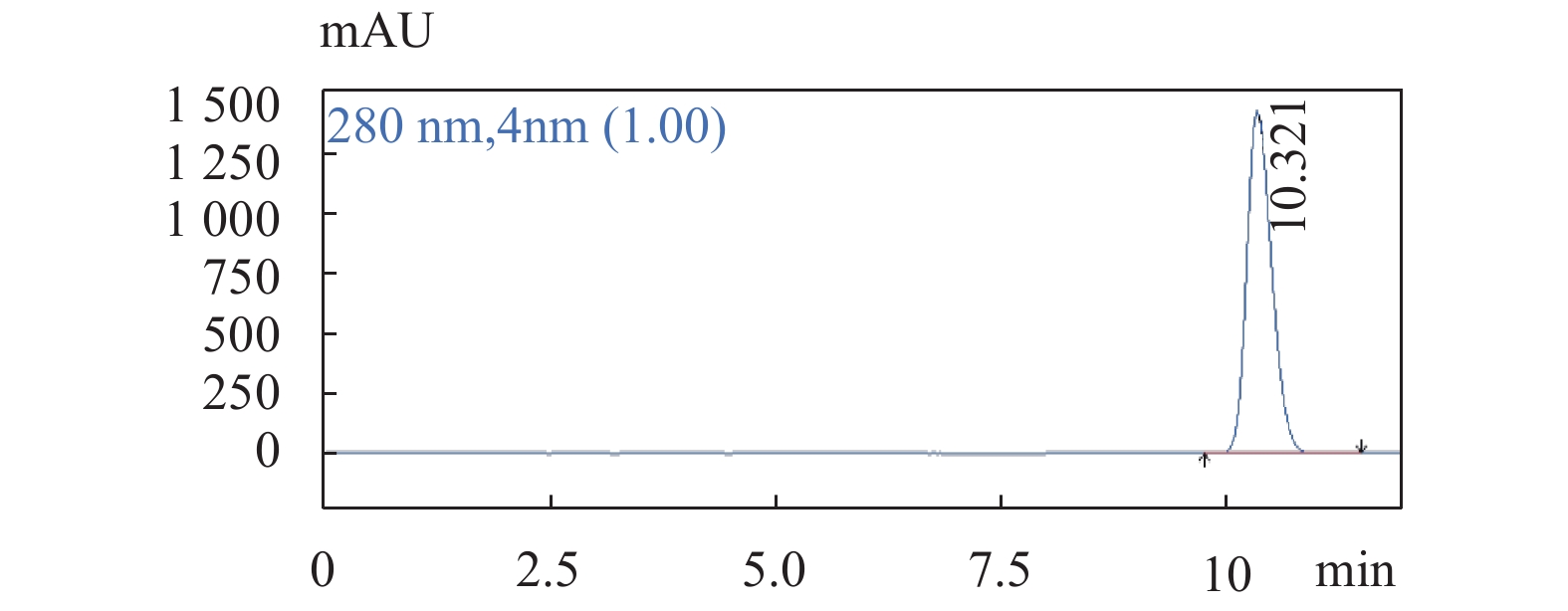 图 2 鞣花酸对照品溶液色谱图色谱条件:流速:1.0 mL/min;柱温:25 ℃;检测波长:280 nm;梯度洗脱程序见表1。Figure 2. The chromatogram of Ellagic acid
图 2 鞣花酸对照品溶液色谱图色谱条件:流速:1.0 mL/min;柱温:25 ℃;检测波长:280 nm;梯度洗脱程序见表1。Figure 2. The chromatogram of Ellagic acid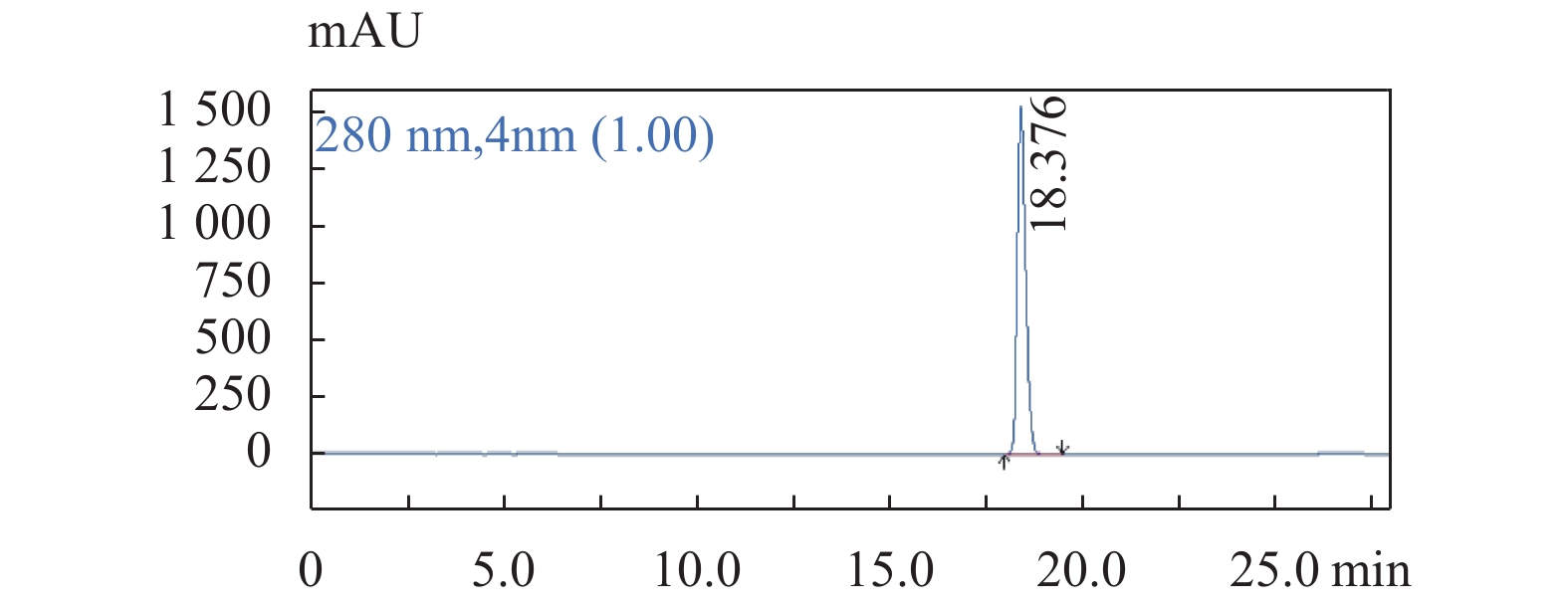 图 3 原儿茶酸对照品溶液色谱图色谱条件:流速:1.0 mL/min;柱温:25 ℃;检测波长:280 nm;梯度洗脱程序见表1。Figure 3. The chromatogram of Protocatechuic acid
图 3 原儿茶酸对照品溶液色谱图色谱条件:流速:1.0 mL/min;柱温:25 ℃;检测波长:280 nm;梯度洗脱程序见表1。Figure 3. The chromatogram of Protocatechuic acid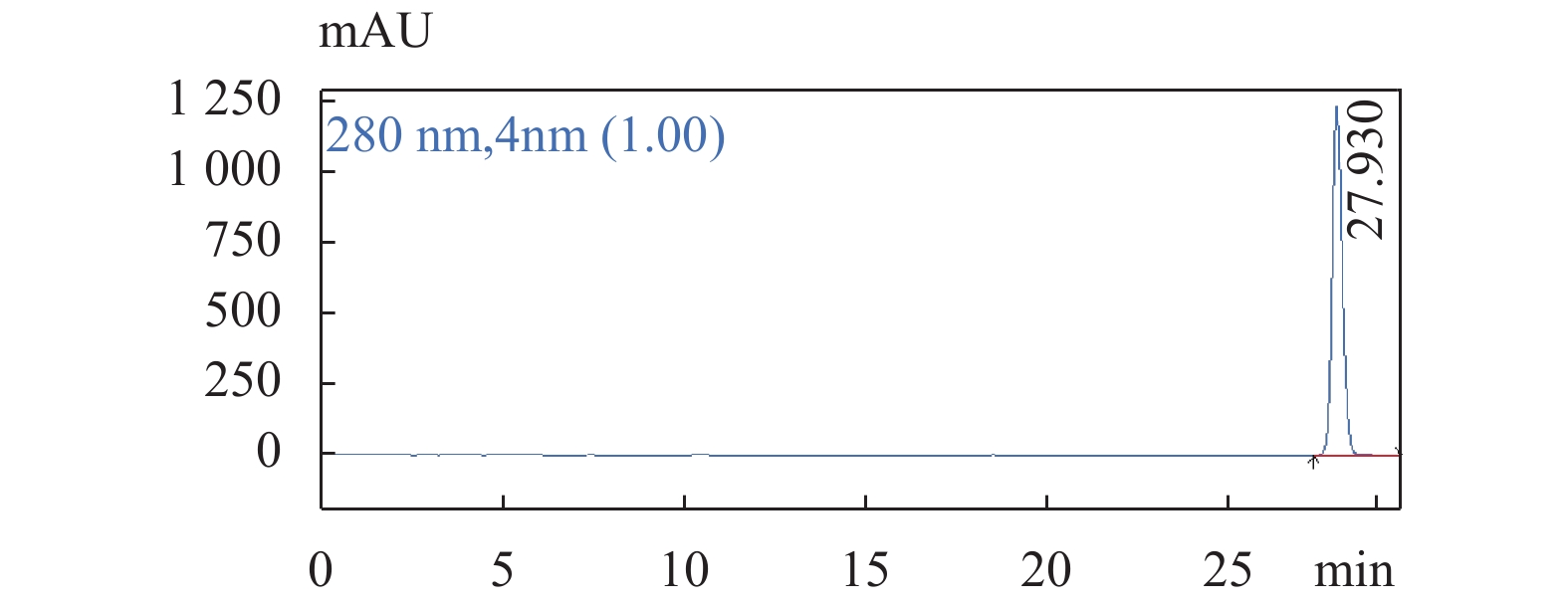 图 4 柯里拉京对照品溶液色谱图色谱条件:流速:1.0 mL/min;柱温:25 ℃;检测波长:280 nm;梯度洗脱程序见表1。Figure 4. The chromatogram of Corilagin
图 4 柯里拉京对照品溶液色谱图色谱条件:流速:1.0 mL/min;柱温:25 ℃;检测波长:280 nm;梯度洗脱程序见表1。Figure 4. The chromatogram of Corilagin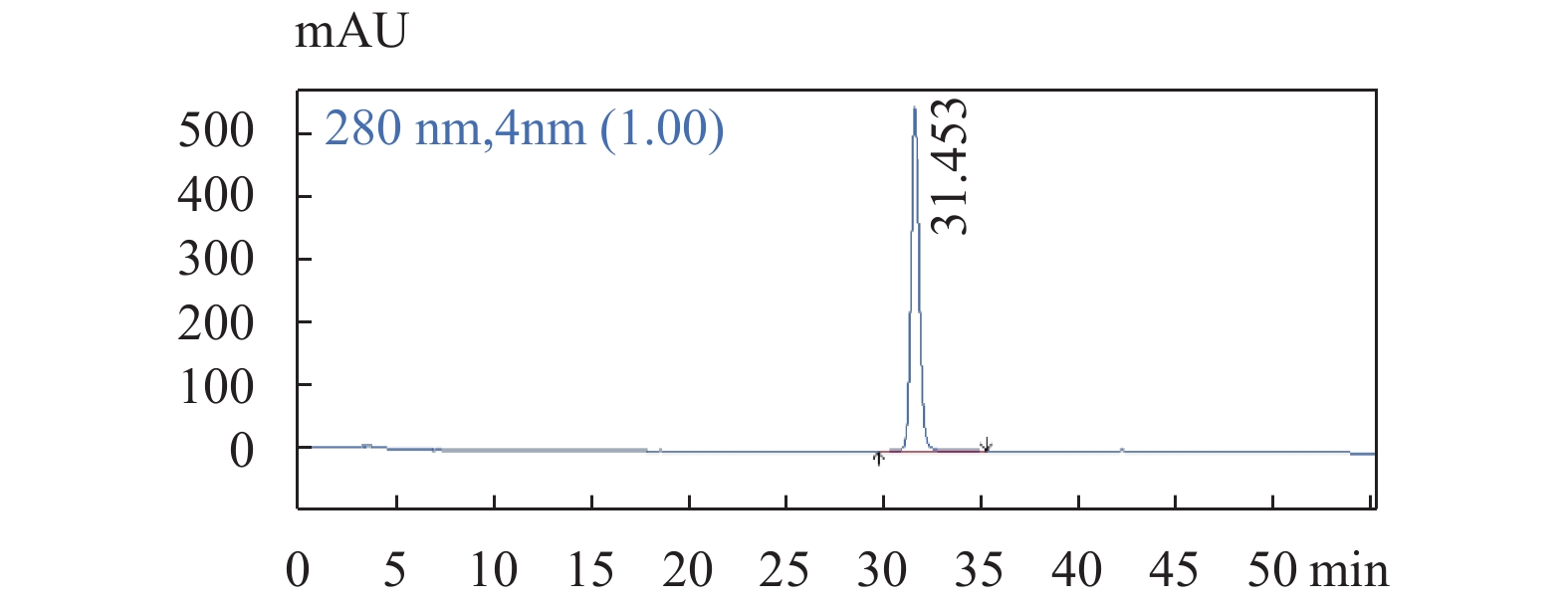 图 5 老鹳草素对照品溶液色谱图色谱条件:流速:1.0 mL/min;柱温:25 ℃;检测波长:280 nm;梯度洗脱程序见表1。Figure 5. The chromatogram of Geraniin
图 5 老鹳草素对照品溶液色谱图色谱条件:流速:1.0 mL/min;柱温:25 ℃;检测波长:280 nm;梯度洗脱程序见表1。Figure 5. The chromatogram of Geraniin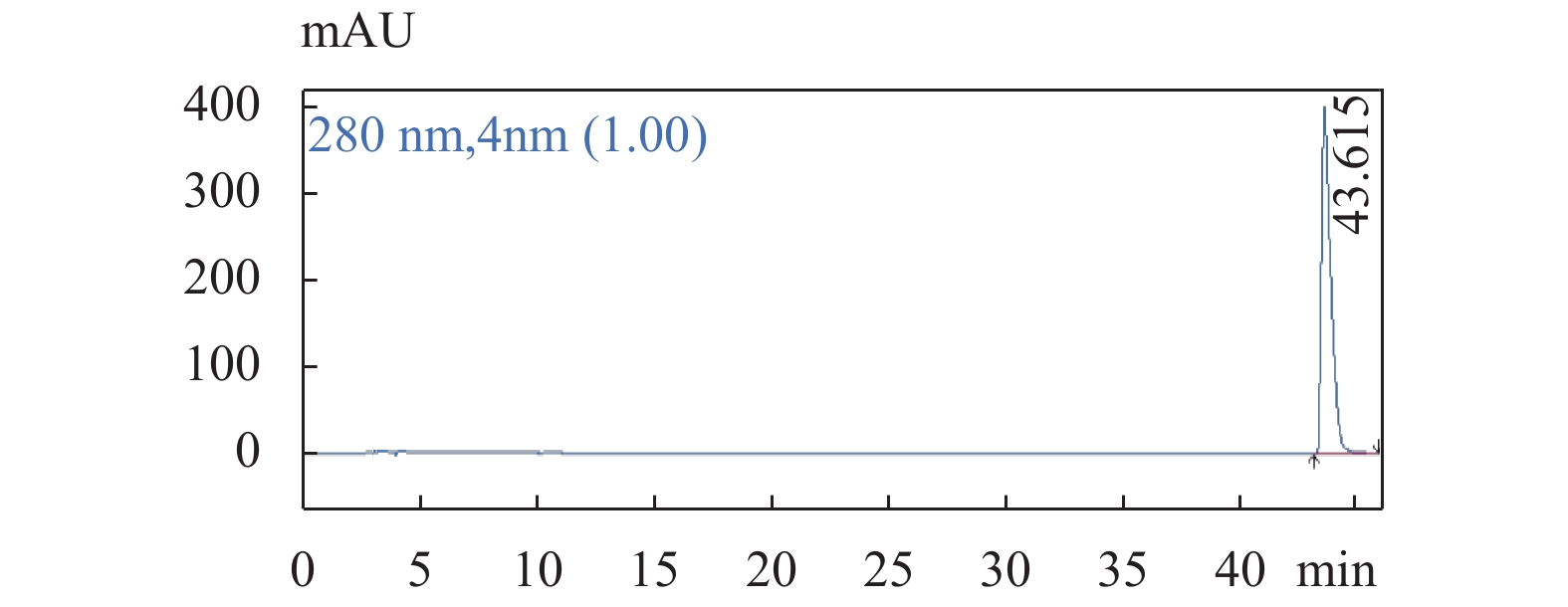 图 6 没食子酸对照品溶液色谱图色谱条件:流速:1.0 mL/min;柱温:25 ℃;检测波长:280 nm;梯度洗脱程序见表1。Figure 6. The chromatogram of Gallic acid
图 6 没食子酸对照品溶液色谱图色谱条件:流速:1.0 mL/min;柱温:25 ℃;检测波长:280 nm;梯度洗脱程序见表1。Figure 6. The chromatogram of Gallic acid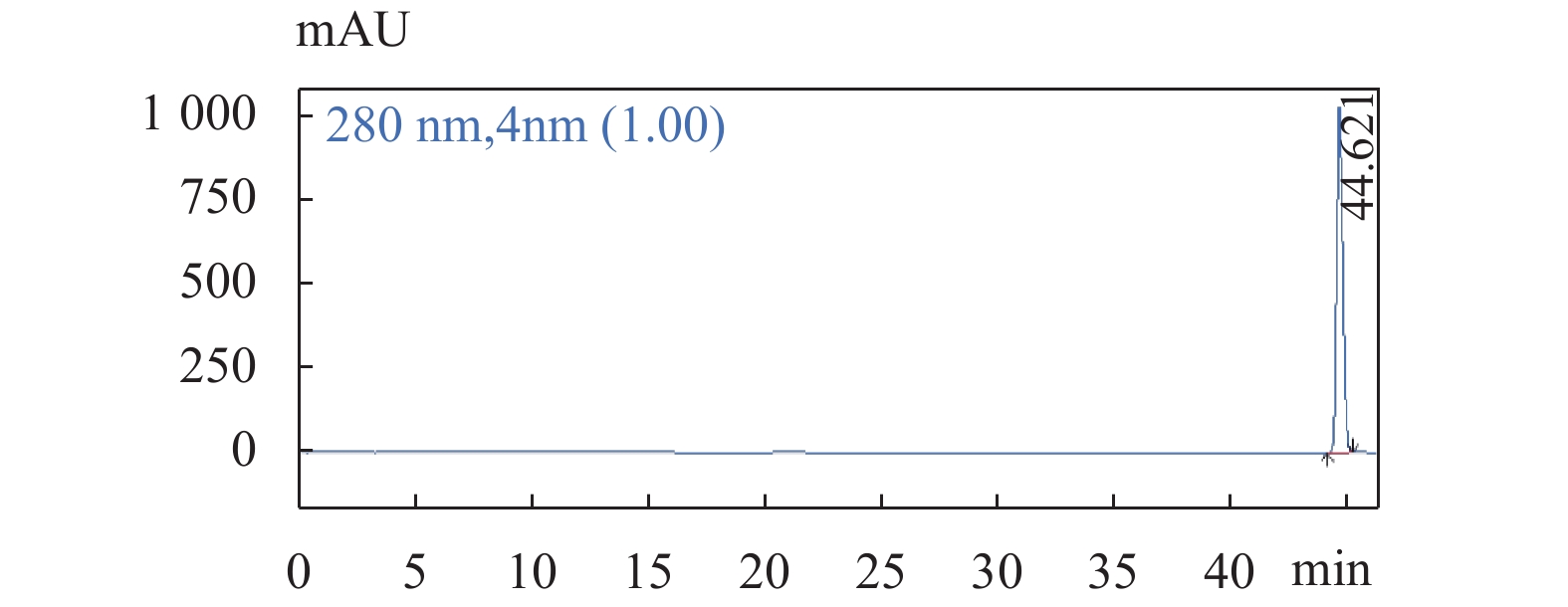 图 7 金丝桃苷对照品溶液色谱图色谱条件:流速:1.0 mL/min;柱温:25 ℃;检测波长:280 nm;梯度洗脱程序见表1。Figure 7. The chromatogram of Hyperoside
图 7 金丝桃苷对照品溶液色谱图色谱条件:流速:1.0 mL/min;柱温:25 ℃;检测波长:280 nm;梯度洗脱程序见表1。Figure 7. The chromatogram of Hyperoside2.2 单因素实验结果
2.2.1 料液比
按照1.5.1项下操作,通过高效液相色谱仪记录六种成分的峰面积,以料液比为横坐标,6种成分的峰面积为纵坐标作图8。在料液比1∶20(g/mL)之前,6种有效成分的峰面积随着料液比的增大而增大;在料液比1∶20(g/ mL)以后,6种有效成分的峰面积随着料液比的增大呈下降的趋势;因为料液比大于1∶10(g/ mL)时,溶液浓度很大导致滤头无法滤过滤过而影响实验结果,故不考虑选择;综合分析后选取1∶10(g/ mL)、1∶15(g/ mL)、1∶20(g/ mL)三个水平进行正交实验。
2.2.2 提取时间
按照1.5.2项下操作,通过高效液相色谱仪记录六种成分的峰面积,以提取时间为横坐标,6种成分的峰面积为纵坐标作图9。对于没食子酸的峰面积变化可以看出20 min至40 min的提取时间峰面积呈现增长的趋势,40 min至60 min后呈现下降的趋势;其他五种有效成分也呈现类似的趋势;因为60 min的提取时间过长且可能会导致有效成分的挥发而影响时间结果,故选取超声提取时间为20、30、40 min三个水平进行正交试验。
2.2.3 甲醇体积分数
按照1.5.3项下操作,通过高效液相色谱仪记录6种成分的峰面积,以甲醇体积分数为横坐标,6种成分的峰面积为纵坐标作图10。甲醇通过超声提取有强烈的挥发性,当甲醇溶剂体积分数为100%时,甲醇几乎全部挥发影响实验结果。40%以下的甲醇体积分数的峰面积呈现下降的趋势,导致提取物太少,故选择40%,60%和80%的3个水平的甲醇体积分数进行正交实验。
2.3 正交实验结果
依据2.2项下实验结果,以提取时间为A因素、甲醇体积分数为B因素、料液比为C因素,设计因素水平见表2。
通过表3老鹳草素的正交实验表可以看出:R1 > R3 > R2,3个因素对6种有效成分的作用大小为A > C > B(A为超声提取时间,B为甲醇体积分数,C为料液比),结合m值的比较可以得出老鹳草素的最佳提取条件为A3B2C1,即:提取时间40 min、料液比为1∶10(g/mL),甲醇体积分数为60%。
表 3 L9(34)正交试验表(老鹳草素)Table 3. The results of orthogonal experiment (Geraniin)试验 因素 老鹳草素
峰面积A B C 1 1 1 1 1103857 2 1 2 2 4837849 3 1 3 3 3661222 4 2 1 2 2185068 5 2 2 3 3675299 6 2 3 1 6156247 7 3 1 3 1361929 8 3 2 1 6736777 9 3 3 2 4823928 M1 9602928 4650854 13996881 T = 34542176 M2 12016614 15249925 11846845 y = 3838020 M3 12922634 14641397 8698450 m1 3200976 1550285 4665627 m2 4005538 5083308 3948948 m3 4307545 4880466 2899483 Rj R1 = 9346027 R2 = 3403080 R3 = 5942947 Sj S1 = 1963022889647 S2 = 23613509157143 S3 = 4734267721180 A:提取时间;B:料液比;C:溶剂体积分数; m1:实验因素一水平实验结果平均值;m2:实验因素二水平实验结果平均值;
m3:实验因素三水平实验结果平均值;Rj :M 值极差;Sj :反映了正交表上第 j 列所排因素的不同水平之间的差异程度,称 Sj 为第 j 列变差平方和;T:9 次实验结果之和;y:9 次实验结果平均值。在一定范围内,R值极差的大小与提取量的影响大小正比,m值大小与最佳提取条件因素成正比。通过表4没食子酸的正交实验表可以看出:R1 > R2 > R3,所以3个因素对提取量的影响大小为A > B > C,结合m值的比较可以得出没食子酸的最佳提取条件为A3B2C1,即:提取时间40 min、料液比为1∶10(g/mL),甲醇体积分数为60%。
表 4 L9(34)正交试验表(没食子酸)Table 4. The results of orthogonal experiment (Gallic acid)试验 因素 没食子酸
峰面积A B C 1 1 1 1 7314190 2 1 2 2 16280524 3 1 3 3 12562631 4 2 1 2 13938578 5 2 2 3 13910940 6 2 3 1 19201446 7 3 1 3 7694251 8 3 2 1 24710211 9 3 3 2 18204393 M1 36157345 28947018 51225846 T = 133817162 M2 47050964 54901675 48423495 y = 14868574 M3 50608854 49968469 34167822 m1 12052448 9649006 17075282 m2 15683655 18300558 16141165 m3 16869618 16656156 11389274 Rj R1 = 22278828 R2 = 7850711 R3 = 5480344 Sj S1 = 37797292060705 S2 = 126653568075958 S3 = 55783729621634 通过表5柯里拉京的正交实验表可以看出:R3 > R1 > R2,所以3个因素对提取量的影响大小为C > A > B,结合m值的比较可以得出柯里拉京的最佳提取条件为A3B2C1,即:提取时间40 min、料液比为1∶10(g/mL),甲醇体积分数为60%。
表 5 L9(34)正交试验表(柯里拉京)Table 5. The results of orthogonal experiment (Corilagin)试验 因素 柯里拉京
峰面积A B C 1 1 1 1 1904530 2 1 2 2 3202766 3 1 3 3 1869245 4 2 1 2 3343529 5 2 2 3 2315514 6 2 3 1 3106967 7 3 1 3 2468583 8 3 2 1 4962727 9 3 3 2 2490196 M1 6976540 7716642 9974224 T = 25664055 M2 8766010 10481006 9036490 y = 2851562 M3 9921506 7466408 6653342 m1 2325513 2572214 3324741 m2 2922003 3493669 3012163 m3 3307169 2488803 2217781 Rj R1 = 2997684 R2 = 1714997 R3 = 3268164 Sj S1 = 14677993215 S2 = 18657924733 S3 = 1954110905333 通过表6金丝桃苷的正交实验表可以看出,R1 > R3 > R2,所以3个因素对提取量的影响大小为A > C > B,结合m值的比较可以得出金丝桃苷的最佳提取条件为A3B2C1,即:提取时间40 min、料液比为1∶10(g/mL),甲醇体积分数为60%。
表 6 L9(34)正交试验表(金丝桃苷)Table 6. The results of orthogonal experiment (Hyperoside)试验 因素 金丝桃苷
峰面积A B C 1 1 1 1 477208 2 1 2 2 482219 3 1 3 3 332298 4 2 1 2 553354 5 2 2 3 325225 6 2 3 1 640846 7 3 1 3 402090 8 3 2 1 716723 9 3 3 2 450258 M1 901338 364870 1480455 T = 3378937 M2 1107751 1535964 1187781 y = 375437 M3 1369849 1478104 710701 m1 300446 121623 493485 m2 369250 511988 395927 m3 456616 492701 236900 Rj R1 = 1115586 R2 = 428214 R3 = 767403 Sj S1 = 36756108434 S2 = 290455567127 S3 = 100642735244 通过表7鞣花酸的正交实验表可以看出:R1 > R3 > R2,所以3个因素对提取量的影响大小为A > C > B,结合m值的比较可以得出鞣花酸的最佳提取条件为A1B1C 1,即:提取时间20 min、料液比为1∶10(g/mL),甲醇体积分数为40%。
表 7 L9(34)正交试验表(鞣花酸)Table 7. The results of orthogonal experiment (Ellagic acid)试验 因素 鞣花酸
峰面积A B C 1 1 1 1 2275146 2 1 2 2 1022426 3 1 3 3 716270 4 2 1 2 1485928 5 2 2 3 704427 6 2 3 1 1477116 7 3 1 3 868155 8 3 2 1 1545919 9 3 3 2 1044160 M1 4013842 4629228 5298180 T = 11139544 M2 3667471 3272771 3552514 y = 1237727.1 M3 3458233 3237545 2288851 m1 1337947 1543076 1766060 m2 1222490 1090924 1184171 m3 1152744 1079182 762950 Rj R1 = 428113 R2 = 131567 R3 = 389794 Sj S1 = 52494689151 S2 = 419777559602 S3 = 1522250079819 通过表8原儿茶酸的正交实验表可以看出:R1 > R3 > R2,所以3个因素对提取量的影响大小为A > C > B,结合m值的比较可以得出原儿茶酸的最佳提取条件为A3B2C1,即:提取时间40 min、料液比为1∶10(g/mL),甲醇体积分数为60%。
表 8 L9(34)正交试验表(原儿茶酸)Table 8. The results of orthogonal experiment (Protocatechuic acid)试验 因素 原儿茶酸
峰面积A B C 1 1 1 1 477208 2 1 2 2 482219 3 1 3 3 332298 4 2 1 2 553354 5 2 2 3 325225 6 2 3 1 640846 7 3 1 3 402090 8 3 2 1 716723 9 3 3 2 450258 M1 1291724 1432652 1834777 T = 4380219 M2 1519425 1524167 1485830 y = 486691 M3 1569071 1423401 1059613 m1 430575 477551 611592 m2 506475 508056 495277 m3 523024 474467 353204 Rj R1 = 543053 R2 = 38337 R3 = 509458 Sj S1 = 14581556354 S2 = 2068250330 S3 = 100478249341 3. 讨论
本实验选用了超声提取法[11-12]对老鹳草中的老鹳草素、没食子酸、柯里拉京、金丝桃苷、鞣花酸、原儿茶酸六种物质进行提取分析,此种方法具有提取效率高、提取时间短、等优点[13-15]。
试验结果表明,在提取过程中,超声提取时间、料液比值、溶剂体积分数、3个因素对提取工艺均有影响。采用L9(34)正交试验设计,根据试验及实际情况,综合六种有效成分的最佳提取条件为:提取时间40 min、料液比为1∶10(g/mL),甲醇体积分数为60%。
-
表 1 87例入组人群的一般临床资料( $\bar x \pm s $)
Table 1. General clinical information of the 87 enrolled cases ( $\bar x \pm s $)
项目 正常(n = 41) 结直肠腺瘤(n = 21) 结直肠癌(n = 25) F/χ2 P 性别(男/女) 36/5 18/3 17/8 4.369 0.113* 年龄(岁) 42.17 ± 13.54 42.38 ± 14.19 54.24 ± 11.79 7.330 0.001* SDC2甲基化检测
(阳性/阴性)2/39 12/9 21/4 43.727 <0.001* *P < 0.05。 表 2 粪便SDC2基因甲基化在结直肠腺瘤和结直肠癌中的表达情况比较( $\bar x \pm s $)
Table 2. Comparison of fecal SDC2 gene methylation expression in colorectal adenoma and colorectal cancer ( $\bar x \pm s $)
项目 正常(n = 41) 结直肠腺瘤(n = 21) 结直肠癌(n = 25) F P Ct值 40.15 ± 1.24 38.19 ± 2.94 36.62 ± 2.47 21.928 < 0.001* *P < 0.05。 表 3 粪便SDC2基因甲基化检测结直肠腺瘤和结直肠癌的阳性率[(n)%]
Table 3. Positive fecal SDC2 gene methylation for detection of colorectal adenoma and colorectal cancer [(n)%]
SDC2甲基化检测 结直肠镜(金标准) 合计 χ2 P 结直肠腺瘤 结直肠癌 阳性 12(57.14) 21(84.00) 33(71.74) 4.060 0.044* 阴性 9(42.86) 4(16.00) 13(28.26) 合计 21 25 46 *P < 0.05。 表 4 粪便SDC2基因甲基化在结直肠腺瘤和结直肠癌中的诊断价值
Table 4. Diagnostic value of fecal SDC2 gene methylation in colorectal adenoma and colorectal cancer
项目 AUC(95%CI) Cutoff值(%) 灵敏度(%) 特异度(%) 约登指数 P SDC基因甲基化 结直肠腺瘤 0.715(0.587~0.823) 37.63 57.14 95.12 0.523 0.010* 结直肠癌 0.918(0.824~0.971) 38.89 88.00 92.68 0.807 < 0.001* *P < 0.05。 -
[1] Wong M C S,Huang J,Lok V,et al. Differences in incidence and mortality trends of colorectal cancer worldwide based on sex,age,and anatomic location[J]. Clinical Gastroenterology and Hepatology,2020,19(5):955-966. [2] Sung H,Ferlay J,Siegal R L,et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA:A Cancer Journal for Clinicians,2021,71(3):209-249. doi: 10.3322/caac.21660 [3] Huybrechts I,Kliemann N,Perol O,et al. Feasibility study to assess the impact of a lifestyle intervention during colorectal cancer screening in France[J]. Nutrients,2021,13(11):3685. doi: 10.3390/nu13113685 [4] Edwardson N,Cartwright K,Sheche J,et al. Colorectal cancer screening among adults in Zuni Pueblo: Factors associated with FOBT and colonoscopy utilization[J]. J Community Health,2023,48(1):1-11. [5] Luo L,Liu Y,Zhang L,et al. Optimizing bowel preparation for colonoscopy: A cross-sectional study of the Chinese population[J]. Front Public Health,2022,10(1):953441. doi: 10.3389/fpubh.2022.953441 [6] Agrawal R,Majeed M,Attar B M,et al. Predictors of poor bowel preparations and colonoscopy cancellations in inpatient colonoscopies,a single center retrospective study[J]. Transl Gastroenterol Hepatol,2022,7(1):4. doi: 10.21037/tgh.2020.02.13 [7] 刘宇英,魏君丽,李艳红,等. 结直肠镜漏诊结直肠癌情况一致性的评价研究[J]. 中华疾病控制杂志,2020,24(8):961-964. doi: 10.16462/j.cnki.zhjbkz.2020.08.019 [8] Imperiale T F,Gruber R N,Stump T E,et al. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: A systematic review and meta-analysis[J]. Annals of Internal Medicine,2019,170(5):319-329. doi: 10.7326/M18-2390 [9] Duran-Sanchon S,Herrera-Pariente C,Moreira L. New non-invasive biomarkers for colorectal cancer screening[J]. Rev Esp Enferm Dig,2020,112(8):642-648. [10] Nagtegaal I D,Odze R D,Klimstra D,et al. The 2019 WHO classification of tumours of the digestive system[J]. Histopathology,2020,76(2):182-188. doi: 10.1111/his.13975 [11] Kværner A S,Birkeland E,Bucher-Johannessen C,et al. The CRCbiome study: A large prospective cohort study examining the role of lifestyle and the gut microbiome in colorectal cancer screening participants[J]. BMC Cancer,2021,21(1):930. doi: 10.1186/s12885-021-08640-8 [12] 夏晨静,陈志荣. 粪便DNA甲基化检测对结直肠癌诊断及预后评估临床价值研究进展[J]. 临床军医杂志,2019,47(6):657-659. doi: 10.16680/j.1671-3826.2019.06.45 [13] Shaukat A,Kahi C J,Burke C A,et al. ACG clinical guidelines: Colorectal cancer screening 2021[J]. American Journal of Gastroenterology,2021,116(3):458-479. doi: 10.14309/ajg.0000000000001122 [14] Ahnen D J,Patel S G. Cost-effectiveness and national effects of initiating colorectal cancer screening for average-risk persons at age 45 years instead of 50 years[J]. Gastroenterology,2019,157(6):1691-1692. doi: 10.1053/j.gastro.2019.04.056 [15] Zhong G C,Sun W P,Wan L,et al. Efficacy and cost-effectiveness of fecal immunochemical test versus colonoscopy in colorectal cancer screening: A systematic review and meta-analysis[J]. Gastrointestinal Endoscopy,2020,91(3):684-697. doi: 10.1016/j.gie.2019.11.035 [16] Chan S C H,Liang J Q. Advances in tests for colorectal cancer screening and diagnosis[J]. Expert Review of Molecular Diagnostics,2022,22(4):449-460. doi: 10.1080/14737159.2022.2065197 [17] Zhang J,Yang C,Wu C,et al. DNA Methyltransferases in cancer: Biology,paradox,aberrations,and targeted therapy[J]. Cancers,2020,12(8):2123. doi: 10.3390/cancers12082123 [18] Fatemi N,Tierling S,Es H A,et al. DNA methylation biomarkers in colorectal cancer: Clinical applications for precision medicine[J]. International Journal of Cancer,2022,151(12):2068-2081. doi: 10.1002/ijc.34186 [19] Hua R,Yu J,Yan X,et al. Syndecan-2 in colorectal cancer plays oncogenic role via epithelial-mesenchymal transition and MAPK pathway[J]. Biomed Pharmacother,2020,121(1):109630. doi: 10.1016/j.biopha.2019.109630 [20] Wang L,Liu Y,Zhang D,et al. Diagnostic accuracy of DNA-based SDC2 methylation test in colorectal cancer screening: A meta-analysis[J]. BMC Gastroenterol,2022,22(1):314. doi: 10.1186/s12876-022-02395-7 [21] Han Y D,Oh T J,Chung T H,et al. Early detection of colorectal cancer based on presence of methylated syndecan-2 (SDC2) in stool DNA[J]. Clin Epigenetics,2019,11(1):51. doi: 10.1186/s13148-019-0642-0 [22] Wang J,Liu S,Wang H,et al. Robust performance of a novel stool DNA test of methylated SDC2 for colorectal cancer detection: A multicenter clinical study[J]. Clinical Epigenetics,2020,12(1):162. doi: 10.1186/s13148-020-00954-x [23] Syed A R,Thakkar P,Horne Z D,et al. Old vs new: Risk factors predicting early onset colorectal cancer[J]. World Journal of Gastrointestinal Oncology,2019,11(11):1011-1020. doi: 10.4251/wjgo.v11.i11.1011 期刊类型引用(2)
1. 汪鑫邦,胡安,赵喜鹏,汪炎,丁伟,操礼鹏,古禹,许轲,张云峰. Ommaya囊治疗脑出血并发急性期脑积水疗效及对血脑屏障功能的影响. 昆明医科大学学报. 2024(10): 96-104 .  本站查看
本站查看2. 黄连飘,农晓妮,王柳宁,杨巧妙,何华伟. 中枢神经系统结核病Ommaya储液囊植入术的护理观察. 名医. 2022(04): 105-107 .  百度学术
百度学术其他类型引用(1)
-






 下载:
下载:
 下载:
下载: