Shikonin Induces Ferroptosis through ROS/JNK Pathway to Intervene in the Malignant Behavior of Pancreatic Cancer
-
摘要:
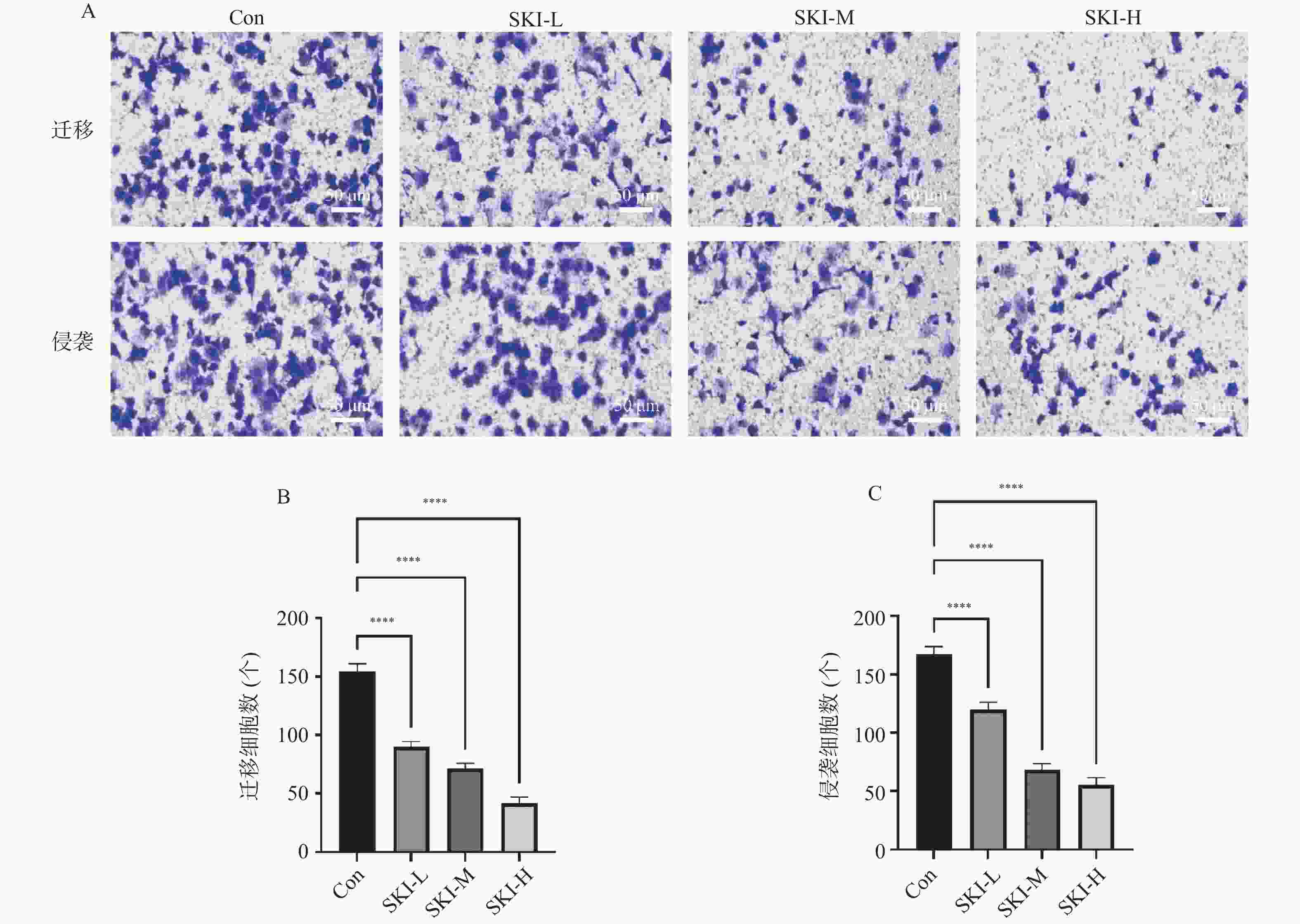
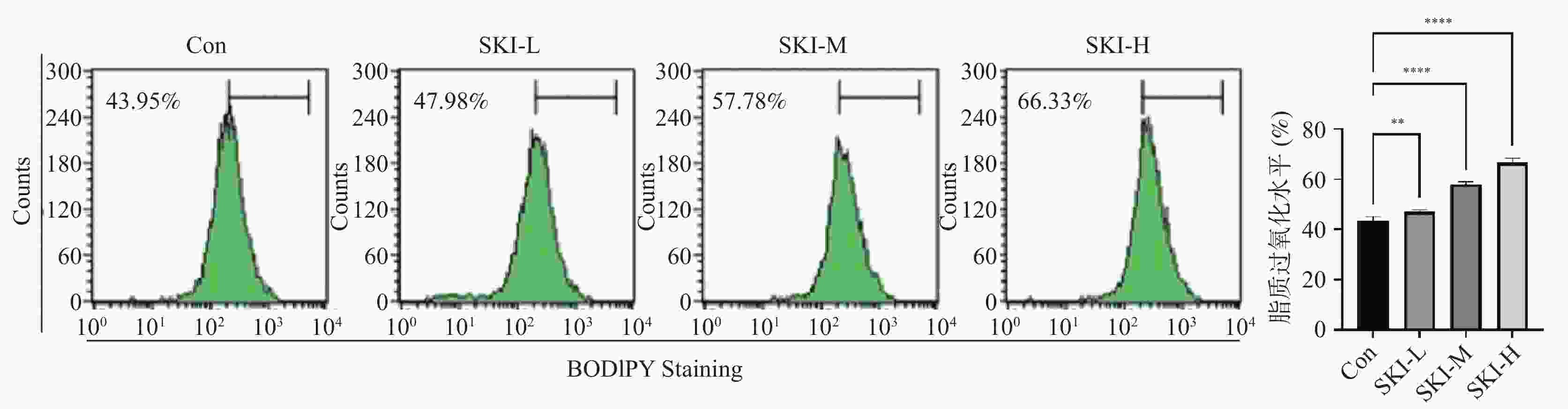
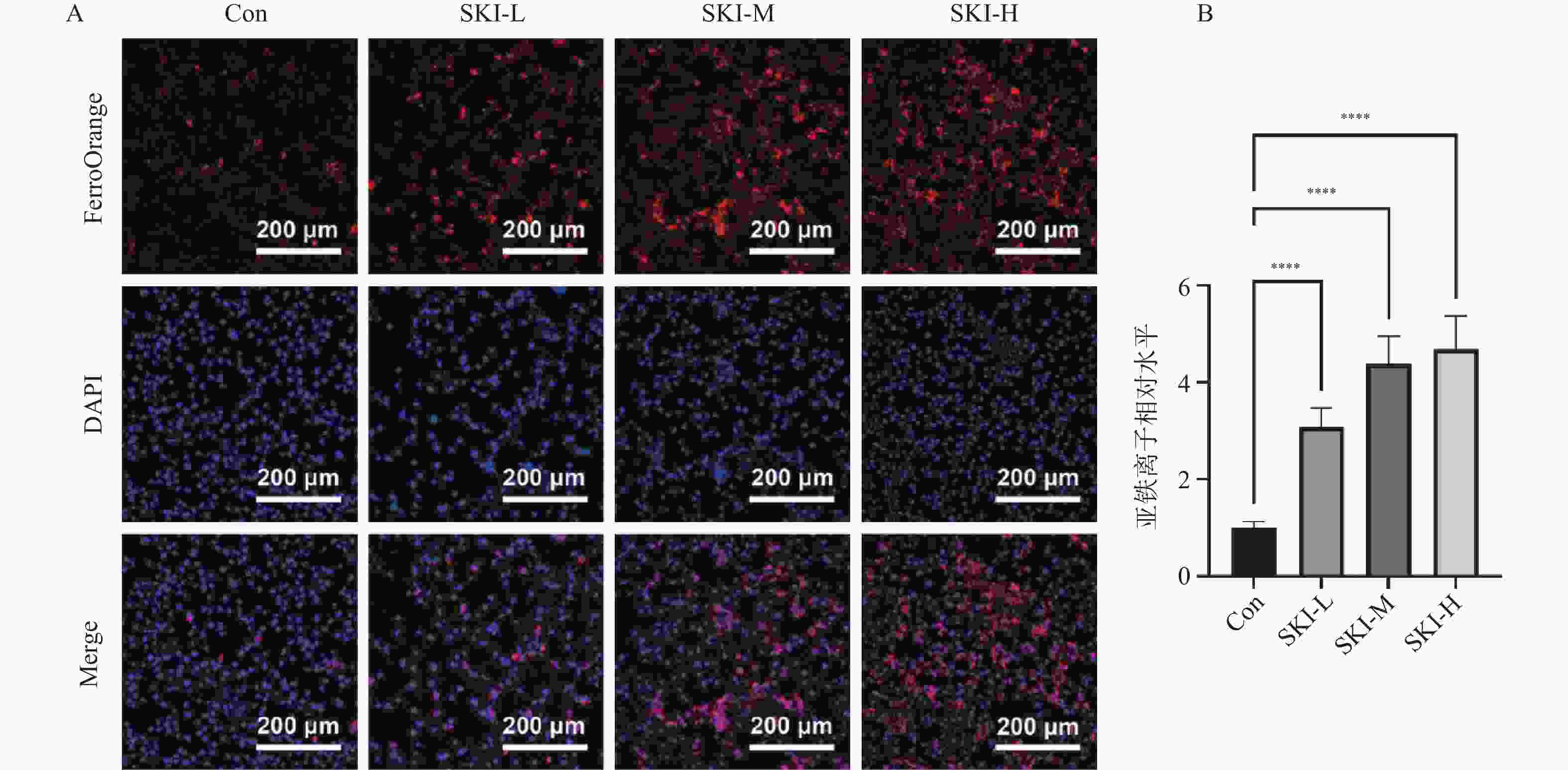
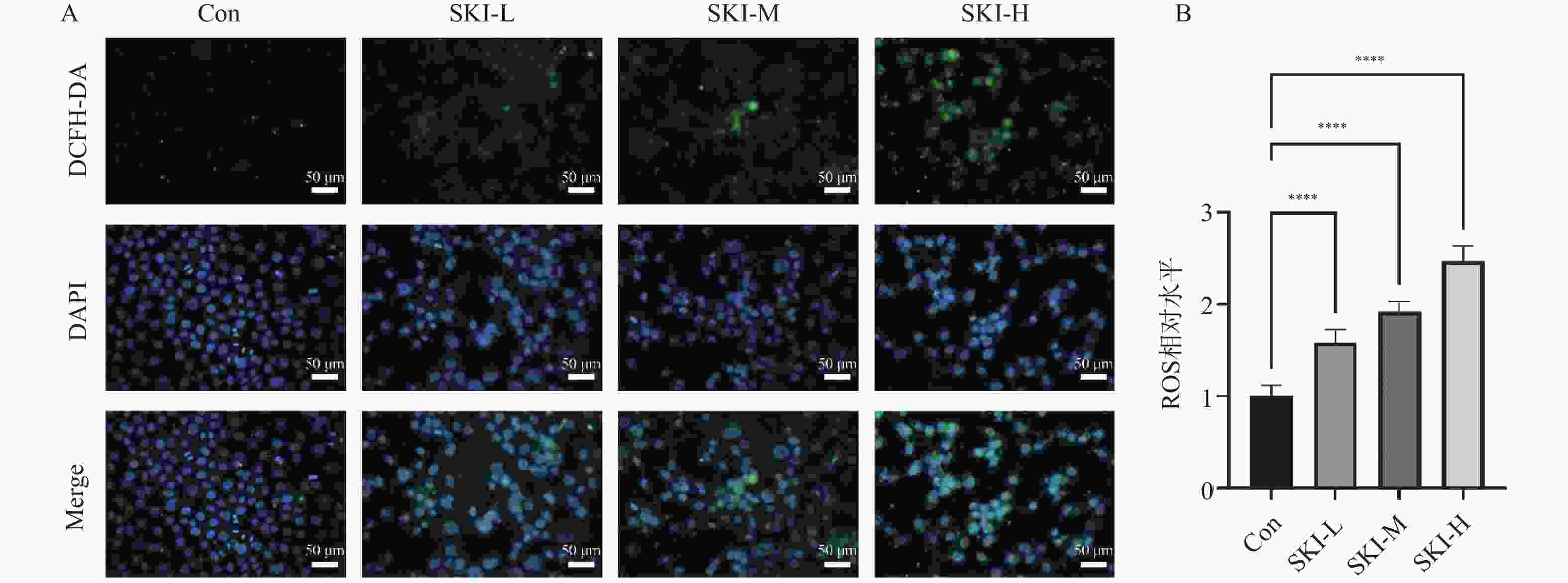
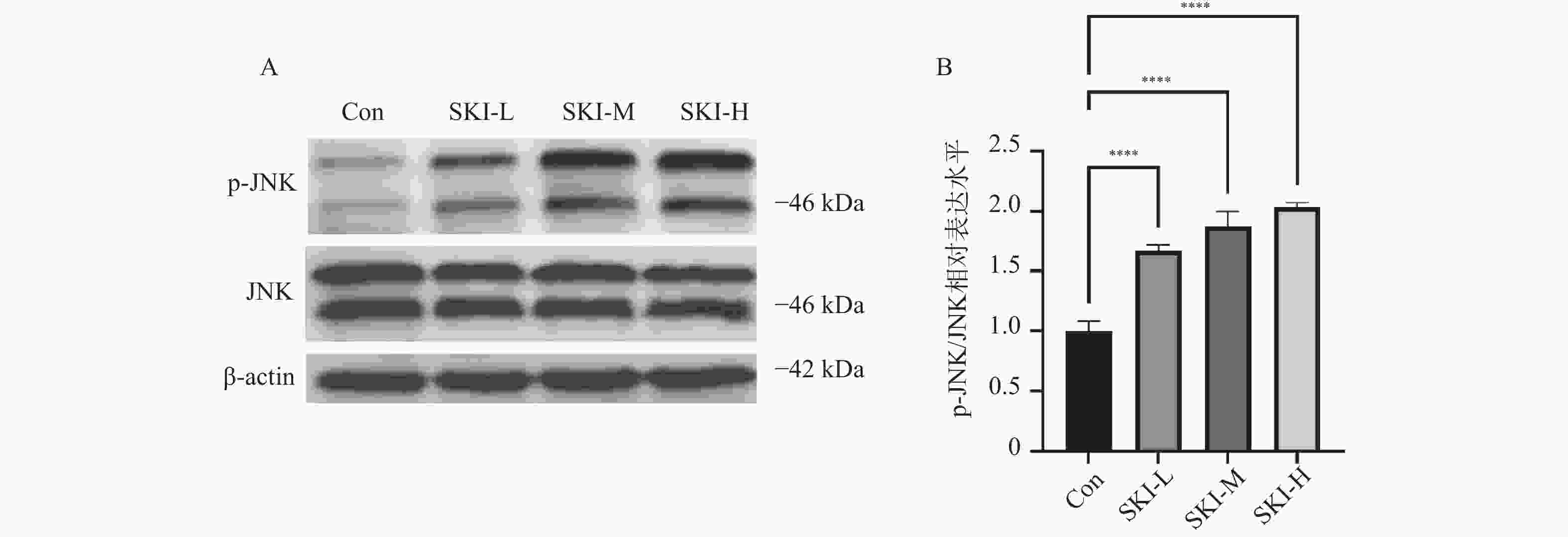
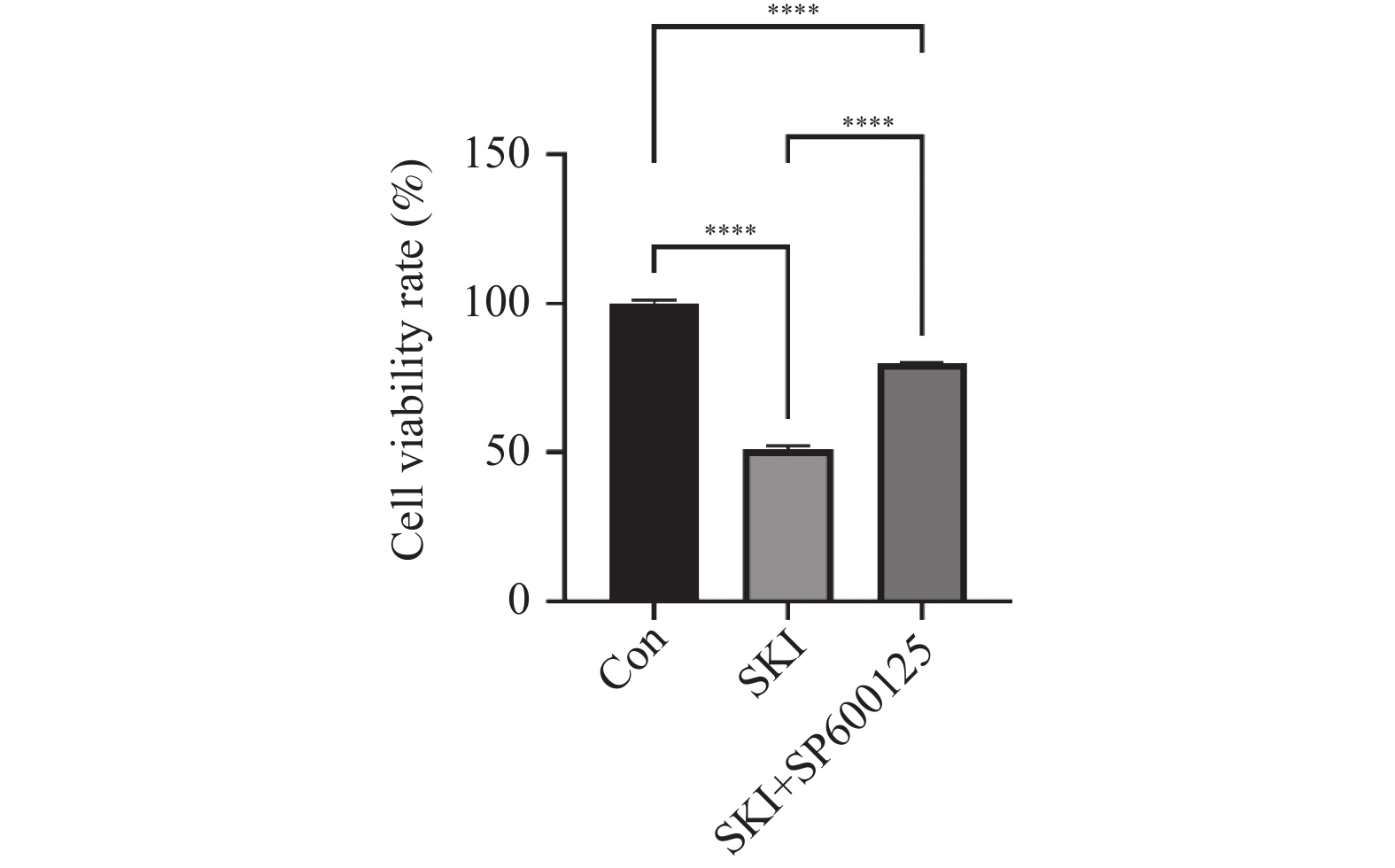
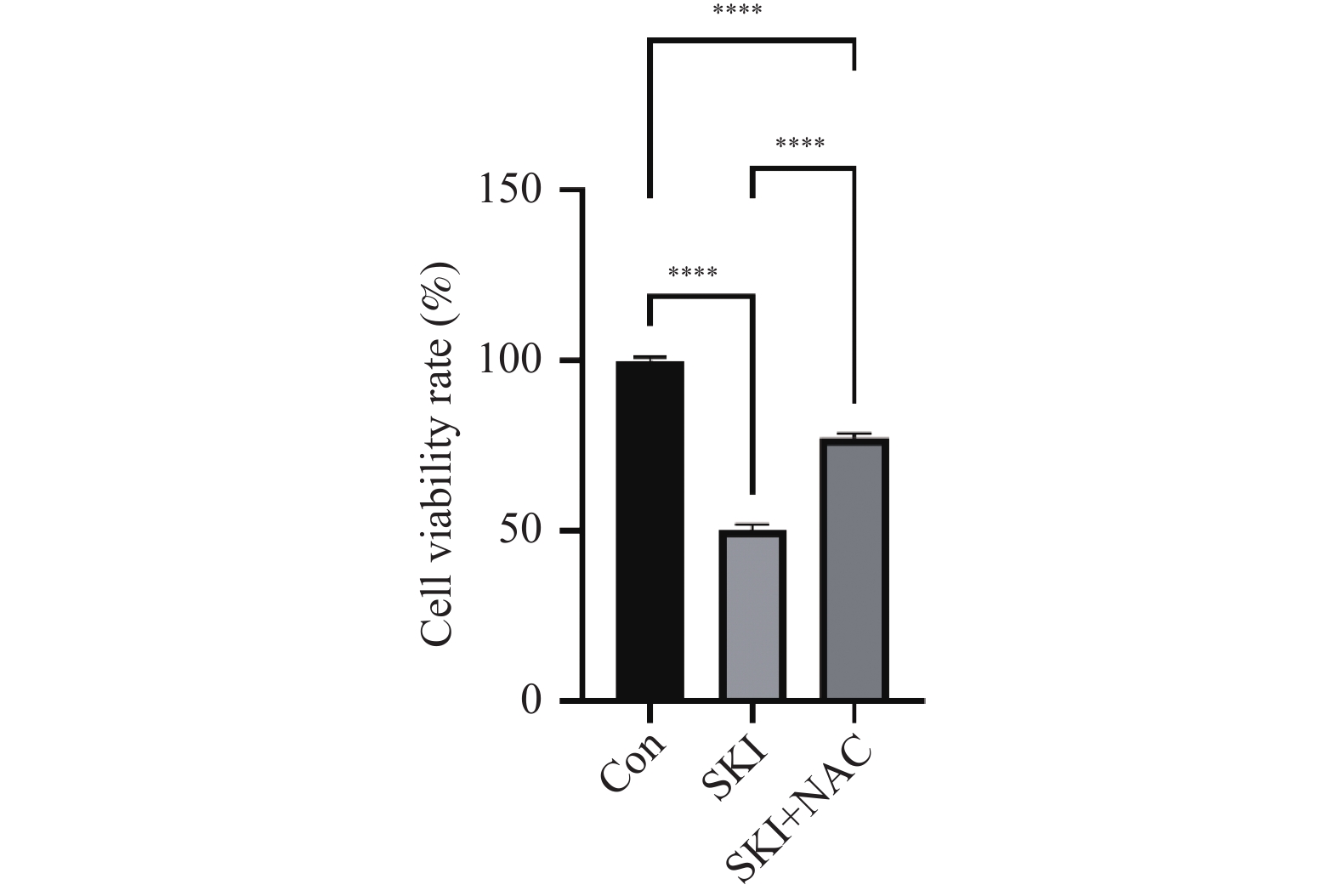
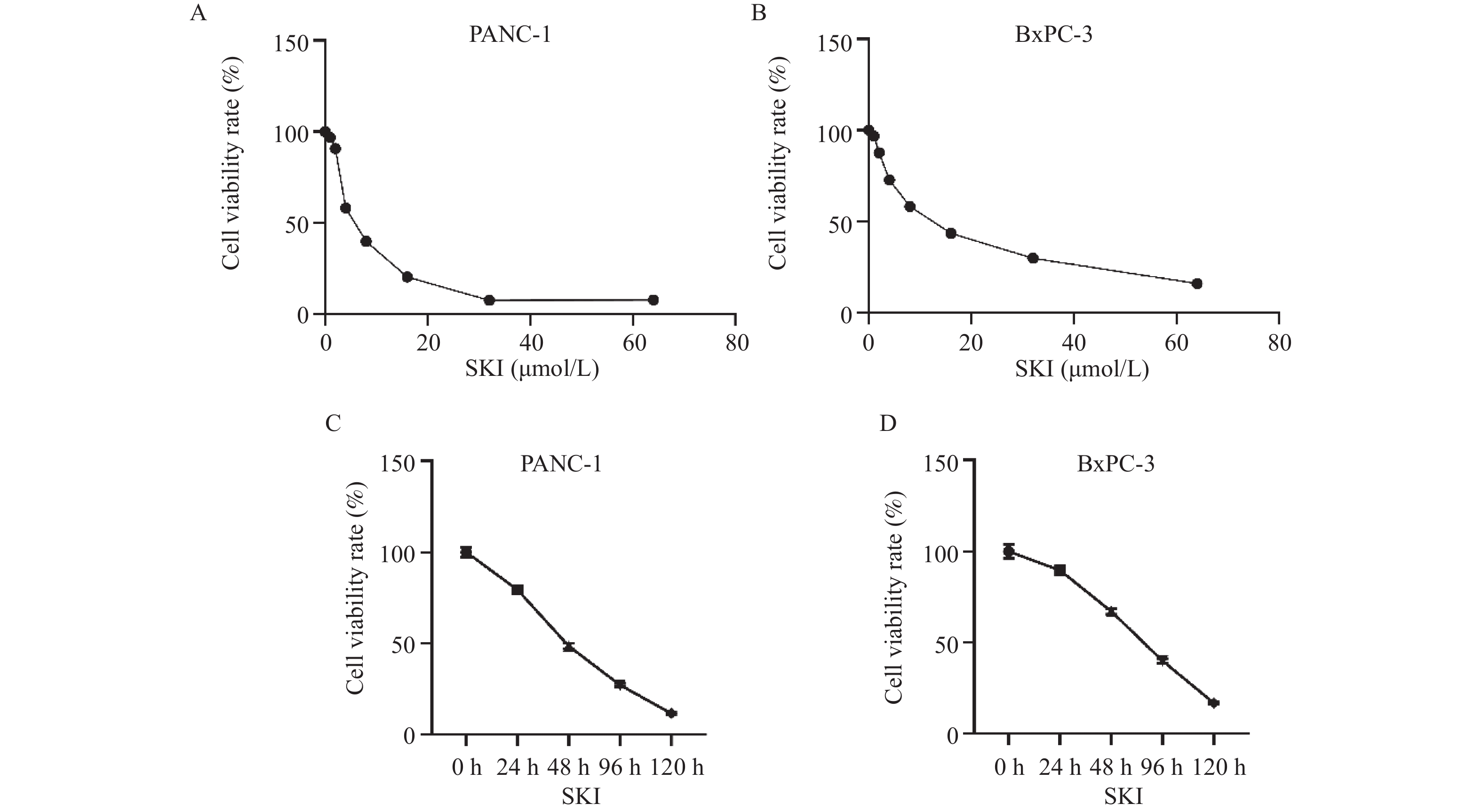
目的 探讨紫草素(Shikonin,SKI)能否通过ROS/JNK通路诱导铁死亡从而抑制胰腺癌恶性行为。 方法 选用人胰腺癌PANC-1细胞或BxPC-3细胞,药效实验设置空白组(Con组)、SKI低、中、高剂量组(2,4,8 μmol/L),JNK相关机制实验分为空白组(Con组)、SKI组(SKI组)、SKI+JNK抑制剂组(SKI+SP600125组),ROS相关机制实验分为空白组(Con组)、SKI组(SKI组)、SKI+ROS清除剂组(SKI+NAC组)。采用CCK-8法检测细胞活力并计算半数抑制浓度(IC50);Transwell实验评估细胞迁移与侵袭能力;使用C11 BODIPY 581/591探针通过流式细胞术检测脂质过氧化水平,FerroOrange荧光探针测定亚铁离子水平;活性氧检测试剂盒检测ROS水平;Western blot法检测铁死亡关键蛋白(SLC7A11、GPX4)、凋亡相关蛋白(Caspase3、PARP)及JNK通路蛋白(JNK、p-JNK)的表达;采用体内异种移植瘤模型检测体内肿瘤增殖情况。 结果 SKI处理可浓度依赖性和时间依赖性地显著抑制PANC-1细胞(IC50为6.04 μmol/L,P < 0.0001 )和BxPC-3细胞(IC50为12.27 μmol/L,P <0.0001 )活力,并明显减少迁移和侵袭的细胞数(P <0.0001 ),8 μmol/L SKI处理组迁移细胞数降至对照组的约30%(P <0.0001 )。机制上,SKI诱导细胞内脂质过氧化水平升高、Fe2+积累以及ROS大量生成(P <0.0001 ),同时显著下调SLC7A11和GPX4的蛋白表达(GPX4蛋白表达降至对照组的40%,P <0.0001 ),并激活JNK磷酸化(p-JNK/JNK比值增至2.8倍,P <0.0001 )。而使用JNK特异性抑制剂SP600125或ROS清除剂NAC预处理,均能有效逆转SKI对细胞活力的抑制或对SLC7A11/GPX4蛋白的下调作用(均P < 0.01)。SKI也能在体内抑制胰腺癌肿瘤细胞的增殖(P <0.0001 )。结论 SKI通过激活ROS/JNK通路诱导铁死亡,进而抑制胰腺癌增殖、迁移与侵袭。 Abstract:Objective To investigate if Shikonin (SKI) can induce ferroptosis via the ROS/JNK pathway to inhibit the malignant behavior of pancreatic cancer. Methods Human pancreatic cancer PANC-1 or BxPC-3 cells were selected. Drug efficacy experiments were established with a blank control group (Con group) and low, medium, and high dose SKI groups (2, 4, 8 μmol/L). JNK-related mechanism experiments were categorized into a blank control group (Con group), SKI group, and SKI+JNK inhibitor group (SKI+SP600125 group). ROS-related mechanism experiments were divided into a blank control group (Con group), SKI group, and SKI+ROS scavenger group (SKI+NAC group). Cell viability was assessed using the CCK-8 method to calculate IC50; Transwell experiments evaluated cell migration and invasion capabilities; the C11 BODIPY 581/591 probe was utilized for flow cytometry to detect lipid peroxidation levels, while the FerroOrange fluorescent probe measured ferrous ion levels; ROS levels were determined using a ROS detection kit; the Western blot method identified ferroptosis-related key proteins (SLC7A11, GPX4), apoptosis-related proteins (Caspase3, PARP), and JNK pathway proteins (JNK, p-JNK); an in vivo xenograft tumor model was employed to assess tumor proliferation. Results SKI treatment significantly and dose-dependently inhibited PANC-1 cell viability (IC50: 6.04 μmol/L, P < 0.0001 ) and BxPC-3 cell viability (IC50: 12.27 μmol/L, P <0.0001 ), and significantly reduced migrating and invasive cell numbers (P <0.0001 ), with migration cell numbers dropping to about 30% of the control group at 8 μmol/L SKI treatment (P <0.0001 ). Mechanistically, SKI induced increased intracellular lipid peroxidation, Fe2+ accumulation, and significant ROS production (P <0.0001 ), significantly downregulated SLC7A11 and GPX4 protein expression (GPX4 protein expression reduced to 40% of that in the control group, P <0.0001 ), and activated JNK phosphorylation (p-JNK/JNK ratio increased to 2.8-fold, P <0.0001 ). Pretreatment with the JNK-specific inhibitor SP600125 or ROS scavenger NAC effectively reversed SKI's inhibition of cell viability and downregulation of SLC7A11/GPX4 protein (all P < 0.01). SKI also inhibited pancreatic cancer tumor cell proliferation in vivo (P <0.0001 ).Conclusion SKI induces ferroptosis by activating the ROS/JNK pathway, thereby inhibiting pancreatic cancer proliferation, migration, and invasion. -
Key words:
- Shikonin /
- Ferroptosis /
- Pancreatic cancer /
- Reactive oxygen species /
- JNK
-
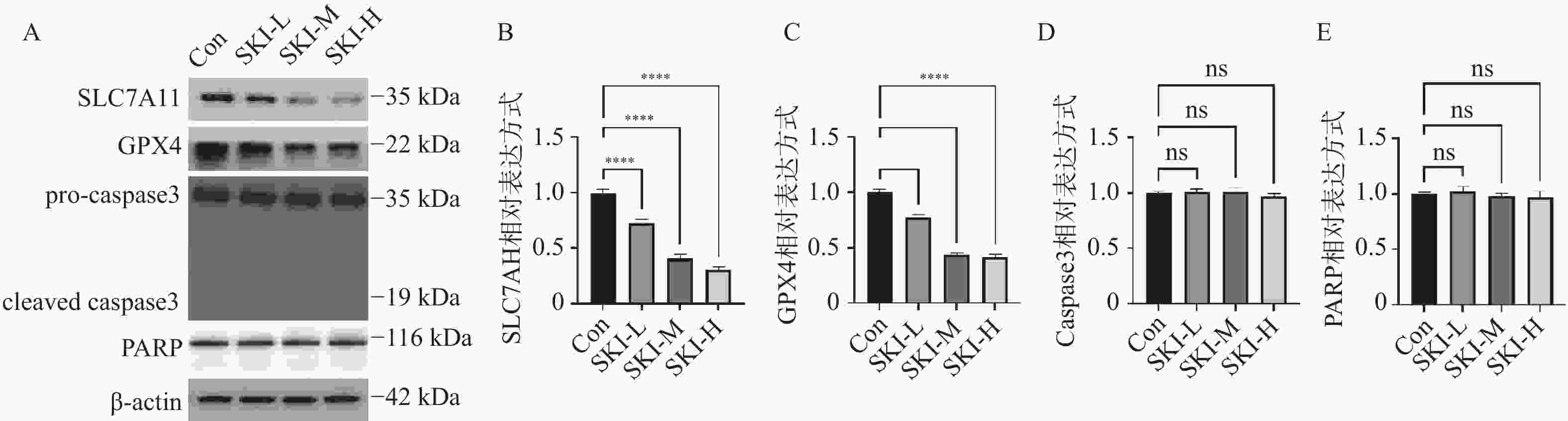
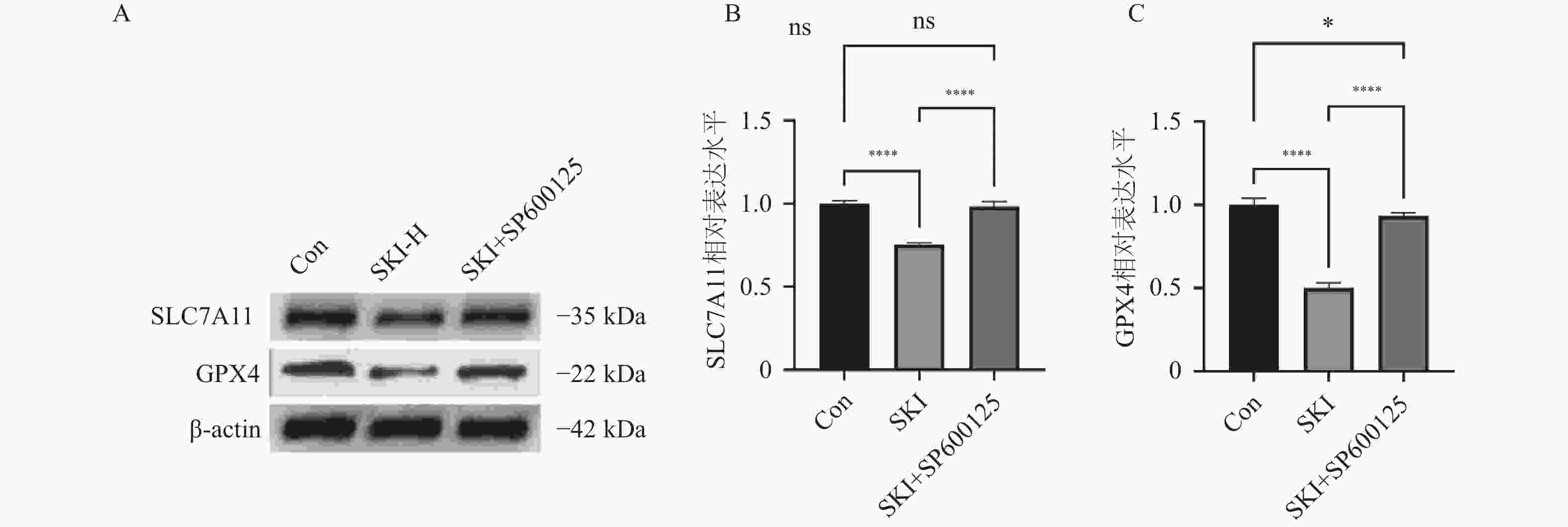
图 5 PANC-1细胞内SLC7A11、GPX4、Caspase3、PARP蛋白表达水平(n = 5)
A:SKI给药干预PANC-1细胞后Western blot结果图;B:SKI给药干预PANC-1细胞后SLC7A11相对表达水平;C:SKI给药干预PANC-1细胞后GPX4相对表达水平;D:SKI给药干预PANC-1细胞后Caspase3相对表达水平;E:SKI给药干预PANC-1细胞后PARP相对表达水平;****P < 0.0001;ns,no significance。
Figure 5. Expression levels of SLC7A11,GPX4,Caspase3,and PARP proteins in PANC-1 cells (n = 5)
-
[1] Halbrook C J, Lyssiotis C A, Pasca di Magliano M, et al. Pancreatic cancer: Advances and challenges[J]. Cell, 2023, 186(8): 1729-1754. doi: 10.1016/j.cell.2023.02.014 [2] Klein A P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors[J]. Nat Rev Gastroenterol Hepatol, 2021, 18(7): 493-502. doi: 10.1038/s41575-021-00457-x [3] Yu B F, Shao S W, Ma W X. Frontiers in pancreatic cancer on biomarkers, microenvironment, and immunotherapy[J]. Cancer Lett, 2025, 610: 217350. doi: 10.1016/j.canlet.2024.217350 [4] Heinrich M A, Mostafa A M R H, Morton J P, et al. Translating complexity and heterogeneity of pancreatic tumor: 3D in vitro to in vivo models[J]. Adv Drug Deliv Rev, 2021, 174(2021): 265-293. [5] Grunwald B T, Devisme A, Andrieux G, et al. Spatially confined sub-tumor microenvironments in pancreatic cancer[J]. Cell, 2021, 184(22): 5577-5592 e18. [6] Gupta T, Murtaza M. Advancing targeted therapies in pancreatic cancer: Leveraging molecular abberrations for therapeutic success[J]. Prog Biophys Mol Biol, 2025, 196: 19-32. doi: 10.1016/j.pbiomolbio.2025.02.003 [7] Jiang X, Stockwell B R, Conrad M. Ferroptosis: Mechanisms, biology and role in disease[J]. Nat Rev Mol Cell Biol, 2021, 22(4): 266-282. doi: 10.1038/s41580-020-00324-8 [8] Zheng J S, Conrad M. Ferroptosis: When metabolism meets cell death[J]. Physiol Rev, 2025, 105(2): 651-706. doi: 10.1152/physrev.00031.2024 [9] Lei G, Zhuang L, Gan B. The roles of ferroptosis in cancer: Tumor suppression, tumor microenvironment, and therapeutic interventions[J]. Cancer Cell, 2024, 42(4): 513-534. doi: 10.1016/j.ccell.2024.03.011 [10] Cheng X F, Zhao F, Ke B X, et al. Harnessing ferroptosis to overcome drug resistance in colorectal cancer: Promising therapeutic approaches[J]. Cancers (Basel), 2023, 15(21): 5209-5216. doi: 10.3390/cancers15215209 [11] Liu J, Kang R, Tang D L. The art of war: Ferroptosis and pancreatic cancer[J]. Front Pharmacol, 2021, 12: 773909. doi: 10.3389/fphar.2021.773909 [12] Lyu H, Kong J H, Chen J S, et al. The emerging scenario of ferroptosis in pancreatic cancer tumorigenesis and treatment[J]. Int J Mol Sci, 2024, 25(24): 13334. doi: 10.3390/ijms252413334 [13] Guo Y M, Zhou M M, Mu Z Z, et al. Recent advances in shikonin for the treatment of immune-related diseases: Anti-inflammatory and immunomodulatory mechanisms[J]. Biomedicine & Pharmacotherapy, 2023, 165: 115138. [14] Yadav S, Sharma A, Nayik G A, et al. Review of shikonin and derivatives: Isolation, chemistry, biosynthesis, pharmacology and toxicology[J]. Front Pharmacol, 2022, 13: 905755. doi: 10.3389/fphar.2022.905755 [15] Qi K, Li J Y, Hu Y, et al. Research progress in mechanism of anticancer action of shikonin targeting reactive oxygen species[J]. Frontiers in Pharmacology, 2024, 15: 1416781. doi: 10.3389/fphar.2024.1416781 [16] Dong H, Chang C D, Gao F, et al. The anti-leukemia activity and mechanisms of shikonin: A mini review[J]. Front Pharmacol, 2023, 14: 1271252. doi: 10.3389/fphar.2023.1271252 [17] Wang B Q, Wang Y, Zhang J, et al. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis[J]. Arch Toxicol, 2023, 97(6): 1439-1451. doi: 10.1007/s00204-023-03476-6 [18] Zheng Y L, Liu F Y, Huang Y H, et al. SNORA58 facilitates radioresistance via suppressing JNK1-mediated ferroptosis in esophageal squamous cell carcinoma[J]. Adv Sci (Weinh), 2025, e08515. [19] Koeberle S C, Kipp A P, Stuppner H, et al. Ferroptosis-modulating small molecules for targeting drug-resistant cancer: Challenges and opportunities in manipulating redox signaling[J]. Med Res Rev, 2023, 43(3): 614-682. doi: 10.1002/med.21933 [20] Tsai M F, Chen S M, Ong A Z, et al. Shikonin induced program cell death through generation of reactive oxygen species in renal cancer cells[J]. Antioxidants (Basel), 2021, 10(11): 1831-1844. doi: 10.3390/antiox10111831 [21] Valipour M. Recent advances of antitumor shikonin/alkannin derivatives: A comprehensive overview focusing on structural classification, synthetic approaches, and mechanisms of action[J]. Eur J Med Chem, 2022, 235: 114314. doi: 10.1016/j.ejmech.2022.114314 [22] Wu J J, Shi Y T, Zhou M, et al. Nutrient vitamins enabled metabolic regulation of ferroptosis via reactive oxygen species biology[J]. Front Pharmacol, 2024, 15: 1434088. doi: 10.3389/fphar.2024.1434088 [23] Ye S Y, Hu X Y, Sun S W, et al. Oridonin promotes RSL3-induced ferroptosis in breast cancer cells by regulating the oxidative stress signaling pathway JNK/Nrf2/HO-1[J]. Eur J Pharmacol, 2024, 974: 176620. doi: 10.1016/j.ejphar.2024.176620 [24] Qi H, Zhang X, Liu H H, et al. Shikonin induced apoptosis mediated by endoplasmic reticulum stress in colorectal cancer cells[J]. J Cancer, 2022, 13(1): 243-252. doi: 10.7150/jca.65297 [25] Lee J H, Han S H, Kim Y M, et al. Shikonin inhibits proliferation of melanoma cells by MAPK pathway-mediated induction of apoptosis[J]. Biosci Rep, 2021, 41(1): BSR20203834. doi: 10.1042/BSR20203834 [26] Kiraly J, Szabo E, Fodor P, et al. Shikonin causes an apoptotic effect on human kidney cancer cells through Ras/MAPK and PI3K/AKT pathways[J]. Molecules, 2023, 28(18): 6725. doi: 10.3390/molecules28186725 [27] Liu S Y, Li L Y, Ren D M. Anti-cancer potential of phytochemicals: The regulation of the epithelial-mesenchymal transition[J]. Molecules, 2023, 28(13): 5069. doi: 10.3390/molecules28135069 -





 下载:
下载: