Protective Effect of Corilagin on OX-LDL-Induced HUVECs Cell Damage and Its Impact on the Expression of the MyD88 Signaling Pathway
-
摘要:
目的 探讨不同浓度和时间的柯里拉京(Corilagin)处理对氧化性低密度脂蛋白(oxidative low-density lipoprotein,ox-LDL)诱导人脐静脉内皮细胞(human umbilical vein endothelial cells,HUVECs)损伤的保护作用,以及其对MyD88信号通路表达调控的影响。 方法 体外培养HUVECs复制ox-LDL诱导损伤模型,利用形态学及免疫学方法鉴定HUVECs细胞,MTT法确定复制ox-LDL损伤HUVECs模型的最佳条件,根据不同处理将细胞分为正常组(Normal)、模型组(Model)、Corilagin(3.125、6.25、12.5、25、50 µmol/L)组、阳性对照(VE 10 µmol/L、Simvastain 1 µmol/L)组。观察Corilagin对ox-LDL诱导损伤的HUVECs保护作用;Western blot和RT-qPCR 方法检测HUVECs细胞中MyD88、P65、TNF-α、MCP-1表达变化。 结果 经形态学及免疫学方法鉴定,所培养细胞为HUVECs;70 mg/L的ox-LDL刺激12 h是复制ox-LDL损伤HUVECs模型的最佳条件;MTT结果显示,与ox-LDL组比较,Corilagin组细胞活力明显升高(P < 0.01);RT-qPCR和Western blot结果表明,与Normal组比,ox-LDL组MyD88、P65、TNF-α及MCP-1的mRNA和蛋白表达升高(P < 0.01);与ox-LDL组比较,Corilagin组及阳性对照组的MyD88、P65、TNF-α及MCP-1的mRNA和蛋白表达降低(P < 0.01),且Corilagin组呈现剂量依赖下调MyD88、P65、TNF-α及MCP-1的mRNA和蛋白表达。 结论 随着时间和浓度的增加,Corilagin能明显提高ox-LDL诱导损伤的HUVECs细胞活力,其保护作用与抑制MyD88信号通路有关。 -
关键词:
- Corilagin /
- 人脐静脉内皮细胞HUVECs /
- 氧化型低密度脂蛋白 /
- 动脉粥样硬化
Abstract:Objective To investigate the protective effects of different concentrations and durations of corilagin treatment on oxidative low-density lipoprotein (ox-LDL)-induced damage in human umbilical vein endothelial cells (HUVECs), as well as its effects on the expression regulation of the MyD88 signaling pathway. Methods In vitro cultured HUVECs were used to replicate the ox-LDL-induced damage model. Morphological and immunological methods were employed to identify HUVECs cells. The MTT assay was used to determine the optimal conditions for replicating the ox-LDL-induced damage model in HUVECs. Cells were divided into different groups based on different treatments: normal group, model group, Corilagin (3.125, 6.25, 12.5, 25, 50 µmol/L) group, and positive control (VE 10 µmol/L, Simvastatin 1 µmol/L) group. The protective effect of corilagin on ox-LDL-induced damage in HUVECs was observed. Western blot and RT-qPCR methods were used to detect the expression changes of MyD88, P65, TNF-α, and MCP-1 in HUVECs cells. Results The cultured cells were confirmed as HUVECs by using morphological and immunological methods. The best condition for replicating ox-LDL-induced damage to HUVECs was 12 hours of treatment with 70 mg/L of ox-LDL. MTT results showed that compared with the ox-LDL group, the corilagin group had a significantly higher cell viability (P < 0.01). RT-qPCR and Western blot results showed that compared with the control group, the mRNA and protein expression of MyD88, P65, TNF-α, and MCP-1 were increased in the ox-LDL group (P < 0.01). Compared with the ox-LDL group, the mRNA and protein expression of MyD88, P65, TNF-α, and MCP-1 were decreased (P < 0.01) in the corilagin group and positive control group, and the corilagin group showed a dose-dependent downregulation of the mRNA and protein expression of MyD88, P65, TNF-α, and MCP-1. Conclusion With the increase of time and concentration, corilagin can significantly improve the cell viability of HUVECs induced by ox-LDL damage, and its protective effect is associated with the inhibition of the MyD88 signaling pathway. -
Key words:
- Corilagin /
- HUVECs /
- Ox-LDL /
- Atherosclerosis
-
动脉粥样硬化(atherosclerosis,AS)是一种以大动脉壁聚集脂质沉积物为主要特征的慢性炎症性疾病,其发病机理是一个复杂的级联反应,ox-LDL的形成和摄取是AS进展的关键步骤[1]。在氧化环境中,低密度脂蛋白(low density lipoprotein,LDL)被氧化修饰为氧化型LDL (oxidized low density lipoprotein,ox-LDL),ox-LDL会与内皮细胞以及单核细胞/巨噬细胞上的多种清道夫受体(scavenger receptors,SRs)结合,最终导致在狭窄的动脉粥样硬化动脉中形成富含血小板和白细胞的凝块[2]。
蛋白质髓样分化初级反应蛋白88 (myeloid differentiation primary response 88,MyD88)是一种重要的衔接分子,通过下调TLR-4/MyD88/P65表达,可减轻单核细胞趋化蛋白-1(monocyte chemoattractant protein-1,MCP-1)及TNF-α介导的内皮细胞凋亡和动脉粥样硬化进展[3-7]。因此,MyD88信号通路在AS发生发展过程中扮演着重要的调控作用。
AS已经成为威胁人类生命健康的主要疾病之一,目前临床仍缺乏有效的治疗药物[8]。虽然应用他汀类为代表的调血脂药取得了一定疗效,但其严重的不良反应限制了其发展。因此,从天然产物中寻找治疗AS的潜在药物具有重要意义。Corilagin是一种从Phyllanthus urinaria L.中分离的天然化合物,具有多种药理作用[9]。研究发现Corilagin在体内外均能抑制ox-LDL诱导的血管平滑肌细胞的增殖和迁移[10],还可降低ox-LDL诱导的HUVECs的LOX-1表达水平[11-12],提示其具有良好的抗AS作用。本研究将通过MyD88信号通路进一步探讨Corilagin干预对ox-LDL诱导损伤的HUVECs细胞的保护作用。
1. 材料与方法
1.1 研究材料
1.1.1 细胞
人脐静脉内皮细胞(HUVECs),购自中国典型物保藏中心。
1.1.2 药物
Corilagin由中科院昆明植物研究所提供,结构式见图1,分子量为634.46,纯度为99.5%,黄褐色粉状物,避光密封4℃保存。辛伐他汀原料药(Q12VK-FO),购自Sigma,分子式C25H38O5,分子量418.57,纯度 > 99%,防潮低温避光保存。维生素E (Vit E)原料药(T-438),购自Sigma,分子式为C29H50O2,分子量为430.71,纯度 > 99%,白色粉末,-20℃保存。
1.1.3 主要试剂
OX -LDL购自广州奕源生物科技有限公司,MTT-705B054购自Solarbio公司,BCA蛋白浓度测定试剂盒购自碧云天公司,TRNzol-A+总RNA提取试剂裂解液,Golden Taq PCR试剂盒,TIANScript M-MLV第一链cDNA合成试剂盒购自Fermentas公司,一抗β-actin购自SAB公司,MYD88(1∶1000),P65(1∶500),TNF-α(1:500),MCP-1(1∶1000)购自爱博泰克生物科技有限公司。
1.2 研究方法
1.2.1 药液配制
Corilagin药液:用PBS溶液配制成2 mmol/L的Corilagin母液。辛伐他汀药液:用PBS溶液配制成10000 µmol/L的辛伐他汀母液。维生素E药液:用PBS溶液配制成10000 µmol/L的维生素E母液。药液均临用前稀释成所需浓度。
1.2.2 细胞鉴定
(1)显微镜观察法:HUVECs的细胞形态于倒置相差显微镜下观察并拍照;(2)免疫组织化学法:将消毒灭菌后的0.5 cm×0.6 cm盖玻片放置在6孔培养板内,并将HUVECs浓度调整为1×105 个/mL,按2 mL/孔接种于6孔板内。培养至HUVECs 融合80%~90%后,取出盖玻片。检测HUVECs的VIII因子、CD34的表达,分别分为实验组和阴性对照组2组,每组设3个复孔,按照免疫组化流程处理后,于荧光显微镜下观察,并拍照保存。
1.2.3 MTT实验方法
将计数后的上述HUVECs接种于96孔板上,加入MTT(5 mg/mL)孵育4 h,再用三联液孵育8 h后,测定570 nm和630 nm处的OD值。
1.2.4 复制ox-LDL诱导HUVECs细胞损伤
用含EDTA的胰蛋白酶消化HUVECs,经细胞计数调整浓度为5×104个/mL,100 μL/孔接种到96孔板内,培养24 h。每次种板4块,分别用于探索HUVECs的损伤时间:6 h、12 h、24 h、48 h,实验重复3次。每块板设正常对照组和ox-LDL组。正常对照组:加入等量的PBS;ox-LDL组:分别加入终浓度为10 mg/L、20 mg/L、30 mg/L、40 mg/L、50 mg/L、60 mg/L、70 mg/L、80 mg/L的ox-LDL。应用改良MTT法测定不同损伤浓度及时间条件下ox-LDL对HUVECs的损伤程度,以确定ox-LDL损伤HUVEC的适宜浓度及时间。
1.2.5 Corilagin对ox-LDL诱导损伤的人脐静脉内皮细胞HUVECs保护作用观察
用含EDTA的胰蛋白酶消化HUVECs,经细胞计数调整浓度为5×104个/mL,并以100 μL/孔接种到96孔板内,培养24 h。每次种板6块,重复3次,每块板上设9个组:正常组;模型组;阳性药组:(Vit E:10 μmol/L、辛伐他汀:1 μmol/L);Corilagin给药组(3.125、6.25、12.5、25、50 μmol/L)。按照分组进行实验,根据改良MTT法测定细胞活力,评价Corilagin对ox-LDL损伤HUVECs的保护作用及其量-效/时-效关系。
1.2.6 RT-qPCR 检测ox-LDL损伤的HUVECs中MyD88、P65、TNF-α、MCP-1 mRNA的表达
将细胞分成正常组,模型组,阳性药组(Vit E:10 μmol/L、辛伐他汀:1 μmol/L);Corilagin组:Corilagin(3.125、6.25、12.5、25、50 μmol/L)一共9个组。孵育24 h,收集细胞提取总RNA进行RT-qPCR定量检测,引物序列见表1。
表 1 引物序列Table 1. Primer sequences基因名 引物序列 长度(bp) β-actin F-CGTGCGTGACATCAAAGAGA 178 R-CAAGAAGGAAGGCTGGAAAA MyD88 F-CTGGGGGCACTGTGGATT 174 R-CGCTGCTGGGGAGAAAAC P65 F-CAACCAAAACAGAGGGGATT 160 R-TTGTGACCAACTGAACGATA TNF-α F-CTCCTCACCCACACCGTC 226 R-AACACCCATTCCCTTCAC MCP-1 F-TGACCCCAAGAAGGAATG 180 R-GAGGTGGTTGTGGAAAAG 1.2.7 Western blot检测ox-LDL损伤的HUVECs及VSMCs中MyD88、P65、TNF-α、MCP-1蛋白的表达
实验分组同1.2.6,细胞培养24 h后,按照分组加入对应的药物浓度,孵育24 h,所有给药组给予70 mg/L的ox-LDL,刺激12 h,将各组细胞分别置于冰上充分研磨后提取总蛋白,总蛋白浓度用BCA蛋白检测试剂盒测定。蛋白质样品用12%、15%十二烷基硫酸钠-聚丙烯酰胺凝胶电泳分离,然后转移到聚偏二氟乙烯膜。用无蛋白快速封闭液封闭膜20 min。将PVDF膜与一抗在4℃孵育过夜,用TBST洗涤3次后与二抗孵育2 h。TBST洗涤3次,用Bio-Rad凝胶成像系统显影、收集和分析靶蛋白的图像,采用ImageJ软件统计数据。
1.3 统计学处理
实验数据采用“均值±标准差(
$\bar x \pm s $ )”表示,并使用SPSS软件进行统计学分析。多组数据间的比较使用One-way ANOVA单因素方差分析法。方差齐时,使用LSD法进行组间两两比较,P < 0.05时各组间差异具有统计学意义。2. 结果
2.1 HUVECs的鉴定
2.1.1 形态学鉴定
倒置显微镜下显示,HUVECs细胞呈扁平的卵圆形、梭形、多角形,细胞均匀透亮,边界清晰,胞核清晰,呈圆形或椭圆形,有2~3个核仁。胞浆内含小颗粒,结构完整,符合血管内皮细胞的基本形态,见图2A~2B。
2.1.2 免疫学鉴定
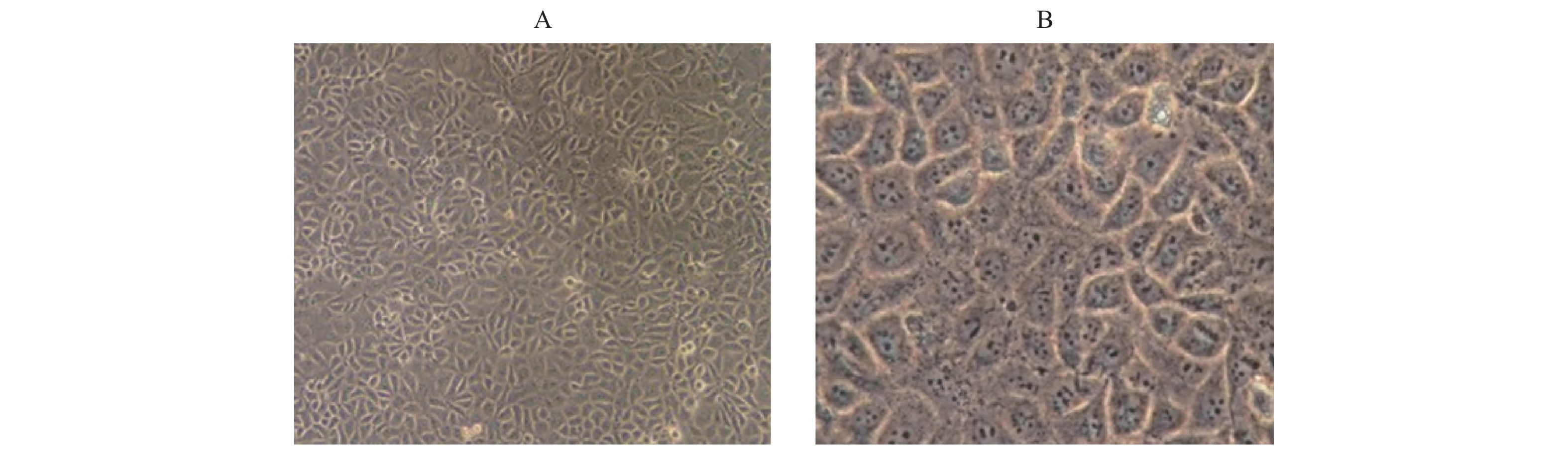
内皮细胞的特异性蛋白CD34、Ⅷ因子既是胞浆蛋白也是胞膜蛋白。通过免疫化学鉴定结果表明,阴性对照细胞形态完整,背景清晰均匀,而阳性组细胞边界清晰,呈棕红色,胞浆和胞膜边缘颜色更深,细胞核明显,见图3A。HUVEC Ⅷ因子的阳性表达,见图3B;CD34阳性表达,进一步证实培养的细胞为HUVECs,见图3D。
2.2 OX-LDL刺激HUVECs损伤模型
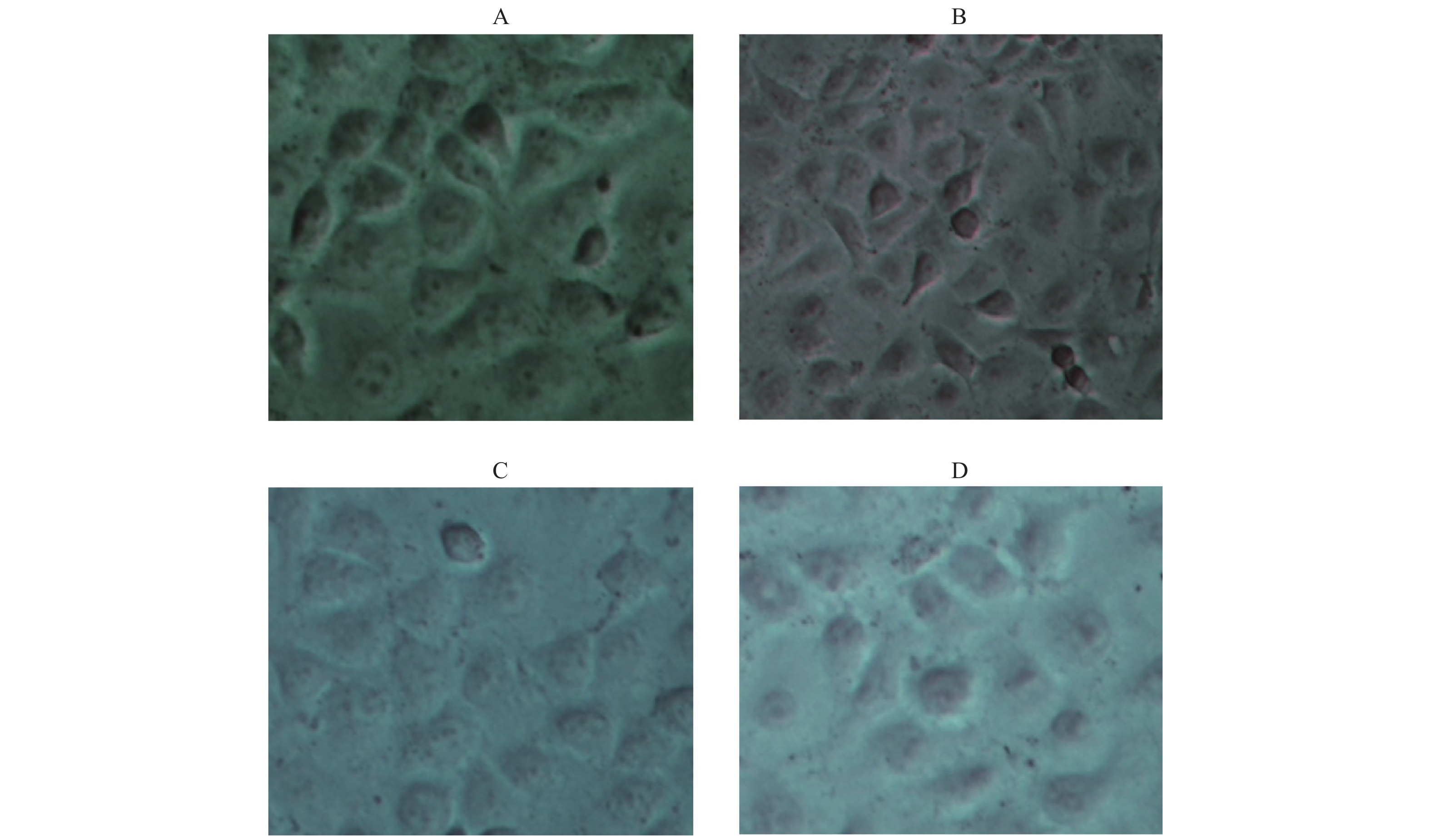
寻找最佳的ox-LDL刺激HUVECs损伤模型的条件。结果显示,随着ox-LDL浓度的增加,OD值下降,说明ox-LDL对HUVECs具有损伤作用。与正常对照组比较,刺激6 h,70 mg/L和80 mg/L浓度的ox-LDL明显损伤了HUVECs(P < 0.01);刺激12、24、48 h,60~80 mg/L浓度的ox-LDL明显损伤HUVECs(P < 0.01)。因此,笔者最终选取终浓度为70 mg/L的ox-LDL刺激HUVECs 12 h作为复制ox-LDL损伤HUVECs模型的最佳条件,见图4。
2.3 Corilagin对ox-LDL损伤HUVECs的保护作用
与正常对照组比较,ox-LDL损伤模型组的细胞活力明显降低(P < 0.01);与模型比较,孵育12 h,6.25~50 μmol/L的Corilagin处理组的细胞活力明显提高,25 μmol/L的Corilagin保护作用最明显,细胞活力较模型组提高50%,50 μmol/L的Corilagin组细胞活力与25 μmol/L组基本持平(P < 0.01);孵育24 h,3.125~50 μmol/L的Corilagin处理组的细胞活力明显提高,25 μmol/L的Corilagin组细胞活力最高,接近正常组水平(P < 0.01);孵育48 h,仅25 μmol/L和50 μmol/L的Corilagin处理组的细胞活力明显提高,较模型组提高30%(P < 0.01),见图5。这表明,Corilagin能对ox-LDL损伤的HUVECs具有保护作用,其中以25 μmol/L和50 μmol/L的浓度效果最显著。
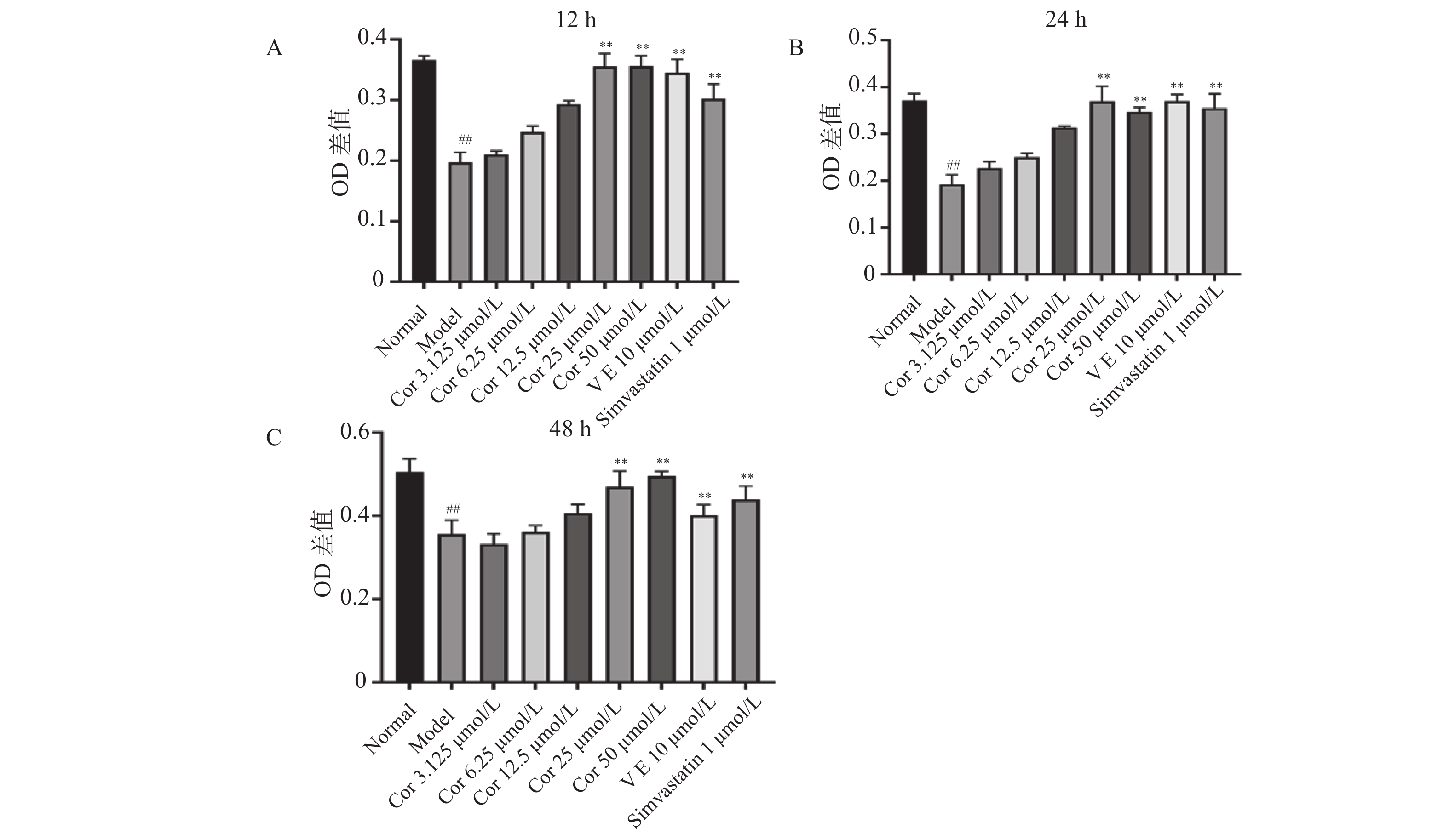 图 5 Corilagin对OX-LDL损伤HUVECs的保护作用(
图 5 Corilagin对OX-LDL损伤HUVECs的保护作用($\bar x \pm s $ ,n = 6)A:Corilagin对OX-LDL损伤HUVECs作用12 h对细胞活力的影响;B:Corilagin对OX-LDL损伤HUVECs作用24 h对细胞活力的影响;C:Corilagin对OX-LDL损伤HUVECs作用48 h对细胞活力的影响。与正常组相比,##P < 0.01,与模型组相比,**P < 0.01。Figure 5. Protective effect of Corilagin on OX-LDL-damaged HUVECs ($\bar x \pm s $ ,n = 6)2.4 Corilagin对ox-LDL损伤HUVECs中MyD88、P65、TNF-α、MCP-1 mRNA表达的影响
与正常对照组比较,ox-LDL模型组的MyD88、NF-kB、TNF-α、MCP-1mRNA的表达量显著升高(P < 0.01)。与ox-LDL模型组比较,不同浓度Corilagin(12.5 μmol/L、25 μmol/L、50 μmol/L)组和阳性药组(辛伐他汀、维生素E)的MyD88、P65、MCP-1、TNF-α mRNA的表达量均显著降低(P < 0.05),见图6。以上结果表明,Corilagin可抑制ox-LDL诱导HUVECs的HUVECs损伤中的MyD88、P65、TNF-α、MCP-1的mRNA表达。
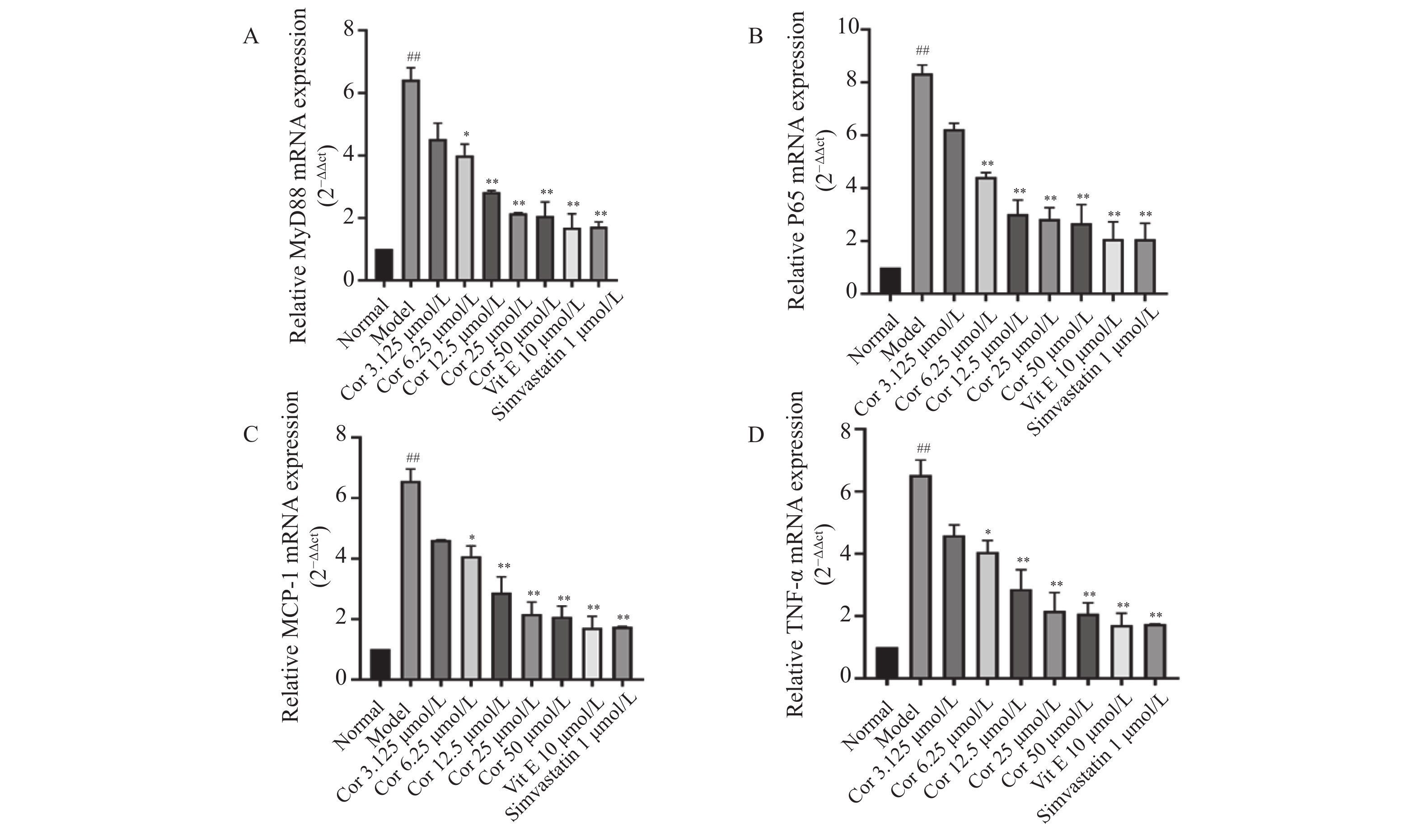 图 6 Corilagin对OX-LDL诱导的HUVECs中P65、MCP-1、MyD88和TNF-α的mRNA表达的影响(
图 6 Corilagin对OX-LDL诱导的HUVECs中P65、MCP-1、MyD88和TNF-α的mRNA表达的影响($\bar x \pm s $ ,n = 6)A:不同浓度Corilagin对OX-LDL诱导的HUVECs中MYD88 mRNA表达的影响;B:不同浓度Corilagin对OX-LDL诱导的HUVECs中P65 mRNA表达的影响;C:不同浓度Corilagin对OX-LDL诱导的HUVECs中MCP-1mRNA表达的影响;D:不同浓度Corilagin对OX-LDL诱导的HUVECs中TNF-αmRNA表达的影响。与正常组相比,##P < 0.01,与模型组相比,*P < 0.05,**P < 0.01。Figure 6. Effect of Corilagin on mRNA expression of P65,MCP-1,MyD88 and TNF-α in OX-LDL-induced HUVECs($\bar x \pm s $ ,n = 6)2.5 Corilagin对ox-LDL损伤HUVECs中MyD88、P65、TNF-α、MCP-1蛋白表达的影响
与正常对照组比较,ox-LDL模型组MyD88、P65、TNF-α、MCP-1蛋白表达均显著升高(P < 0.01)。与模型组比较,Vit E 10 μmol/L、辛伐他汀1 μmol/L组和不同浓度Corilagin(12.5 μmol/L、25 μmol/L、50 μmol/L)组MyD88、P65、TNF-α、MCP-1蛋白表达均出现显著降低(P < 0.05),见图7。以上结果显示,Corilagin可抑制ox-LDL损伤的HUVECs中MyD88、P65、TNF-α、MCP-1蛋白表达。
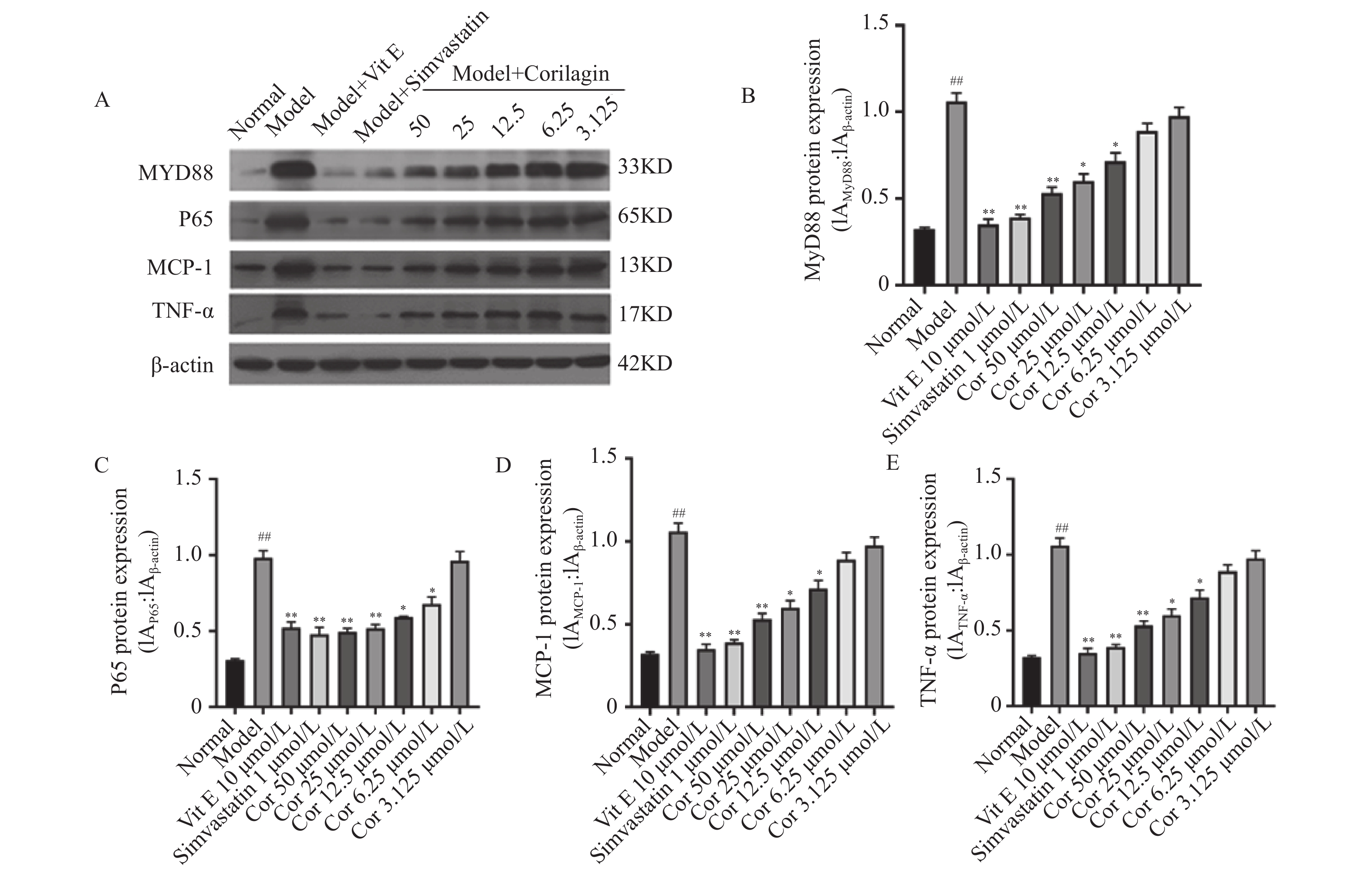 图 7 Corilagin对OX-LDL诱导的HUVECs中P65、MCP-1、MyD88和TNF-α的蛋白表达的影响(
图 7 Corilagin对OX-LDL诱导的HUVECs中P65、MCP-1、MyD88和TNF-α的蛋白表达的影响($\bar x \pm s $ ,n = 3)A:Western blot 检测Corilagin对OX-LDL诱导的HUVECs中P65、MCP-1、MyD88和TNF-α的蛋白表达的影响;B:Corilagin对OX-LDL诱导的HUVECs中MYD88蛋白表达的影响;C:Corilagin对OX-LDL诱导的HUVECs中P65蛋白表达的影响;D:Corilagin对OX-LDL诱导的HUVECs中MCP-1蛋白表达的影响;E:Corilagin对OX-LDL诱导的HUVECs中TNF-α蛋白表达的影响。与正常组相比,##P < 0.01;与模型组相比,*P < 0.05,**P < 0.01。Figure 7. Effect of Corilagin on protein expression of P65,MCP-1,MyD88 and TNF-α in OX-LDL-induced HUVECs ($\bar x \pm s $ ,n = 3)3. 讨论
AS病程复杂,涉及脂质代谢紊乱和免疫细胞向动脉壁募集等病理过程。他汀类药物是治疗血脂异常的一线药物,具有降低LDL-C的功效,然而,部分人对他汀类药物不耐受,且不良反应较多,限制其使用[13],因此,寻找安全有效的抗AS药物是医药学界急需解决的问题。
血管内皮细胞是参与AS发生发展的关键细胞之一,研究选用HUVECs细胞系,通过鉴定发现该细胞符合血管内皮细胞的形态特征并且高表达血管内皮细胞特异性蛋白CD34和Ⅷ,提示该细胞系确为HUVECs。目前普遍认为血管内皮细胞的氧化损伤是AS发展的始动环节,采用ox-LDL诱导后笔者发现60~80 mg/L浓度的ox-LDL 作用12 h可使HUVECs损伤明显,因此本研究选择75 mg/L浓度的ox-LDL 作用12 h为造模条件。在前期研究中笔者已经发现了Corilagin抗AS的作用,本实验中进一步在细胞水平评价了不同时-效/量-效的Corilagin对ox-LDL损伤HUVECs的保护作用,结果显示,Corilagin在12、24、48 h以浓度依赖的方式(3.125、6.25、12.5、25、50 μmol/L)提高细胞活力,表明Corilagin对ox-LDL损伤HUVECs具有保护作用。
炎性反应贯穿AS发生发展的全过程,MyD88是TLR信号转导通路的关键靶标,在炎症反应中发挥着重要作用,它通过调控胞浆募集下游的肿瘤坏死因子受体相关因子-6(tumor-necrosis factor receptor associated factor,TRAF-6),诱导TNF-α和MCP-1等炎症因子的表达。Zhu等[14]研究发现抑制巨噬细胞中TLR4/MyD88/P65信号通路后小鼠AS出现转归,表明以MyD88衔接的炎症反应通路对AS的进展具有重要调控作用。HUVECs作为研究内皮细胞功能调节的模型系统,常应用于血管形成、AS斑块的研究[15]。本研究以MyD88、P65、TNF-α和MCP-1为研究靶标,通过RT-qPCR 和Western blot 技术检测Corilagin对ox-LDL诱导的损伤HUVECs模型中上述因子的表达的影响,在模型组中,MyD88、P65、TNF-α和MCP-1的表达均明显上调,而Corilagin组中这些指标的表达均得到了显著逆转。
综上所述,Corilagin对ox-LDL损伤的HUVECs的保护作用可能是通过下调MyD88基因的表达,抑制p65、炎性因子TNF-α和趋化因子MCP-1的表达来实现的。本研究没有采用MyD88抑制剂佐证Corilagin通过下调MyD88信号通路发挥抗AS作用,因此,在接下来的研究中,笔者将使用MyD88抑制剂在动物-细胞水平进一步验证Corilagin的作用机制,为Corilagin临床用于防治AS 提供理论和实验依据。
-
图 5 Corilagin对OX-LDL损伤HUVECs的保护作用(
$\bar x \pm s $ ,n = 6)A:Corilagin对OX-LDL损伤HUVECs作用12 h对细胞活力的影响;B:Corilagin对OX-LDL损伤HUVECs作用24 h对细胞活力的影响;C:Corilagin对OX-LDL损伤HUVECs作用48 h对细胞活力的影响。与正常组相比,##P < 0.01,与模型组相比,**P < 0.01。
Figure 5. Protective effect of Corilagin on OX-LDL-damaged HUVECs (
$\bar x \pm s $ ,n = 6)图 6 Corilagin对OX-LDL诱导的HUVECs中P65、MCP-1、MyD88和TNF-α的mRNA表达的影响(
$\bar x \pm s $ ,n = 6)A:不同浓度Corilagin对OX-LDL诱导的HUVECs中MYD88 mRNA表达的影响;B:不同浓度Corilagin对OX-LDL诱导的HUVECs中P65 mRNA表达的影响;C:不同浓度Corilagin对OX-LDL诱导的HUVECs中MCP-1mRNA表达的影响;D:不同浓度Corilagin对OX-LDL诱导的HUVECs中TNF-αmRNA表达的影响。与正常组相比,##P < 0.01,与模型组相比,*P < 0.05,**P < 0.01。
Figure 6. Effect of Corilagin on mRNA expression of P65,MCP-1,MyD88 and TNF-α in OX-LDL-induced HUVECs(
$\bar x \pm s $ ,n = 6)图 7 Corilagin对OX-LDL诱导的HUVECs中P65、MCP-1、MyD88和TNF-α的蛋白表达的影响(
$\bar x \pm s $ ,n = 3)A:Western blot 检测Corilagin对OX-LDL诱导的HUVECs中P65、MCP-1、MyD88和TNF-α的蛋白表达的影响;B:Corilagin对OX-LDL诱导的HUVECs中MYD88蛋白表达的影响;C:Corilagin对OX-LDL诱导的HUVECs中P65蛋白表达的影响;D:Corilagin对OX-LDL诱导的HUVECs中MCP-1蛋白表达的影响;E:Corilagin对OX-LDL诱导的HUVECs中TNF-α蛋白表达的影响。与正常组相比,##P < 0.01;与模型组相比,*P < 0.05,**P < 0.01。
Figure 7. Effect of Corilagin on protein expression of P65,MCP-1,MyD88 and TNF-α in OX-LDL-induced HUVECs (
$\bar x \pm s $ ,n = 3)表 1 引物序列
Table 1. Primer sequences
基因名 引物序列 长度(bp) β-actin F-CGTGCGTGACATCAAAGAGA 178 R-CAAGAAGGAAGGCTGGAAAA MyD88 F-CTGGGGGCACTGTGGATT 174 R-CGCTGCTGGGGAGAAAAC P65 F-CAACCAAAACAGAGGGGATT 160 R-TTGTGACCAACTGAACGATA TNF-α F-CTCCTCACCCACACCGTC 226 R-AACACCCATTCCCTTCAC MCP-1 F-TGACCCCAAGAAGGAATG 180 R-GAGGTGGTTGTGGAAAAG -
[1] Shah P K. Inflammation,infection and atherosclerosis[J]. Trends Cardiovas Med,2019,29(8):468-472. doi: 10.1016/j.tcm.2019.01.004 [2] Sharma T,Romeo F,Mehta J L. LOX-1: Implications in atherosclerosis and myocardial ischemia[J]. Excli J,2022,21(1):273-278. [3] Gao W,Liu H,Yuan J,et al. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-α mediated NF-κB pathway[J]. J Cell Mol Med,2016,20(12):2318-2327. doi: 10.1111/jcmm.12923 [4] Xiong X,Lu W,Zhang K,et al. Pterostilbene reduces endothelial cell apoptosis by regulation of the Nrf2-mediated TLR-4/MyD88/NF-κB pathway in a rat model of atherosclerosis[J]. Exp Ther Med,2020,20(3):2090-2098. [5] Chen T,Luo W,Wu G,et al. A novel MyD88 inhibitor LM9 prevents atherosclerosis by regulating inflammatory responses and oxidative stress in macrophages[J]. Toxicol Appl Pharm,2019,370(1):44-55. [6] Hosseini H,Li Y,Kanellakis P,et al. Toll-like receptor (TLR)4 and MyD88 are essential for atheroprotection by peritoneal B1a B cells[J]. J Am Heart Assoc,2016,5(11):e002947. doi: 10.1161/JAHA.115.002947 [7] Basurto L,Gregory M A,Hernández S B,et al. Monocyte chemoattractant protein-1 (MCP-1) and fibroblast growth factor-21 (FGF-21) as biomarkers of subclinical atherosclerosis in women[J]. Exp Gerontol,2019,124(1):110624-110631. [8] Yu H,Cao H,Yu H. MicroRNA-98 inhibition accelerates the development of atherosclerosis via regulation of dysfunction of endothelial cell[J]. Clin Exp Hypertens,2023,45(1):2206068. doi: 10.1080/10641963.2023.2206068 [9] 夏欣,杨媛,李发靖,等. 柯里拉京的心脑血管保护作用及机制研究进展[J]. 医学综述,2022,28(2):229-234. [10] Tao Y,Zhang L,Yang R,et al. Corilagin ameliorates atherosclerosis by regulating MMP-1,-2,and -9 expression in vitro and in vivo[J]. Eur J Pharmacol,2021,906(1):174200-174210. [11] 郭英,陈鹏,谢建平,等. Corilagin对血管内皮细胞的保护作用及LOX-1蛋白表达的影响[J]. 中成药,2012,34(1):151-154. [12] 郭英,陈鹏,谢建平,等. Corilagin对HUVEC的保护作用及LOX-1 mRNA表达的影响[J]. 时珍国医国药,2011,22(10):2419-2421. doi: 10.3969/j.issn.1008-0805.2011.10.044 [13] Gunta S P,O'keefe J H,O'keefe E L,et al. PCSK9 inhibitor,ezetimibe,and bempedoic acid: Evidence-based therapies for statin-intolerant patients[J]. Prog Cardiovasc Dis,2023,13(5):e068915. [14] Zhu K,Wang X,Weng Y,et al. Sulfated galactofucan from sargassum thunbergii attenuates atherosclerosis by suppressing inflammation via the TLR4/MyD88/NF-κB signaling pathway[J]. Cardiovasc Drug Ther,2022,10(1):7383-7397. [15] Park H J,Zhang Y,Georgescu S P,et al. Human umbilical vein endothelial cells and human dermal microvascular endothelial cells offer new insights into the relationship between lipid metabolism and angiogenesis[J]. Stem Cell Rev,2006,2(2):93-102. doi: 10.1007/s12015-006-0015-x -






 下载:
下载:
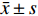
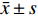
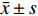
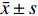
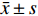
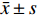
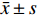
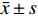
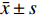
 下载:
下载:

