Analysis of Triterpene Chemical Constituents in Poria cocos 95% Ethanol Extract via UPLC-IT-TOF/MS
-
摘要:
目的 采用超高效液相色谱串联离子阱飞行时间质谱(UPLC-IT-TOF/MS)技术定性分析茯苓95%乙醇提取物的三萜类化学成分。 方法 采用UHPLC XB-C18色谱柱,甲酸/水(0.05/100,v/v)-乙腈作为流动相,梯度洗脱,流速为0.20 mL/min;紫外检测波长范围为190~400 nm。使用ESI离子源,三氟乙酸钠校正,电喷雾正负离子同时检测,Shimadzu Composition Formula Predictor软件定性分析茯苓三萜类化合物。 结果 通过多级质谱数据分析,结合氮规则和Scifinder数据库检索,从茯苓醇提取物中鉴定了22个三萜类成分,结构类型包括羊毛甾-8-烯型三萜、羊毛甾-7,9(11)-二烯型三萜和其他羊毛甾三萜类型。 结论 通过LC-MS快速分析了茯苓醇提取物中三萜类成分,为其药效物质基础和质量控制提供了依据。 -
关键词:
- 茯苓 /
- 三萜 /
- UPLC-IT-TOF/MS
Abstract:Objective To evaluate the triterpene chemical constituents in Poria cocos 95% ethanol extract by ultra-performance liquid chromatography ion trap time-of-flight mass spectrometry (UPLC-IT-TOF/MS) method. Methods UPLC was performed with the UHPLC XB-C18 and methanoic acid/water (0.05/100, v/v)-acetonitrile solution (gradient elution) was employed with a flow rate of 0.2 mL·min-1. The detection wavelength range was 190~400 nm. Triterpene chemical compositions in Poria cocos were qualitatively analyzed using ESI ion with electrospray positive and negative ions simultaneous detection, sodium trifluoroacetace correction and Shimadzu Composition Formula Predictor software. Results Through the analysis of the multistage tandem mass spectrometry, combined with the application of nitrogen rule and the Scifinder database search, 22 triterpenes with lanostan-8-en, lanostan-7, 9(11)-diene type and other lanostane triterpene were identified. Conclusion This study quickly analyzed the triterpenes in the Poria cocos ethanol extract via LC-MS, which provided the basis for the pharmacodyamic material basis and quality control. -
Key words:
- Poria cocos /
- Triterpene /
- UPLC-IT-TOF/MS
-
肺癌是全球最常见的癌症,占所有肿瘤例数的11.6%[1]。根据最新Global cancer statistics统计,每年估计诊断出220万新肺癌病例,约180万例肺癌死亡,造成了重大的社会负担和经济损失[2]。肺癌的早期治疗策略优先考虑解剖性肺切除联合淋巴结清扫。然而,术后治愈率和生存率仍然很低,30%~70%的术后患者出现肿瘤复发或转移,治疗效果非常不理想[3]。因此,确定新型和有效的生物标志物和治疗靶点对于肺癌防治是至关重要。
作为一种重要的细胞内矿物营养物质,铜在几个重要的细胞过程中发挥作用,包括线粒体呼吸、氧代谢、铁摄取以及一些生物途径的调节[4−5]。此前的研究已经观察到铜与疾病状态之间的联系,例如,在各种恶性肿瘤中发现了更高水平的铜[4−5]。最近,Tsvetkov等[6]揭示了一种称为“铜死亡”的新机制,即过高的细内铜浓度会导致细胞死亡。值得注意地。已有研究证实铜死亡参与肺癌的进程。例如,PSMD11被鉴定为铜死亡和免疫相关基因,并且促进肺癌细胞的增殖和转移[7]。此外,AC144450.1/miR-424-5p/CBX2轴被鉴定为铜死亡相关的竞争性内源RNA机制,并且该轴的激活有利于肺癌细胞的生长[8]。这为肺癌治疗干预组合提供了潜在的方向。然而,铜死亡在肺癌中的研究仍然处于初步阶段。
本研究拟通过生物信息学鉴定与肺癌预后和免疫微环境相关的铜死亡基因。旨在为肺癌的治疗提供新型的生物标志物和铜死亡相关的理论基础。
1. 资料与方法
1.1 肺癌数据集和铜死亡相关基因(cuproptosis-related gene,CRGs)的获取
本研究使用的肺癌数据集来源于TCGA(https://portal.gdc.cancer.gov/),包括肺腺癌(lung adenocarcinom,TCGA-LUAD)和肺鳞细胞癌(lung squamous cell carcinomas,TCGA-LUSC)队列。从TCGA数据库提取队列的count和fpkm格式数据。为避免模型构建受到不同队列研究间批次效应的影响,log2转换的fpkm(log2-fpkm)值采用sva包进行批次效应校正,校正中将样本类型(肿瘤或正常组织)作为协变量,避免批次校正掩盖组织类型间生物学效应的差异。最后筛选患者生存信息完整的肿瘤样本数据及所有正常组织样本数据作为分析使用的数据集,其中包括616例肿瘤样本和108例正常组织样本。Count数据用于差异分析,log2-fpkm数据用于预后模型构建。从此前的文献中共检索到38个CRGs[9],包括ATP7B、CDKN2A、DLD、DPYD、FDX1、GLRX5、GLS、ISCA2、LIPT1、MTF1、NDUFA1、NDUFA8、NDUFB10、NDUFB2、NDUFB6、NDUFC1、NDUFC2、NDUFV2、PDHA1、PLAT、POLD1、PPAT、SLC31A1、SDHB、TIMMDC1、DLAT、GCSH、DBT、DLST、LIAS、LIPM、LIPA、LIPT2、PDHB、ACO2、NLRP3、ATP7A和NFE2L2。
1.2 差异表达的CRGs(differentially expressed cuproptosis-related gene,DE-CRGs)的鉴定
采用R软件Deseq2包[10]对TCGA肺癌队列count格式数据执行差异表达分析,以鉴定差异表达基因(differentially expressed genes,DEGs)。阈值为|log2-fold change (FC)|≥2且校准的P-value<0.05。采用R软件ggplot2包[11]可视化DEGs的火山图。采用R软件VennDiagram包[12]获取和可视化DEGs和CRGs的交集,以鉴定DE-CRGs。
1.3 DE-CRGs的富集分析
DE-CRGs的富集分析包括GO和KEGG富集分析,以识别DE-CRGs的生物学功能和可能涉及的信号通路。DE-CRGs采用R软件clusterProfiler包[13]进行GO和KEGG富集分析,并采用R软件ggplot2包[11]进行气泡图的可视化。
1.4 DE-CRGs相关蛋白-蛋白互作(protein-protein interaction,PPI)网络的构建
采用STRING数据库预测DE-CRGs的互作关系。最低要求的互作分数设置为中等可行度(0.4)。DE-CRGs互作关系的TSV从STRING数据库导出,并且导入Cytoscape软件。通过Cytoscape软件的Network Analysis进行PPI网络的可视化,并统计每个节点的连接度、介数中心性和接近中心性。
1.5 DE-CRGs与肺癌患者预后的相关性分析
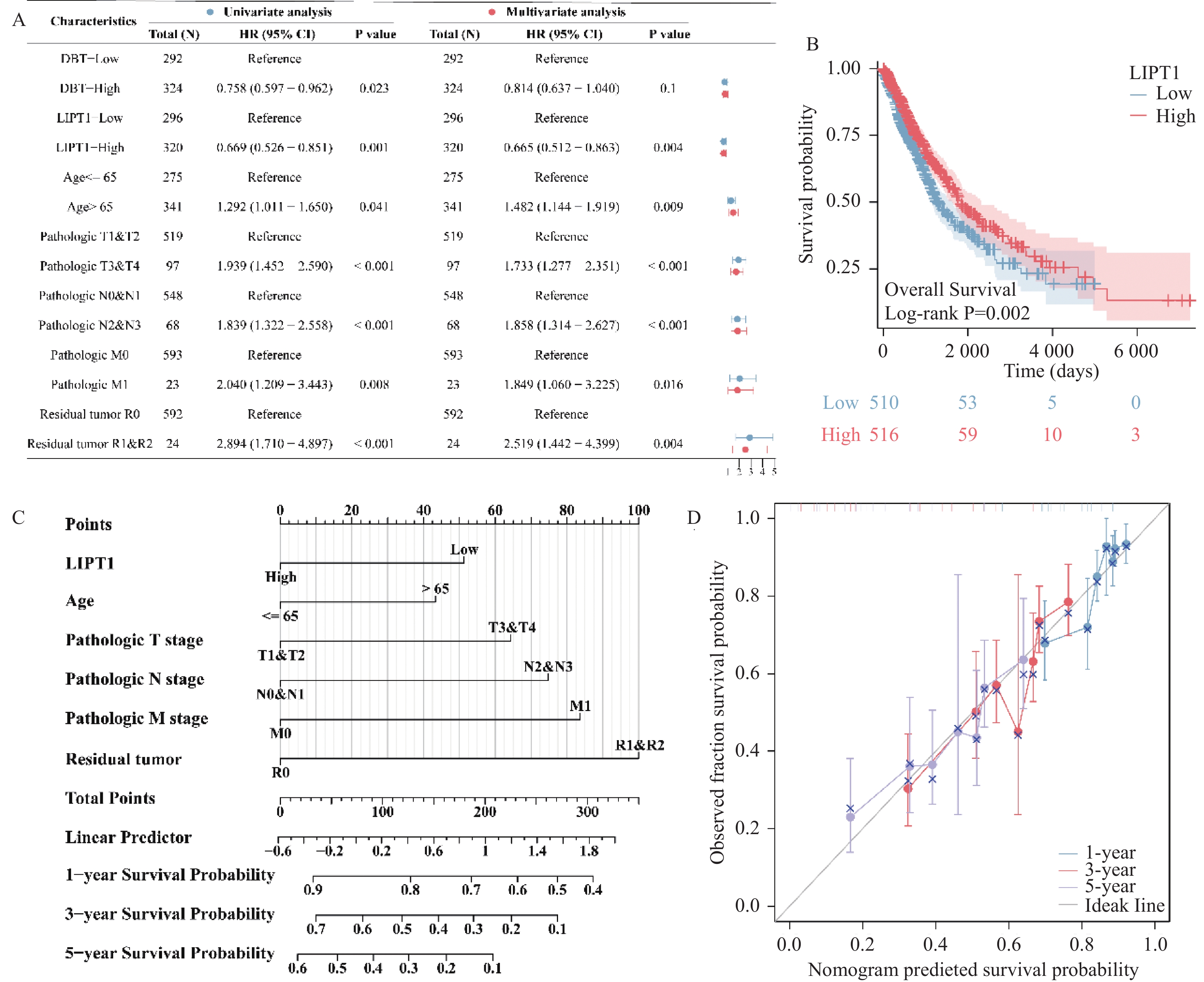
采用R软件Survival包[14]进行TCGA肺癌队列log2-fpkm格式数据(共616个肺癌样本,包括344例死亡和272例存活)的Log-rank检验和Cox回归分析,以识别DE-CRGs表达和临床特征(年龄、性别、是否吸烟、TNM分期、残留肿瘤情况)与肺癌患者预后的相关性,筛选可以独立用于预后风险预测的DE-CRGs。计算每个变量的风险比(hazard ratio,HR)和95%置信区间(confidence interval,CI)。P-value<0.05被认为具有统计学意义。采用R软件survminer包进行森林图和K-M曲线的可视化。将上述筛选出的独立临床因素用于预测列线图的构建。使用R软件rms包[15]构建预测列线图和相应的校准曲线。校准曲线越接近代表最佳预测的45°线,代表列线图的预后预测性能越好。
1.6 DE-CRGs与肺癌患者免疫微环境的相关性分析
本研究采用CIBERSORT和ESTIMATE算法评估LIPT1表达与肺癌患者免疫微环境的相关性。CIBERSORT算法是基于线性支持向量回归原理对人类免疫细胞亚型的表达矩阵进行去卷积的一种算法[16]。本研究通过CIBERSORT算法分析了肺癌队列中22种人类免疫细胞亚群的比例,及LIPT1表达与22种免疫细胞浸润的相关性。Estimate算法通过使用表达数据预测恶性肿瘤组织中的基质细胞和免疫细胞存在的工具[17]。本研究通过ESTIMATE算法评估肿瘤纯度,并分析LIPT1与免疫分数和基质分数之间的相关性。
1.7 统计学分析
采用R软件(版本4.1.1)进行统计分析。Student's t检验和Mann-Whitney检验用于比较肺癌组织和正常组织之间的基因表达。采用Kaplan-Meier(K-M)曲线及Log-rank统计检验对高表达LIPT1和低表达LIPT1的患者生存进行差异分析。使用单变量和多变量Cox回归分析来确定肺癌的独立的预后因素。P<0.05为差异有统计学意义。
2. 结果
2.1 肺癌中DE-CRGs的鉴定
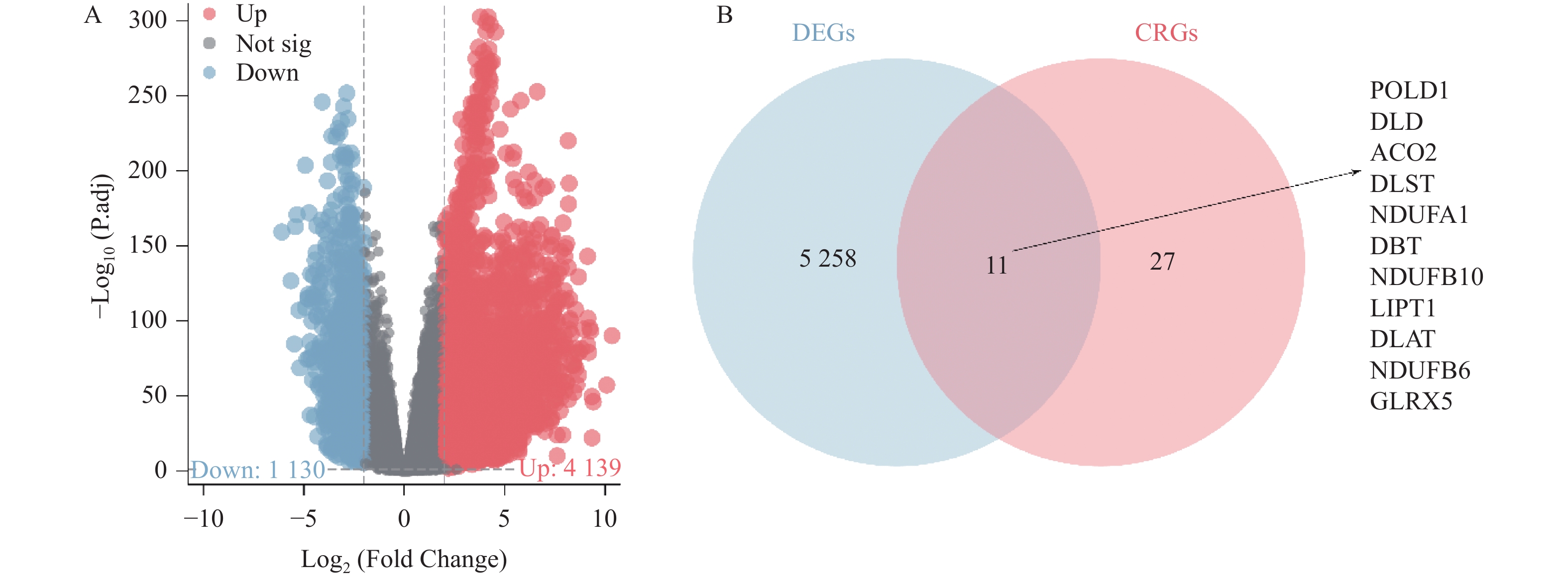
R软件Deseq2包分析显示,相较于正常组织,TCGA-LUAD和TCGA-LUSC队列的肺癌组织中共存在
5269 个DEGs,包含1130 个下调的DEGs和4139 个上调的DEGs,见图1A。韦恩图显示,DEGs和CRGs共存在11个交集,包括POLD1、DLD、ACO2、DLST、NDUFA1、DBT、NDUFB10、LIPT1、DLAT、NDUFB6、GLRX5,见图1B。这些交集为DE-CRGs。2.2 肺癌中DE-CRGs的富集分析
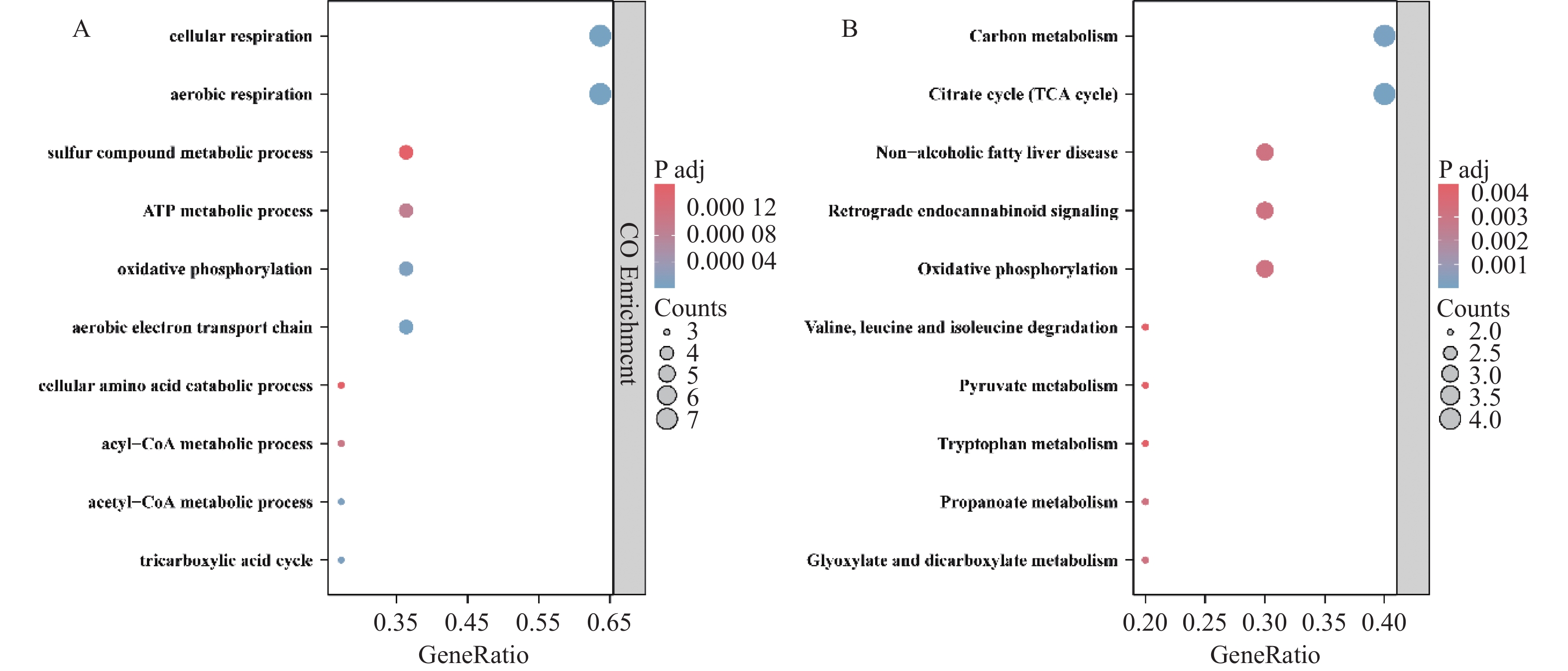
GO富集分析显示,11个DE-CRGs主要参与有氧呼吸、硫化合物代谢过程、氨基酸分解代谢过程、三羧酸循环和ATP代谢过程的调控,见图2A。KEGG富集分析显示,11个DE-CRGs主要调控碳代谢、氧化磷酸化、氨基酸代谢、乙醛酸盐和二羧酸盐代谢和柠檬酸循环,见图2B。
2.3 肺癌中DE-CRGs相关PPI网络的构建
DE-CRGs相关PPI网络包含10个节点和19个互作关系对,其中不包含POLD1,见图3A。该网络中,DLST、ACO2、DLD、DLAT、DBT是连接度排名前五的DE-CRGs,见图3B。
2.4 与肺癌患者预后相关的DE-CRGs鉴定
单因素COX分析表明,11个DE-CRGs中仅有DBT和LIPT1与肺癌患者生存相关,见图4A。进一步的多因素COX分析表明,LIPT1、年龄、TNM分期和残留肿瘤是肺癌患者的独立风险因素,见图4A。Log-rank检验同样表明,相较于高表达LIPT1的肺癌患者,低表达LIPT1的肺癌患者的生存可能更低,见图4B。本研究构建了预测肺癌患者预后的独立风险因素相关列线图,见图4C。校准曲线显示,在第1年、第2年和第3年,列线图预测的患者生存可能与患者实际的生存/结局相近,见图4D。
2.5 LIPT1与肺癌患者免疫微环境相关
ESTIMATE算法分析显示,LIPT1表达与肺癌患者的免疫分数、基质分数和ESTIMATE分数呈现显著的负相关,见图5A。CIBERSORT算法分析显示,LIPT1表达与T细胞滤泡辅助细胞、静息肥大细胞、单核细胞、嗜酸性粒细胞、树突细胞、M1和M2型巨噬细胞、γδ T细胞、活化的自然杀伤细胞细胞、CD8+ T细胞的浸润呈现正相关,与M0型巨噬细胞、激活的肥大细胞、浆细胞、中性粒细胞、静息的自然杀伤细胞和Treg细胞显现显著的负相关,见图5B。
3. 讨论
本研究在肺癌中共鉴定了11个DE-CRGs,包括ACO2、POLD1、DLD、DLST、NDUFA1、DBT、NDUFB10、LIPT1、DLAT、NDUFB6、GLRX5。有趣地,部分DE-CRGs在肺癌进程中的功能已被证实。Shideh等[18]证实,ACO2在非小细胞肺癌组织中低表达,这预示这患者预后变差,并且ACO2通过抑制CISD1导致铁饥饿反应来降低FeS转运,这抑制了癌细胞的增殖。此外,PM2.5通过翻译和转录激活DLAT介导的糖酵解重编程,这加速了非小细胞肺癌的生长[19]。然而,这些研究均不涉及铜死亡的调控。结合本研究的发现提示,ACO2和DLAT对肺癌的抑癌和促癌作用可能与铜死亡有关。值得注意地,富集分析证实,11个DE-CRGs主要调控能量代谢。这是可以预见的。因为,过量的铜会损害线粒体呼吸中的三羧酸循环,并刺激铜中毒,导致脂酰化和FeS簇蛋白的明显丢失以及热休克蛋白70水平的增加,进而引起细胞的程序性死亡[6]。
本研究证实,LIPT1低表达是肺癌的独立风险因子,并且LIPT1相关的列线图对肺癌患者预后具有卓越的预测性能。LIPT1是编码线粒体硫辛酸代谢的重要基因,其编码的LIPT1蛋白将硫辛酰基从硫辛酰腺苷酸转移到甘氨酸裂解系统蛋白H和2-氧酸脱氢酶E2亚基,进而参与硫辛酸代谢[6,20]。目前,有关于LIPT1的研究主要集中于Leigh综合征[21]、硫辛酸代谢相关的致命疾病[22]、恶性肿瘤(膀胱癌、黑色素瘤和肝细胞癌)[23−25]。在膀胱癌和黑色素瘤中,LIPT1的高表达预示着患者预后良好,这与本研究的结果是一致的。然而,在肝细胞癌中,LIPT1表现为高表达,并且是肝细胞癌预后不良的独立风险因素。这表明,LIPT1在不同细胞类型中以完全相反的方式发挥作用,这一点对于理解癌症中的细胞命运决定至关重要。然而,LIPT1在肺癌发生和发展中的功能是未知的,尤其是对铜死亡的调控。值得注意地,在黑色素瘤患者中,上调的LIPT1表达可能会抑制Treg的浸润,从而提高免疫治疗效果[24]。本研究发现,LIPT1表达与Treg的浸润呈现显著的负相关。这个发现提示,LIPT1可能也通过调控Treg的浸润,参与肺癌免疫治疗疗效的干预。此外,本研究还发现,LIPT1表达与T细胞滤泡辅助细胞、肥大细胞、树突细胞、巨噬、γδ T细胞、自然杀伤细胞细胞、CD8+ T细胞和中性粒细胞的浸润有关。这些发现表明,LIPT1可能是肺癌免疫治疗的有效靶点,为肿瘤患者的临床治疗提供了新的希望。然而,LIPT1表达与癌症患者免疫检查点之间的关联仍需在更多的临床前和临床试验中探索。
综上所述,本研究利用生物信息学分析探讨了与肺癌患者预后和免疫微环境相关的铜死亡基因。本研究证实,LIPT1是一种有效预测肺癌患者预后,并且与肿瘤免疫微环境相关的铜死亡生物标志物。本研究为进一步研究LIPT1在肺癌发生和治疗中的具体机制奠定了基础。
-
表 1 茯苓中色谱峰的LC/ESI–MSn鉴定结果(1)
Table 1. Characterization of the peaks in LC/ESI–MSn chromatogram of Poria cocos (1)
色谱峰 保留时间 分子式 分子量 ESI+ (误差,mDa) ESI− (误差,mDa) 化合物名称 1 17.51 C30H48O5 488 [M+Na]+ 511.3328 (−6.6)
MSn: 511-493.3316 (C30H46O4, +2.8),
453.3319 (C30H44O3, −4.4)[M−H]−487.3402 (−2.7) Daedaleanic acid B[10] 2 19.15 C30H46O6 502 [M+Na]+ 525.3301 (+11.4) [M−H]−501.3164 (−5.8) Pinicolic acid F [9] 3 19.90 C31H48O5 500 MSn: 483.3468 (C31H46O4, −0.1), 465.3319 (C31H44O3, −4.4) [M−H]−499.3427 (−0.2) 29α-羟基去氢土莫酸
29α-Hydroxydehy-dropachymic acid[14]4 19.90 C30H48O6 504 [M+Na]+ 527.3331 (−1.2)
MSn: 527- 495.3077 (C29H44O5, +6.3), 437.2886 (C27H42O3, −14.0)[M−H]−503.3373 (−0.5) - 5 21.69 C31H48O6 516 [M+Na]+539.3047 (+6.8)
MSn: 539- 521.2809 (C31H46O5, −6.5), 507.2633 (C30H44O5, −4.6)[M−H]−515.3056 (+4.2) 5,8α-Dioxy-3β,16α-dihydroxylanosta-7,
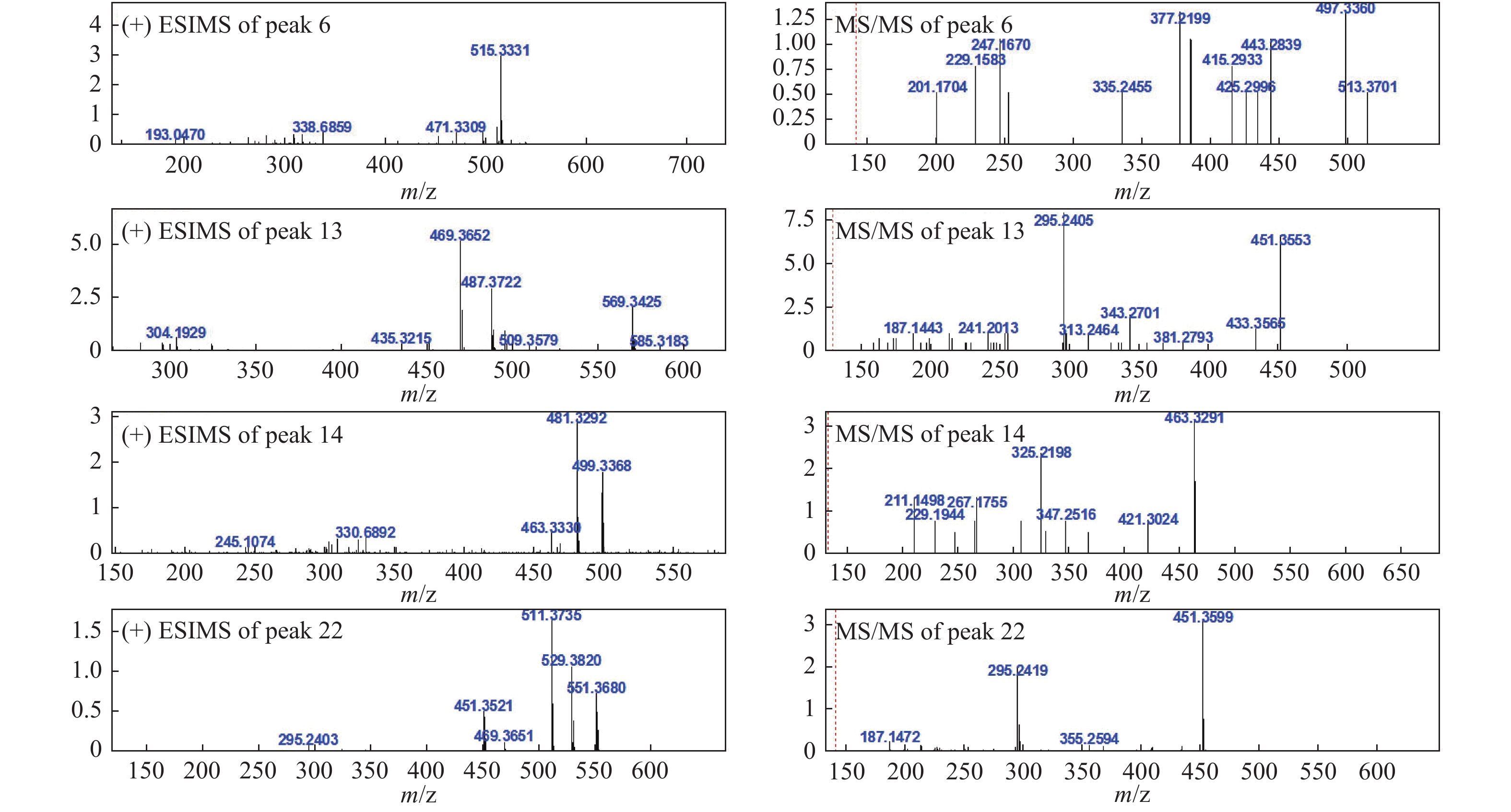
24- dien-21-oic acid [18]6 21.97 C31H46O6 514 [M+H]+515.3331 (−3.6)
MSn: 515- 497.3360 (C31H44O5, +9.8), 443.2839
(C31H38O2, −10.6), 433.3267 (C30H40O2, +16.6), 425.2996
(C28H40O3, −5.4), 415.2933 (C30H38O, −6.2), 385.2397
(C24H32O4, +2.4), 377.2199 (C25H28O3, +8.8), 335.2455
(C24H30O, +8.6), 253.1655 (C18H20O, +6.8), 247.1670
(C16H22O2, −2.3), 229.1583 (C16H20O, −0.4), 201.1704 (C15H20, +6.6)[M−H]−513.3151 (−7.1)
MSn: 513- 483.3167 (C31H46O4, −5.6)5α,8α-过氧化去氢土莫酸
5α,8α-Peroxydehydrotumulosic acid[19]7 22.54 C31H46O5 498 [M+H]+ 499.3287 (−13.1)
MSn: 481.3308 ( (C31H44O4, −0.4), 463.3147 (C31H42O3, −6.0)[M−H]−497.3193 (−7.9)
MSn: 497- 403.2418 (C24H26O5, −7.2), 389.2435 (C27H34O2, −5.1),
371.2336(C27H32O, −4.4), 355.2131 (C26H28O, +6.4)6α-羟基猪苓酸C
6α-Hydroxypolyporenic acid C[11]8 23.69 C33H48O8 572 [M+H]+573.3312 (−11.1) [M−H]−571.3213 (−6.3) 3-(2-羟基乙酰氧基)- 5α,8α-过氧化去氢土莫酸
3-(2-hydroxyacetoxy)- 5α,
8α-peroxydehydrotumulosic acid [20]9 25.78 C31H46O5 498 [M+H]+ 499.3433 (+1.5)
MSn: 481.3408 ( (C31H44O4, +9.6)- 451.3147 (C30H42O3, −1.4),
435.2955 (C29H38O3, +6.2), 325.2132 (C22H28O2, −3.0),
299.2112 (C20H26O2, +10.6)[M−H]−497.3236 (−3.6) - 表 1 茯苓中色谱峰的LC/ESI–MSn鉴定结果(2)
Table 1. Characterization of the peaks in LC/ESI–MSn chromatogram of Poria cocos (2)
色谱峰 保留时间 分子式 分子量 ESI+ (误差,mDa) ESI- (误差,mDa) 化合物名称 10 28.42 C32H50O6 530 [M+Na]+553.3443 (−5.7)
MSn: 553- 535.3422 (C32H48O5, +2.8), 495.3437
(C30H48O4, −0.8), 477.3274 (C30H46O3, −6.5), 435.3364
(C28H44O2,+13.0), 353.2548 (C22H34O2, +9.7)[M−H]−529.3461 (−7.4) 3-乙酰氧基-16α,26-二羟基-羊毛甾-8,24-二烯-21-酸
3- Acetoxy-16α,26-dihydroxy-lanosta-8,24-dien-21-oic acid[10]11 29.01 C33H50O6 542 MSn: 525.3513 (C33H48O5, −6.2)- 507.3459
(C33H46O4, −1.0), 465.3365 (C31H44O3, +0.2),
447.3544 (C32H46O, −7.7)[M−H]−541.3460 (−7.5) 6α-羟基去氢茯苓酸
6α-Hydroxy-dehydropachymic acid[13]12 31.15 C31H48O4 484 [M+Na]+485.3592 (−3.3)
MSn: 485- 467.3492 (C31H46O3, −2.9), 449.3408 (C31H44O2, −0.6),
311.2388 (C22H30O, +1.9), 293.2259 (C22H28, −0.5)[M−H]−483.3413 (−6.7)
MSn: 483- 437.3393 (C30H46O2, −3.2), 421.2978 (C29H42O2, −13.4)3β,15α-二羟基-羊毛甾-7,9(11),24-三烯-21-酸
3β,15α-dihydroxylanosta-7,9(11),24-trien-21-oic acid[15]13 30.27 C31H50O4 486 [M+H]+ 487.3722 (−6.0)
MSn: 487- 469.3719 (C31H48O3, +4.3),
451.3553 (C31H46O2, −1.8), 343.2701
(C23H34O2, +6.9), 313.2464 (C22H32O, −6.2),
295.2405 (C22H30, −1.5)[M−H]−485.3570 (−6.6)
MSn: 483- 423.3371 (C29H44O2,+10.2)土莫酸
Tumulosic acid[10-11]14 31.74 C31H46O5 498 [M+H]+ 499.3368 (−5.0)
MSn: 499- 481.3292 (C31H44O4, −2.0), 463.3291 (C31H42O3, +8.4),
421.3024 (C29H40O2, −7.7), 325.2198 (C22H28O2, +3.8), 307.2102 (C22H26O, +4.6)[M−H]−497.3199 (−7.3)
MSn: 497- 425.2942 (C31H38O, +9.2)29-羟基猪苓酸 C
29-Hydroxypolyporenic acid C[16]15 33.26 C33H52O6 544 [M+H]+ 545.3733 (−10.4)
MSn: 545- 527.3696 (C33H50O5, −3.5), 451.3097 (C31H46O2, +2.6), 433.3267 (C31H44O, −19.8), 295.2433 (C22H30,+2.3)[M−H]−543.3623 (−6.8)
MSn: 543- 467.3227 (C31H48O3, +9.6)25-羟基茯苓酸
25-Hydroxypachimic acid[11]16 33.57 C31H46O4 482 [M+H]+ 483.3420 (−4.9)
MSn: 483- 465.3327 (C31H44O3, −3.6), 447.3246 (C31H42O2, −1.2), 309.2214 (C22H28O, +0.1)[M−H]−481.3266 (−5.7)
MSn: 481- 311.1817 (C21H28O2, −14.0)猪苓酸 C
Polyporenic acid C[11]17 34.28 C31H48O4 484 [M+H]+ 485.33637 (+1.2)
MSn: 483- 467.3486 (C31H46O3, −3.4), 449.3532 (C31H44O2, +11.8), 311.2343 (C22H30O, −2.6), 293.2330 (C22H28,+6.6)[M−H]−483.3378 (−10.2)
MSn:3-表去氢土莫酸
3-epi-Dehydrotumulosic acid acid[11]表 1 茯苓中色谱峰的LC/ESI–MSn鉴定结果(3)
Table 1. Characterization of the peaks in LC/ESI–MSn chromatogram of Poria cocos (3)
色谱峰 保留时间 分子式 分子量 ESI+ (误差,mDa) ESI− (误差,mDa) 化合物名称 18 34.89 C31H48O4 484 [M+H]+ 485.3589 (−3.6)
MSn: 483- 467.3526 (C31H46O3, +0.6), 449.3377 (C31H44O2, −3.7), 311.2408 (C22H30O, +3.9), 293.2265 (C22H28, 0.1)[M−H]−483.3406 (−7.4)
MSn: 481- 337.2465 (C24H34O, −7.2)去氢土莫酸
Dehydrotumulosic acid[17]19 38.63 C33H50O5 526 [M+H]+ 527.3671(−6.0)
MSn: 527- 509.3564 (C33H48O4, −6.1), 449.3398 (C31H44O2, −1.6), 293.2240 (C22H28, −2.4)[M−H]−525.3493 (−9.2)
MSn:3-表去氢茯苓酸
3-epi-Dehydropachymic acid[14]20 39.64 C32H50O5 514 [M+H]+ 515.3708(−2.3)
MSn: 515- 497.3555 (C32H46O4, −7.0), 437.3398 (C30H44O2, −1.6), 295.2422 (C22H30,+0.2)[M−H]−513.3532 (−5.3) 3-O-乙酰基-16α-羟基松苓酸
3-O-Acetyl-16α-hydroxytrametenolic acid[12]21 40.64 C33H50O5 526 [M+H]+ 527.3697 (−3.4)
MSn: 527- 509.3594 (C33H48O4, −3.1), 449.3446 (C31H44O2, +3.2), 353.2501 (C24H32O2, +2.6),
293.2254 (C22H28, −1.0)[M−H]−525.3511 (−7.4)
MSn: 525- 479.3471 (C32H48O3, −6.0), 465.3351 (C31H46O3, −2.3), 355.2295 (C23H32O3, +1.6),去氢茯苓酸dehydropachymic acid[11] 22 41.87 C33H52O5 528 [M+H]+ 529.3820 (−6.8)
MSn: 529- 511.3735 (C33H50O4, −4.7), 451.3599 (C31H46O2, −3.0), 295.2419 (C22H30, −0.1)[M−H]−527.3666 (−7.6)
MSn: 527- 465.3330 (C31H46O3, −4.4)茯苓酸
Pachymic acid[10-11]23 45.90 C30H46O3 454 [M+H]+ 455.3532 (+1.2)
MSn: 455- 437.3457 (C30H44O2, +4.3), 311.2370 (C22H30O, +0.1), 295.2381 (C22H30, −3.9)[M−H]−453.3342 (−3.2) 3-羟基-羊毛甾-7,9(11),24-三烯-21酸
Dehydrotrametenolic acid[12]24 47.30 C30H48O3 456 [M+H]+ 457.3663 (−1.3)
MSn: 457- 439.3502 (C30H46O2, −6.9), 313.2445 (C22H32O, −8.1), 295.2463 (C22H30, +4.3)[M−H]−455.3471 (−6.0) 3-氢化松苓酸
trametenolic acid[13] -
[1] 王萌,张毅,李金田. 从《神农本草经》论茯苓在经方中的应用[J]. 中国中医基础医学杂志,2017,23(8):1149-1151. [2] 邓桃妹,彭代银,俞年军,等. 茯苓化学成分和药理作用研究进展及质量标志物的预测分析[J]. 中草药,2020,51(10):2703-2717. doi: 10.7501/j.issn.0253-2670.2020.10.013 [3] 田婷,陈华,殷璐,等. 茯苓和茯苓皮水和乙醇提取物的利尿作用及其活性成分的分离鉴定[J]. 中国药理学和毒理学杂志,2014,28(1):57-62. [4] Xu H,Wang Y C,Jurutka P W,et al. 16α-Hydroxytrametenolic acid from Poria cocos improves intestinal barrier function through the glucocorticoid receptor-mediated PI3K/Akt/NF-kappaB pathway[J]. J Agric Food Chem,2019,67(39):10871-10879. doi: 10.1021/acs.jafc.9b04613 [5] Gao Y Q,Yan H,Jin R R,et al. Antiepileptic activity of total triterpenes isolated from Poria cocos is mediated suppression of aspartic and glutamic acids in the brain[J]. Pharm Biol,2016,54(11):2528-2535. doi: 10.3109/13880209.2016.1168853 [6] 董建设,赵俊峰,张林超,等. 茯苓酸通过Wnt信号通路对肾癌细胞生物学特性的影响[J]. 中国老年学杂志,2019,39(9):2241-2244. doi: 10.3969/j.issn.1005-9202.2019.09.061 [7] 樊燕青,孙兰池,李大鹏. 茯苓酸对舌鳞状细胞癌细胞CAL-27 增殖、凋亡及细胞周期的影响[J]. 中成药,2021,43(7):1909-1914. doi: 10.3969/j.issn.1001-1528.2021.07.043 [8] Lee S R,Lee S,Moon E,et al. Bioactivity-guided isolation of anti-inflammatory triterpenoids from the sclerotia of Poria cocos using LPS-stimulated Raw264.7 cells[J]. Bioorg Chem,2017,70:94-99. doi: 10.1016/j.bioorg.2016.11.012 [9] Chen B S,Zhang J J,Han J J,et al. Lanostane triterpenoids with glucose-uptake-stimulatory activity from peels of the cultivated edible mushroom Wolfiporia cocos[J]. J Agric Food Chem,2019,67(26):7348-7364. doi: 10.1021/acs.jafc.9b02606 [10] 邹叶挺. 茯苓化学组分表征及其抗顺铂肠损伤作用初步研究[D]. 南京: 南京中医药大学硕士论文. 2019: 10–45. [11] 郑艳,杨秀伟. 中药材规范化种植茯苓化学成分研究[J]. 中国现代中药,2017,19(1):44-50. [12] 李慧,黄帅,单连海,等. 茯苓皮中三萜酸类成分的研究[J]. 华西药学杂志,2016,31(1):6-10. [13] Nukaya H, Yamashiro H, Fukazawa H, et al, Isolation of inhibitors of TPA-induced mouse ear edema from Hoelen, Poria cocos[J]. Chem Pharm Bull, 1996, 44(4): 847–849. [14] 王坤凤. 茯苓化学成分及质量控制方法研究[D]. 北京: 北京中医药大学硕士论文. 2014: 62–85. [15] Zhang H F,Feng B,Liu K,et al. Isolation and purification of two triterpenoids from the Chinese medicinal plant Fomes officinalis ames[J]. Asian J Chem,2013,25(11):6130-6132. doi: 10.14233/ajchem.2013.14285 [16] Zheng Y,Yang X W. Two new lanostane triterpenoids from Poria cocos[J]. J Asian Nat Prod Res,2008,10(4):289-292. doi: 10.1080/10286020701782742 [17] 邹叶挺,徐金娣,龙芳,等. 整合UPLC-QTOF-MS/MS全扫描和模拟MRM方法综合评价茯苓乙醇提取物与后续乙酸乙酯萃取物三萜酸类组分化学一致性[J]. 药学学报,2019,54(1):130-137. [18] Lin H C, Chang T C, Chang W L, et al. Pharmaceutical composition and extract of Poria for enhancing uptake of nutrients: The United States of America, US 9757392 B2 [P]. 2017.09. 12. [19] Akihisa T,Nakamura Y,Tokuda H,et al. Triterpene acids from Poria cocos and their anti-tumor-promoting effects[J]. J Nat Prod,2007,70(6):948-953. doi: 10.1021/np0780001 [20] Li S,Wang Z,Gu R,et al. A new epidioxy-tetracyclic triterpenoid from Poria cocos Wolf[J]. Nat Prod Res,2016,30(15):1712-1717. doi: 10.1080/14786419.2015.1136909 期刊类型引用(2)
1. 赵琳,樊少军,于建云,王文杰. 大客车正面碰撞车内乘员损伤AIS评分的个案分析. 昆明医科大学学报. 2018(04): 125-129 .  本站查看
本站查看2. 屠建权. 道路交通事故致颅脑损伤的法医学伤残评定分析. 世界最新医学信息文摘. 2017(19): 176-177 .  百度学术
百度学术其他类型引用(0)
-






 下载:
下载:



 下载:
下载: