Mechanistic Studies on the Improvement of Diabetic Peripheral Neuropathy by Plantamajoside via Promoting the PINK1/Parkin Mitochondrial Autophagy Pathway
-
摘要:
目的 研究大车前苷(plantamajoside,PMS)对糖尿病周围神经病变(diabetic peripheral neuropathy,DPN)的疗效并从线粒体自噬探讨其作用机制。 方法 C57BL/6J小鼠随机分为6组(n = 10):正常组(Control)、模型组(Model)、阳性药物组(LA)、大车前苷低(L-PMS)、中(M-PMS)、高(H-PMS)剂量组,高糖高脂饮食配合腹腔注射链脲佐菌素构建DPN模型。模型构建成功后,灌胃给药干预4周,取坐骨神经,HE染色和Nissl染色观察其病理学变化;通过试剂盒检测坐骨神经组织中氧化应激指标SOD、MDA、GSH-Px和炎症因子IL-1β、IL-6、TNF-α; Western blotting法检测小鼠坐骨神经VDAC1、TOM20、COX IV、PINK1、Parkin、自噬蛋白Beclin1、LC3、P62表达。 结果 PMS可剂量依赖性地改善DPN小鼠的行为学指标,减轻坐骨神经的病理损伤,使坐骨神经纤维排列紧密,髓鞘结构清晰可见,着色均匀,施旺细胞以及尼氏体数量增加。与模型组比较,M-PMS组、H-PMS组均可提高SOD、GSH-Px的表达水平(P < 0.05),降低MDA表达(P < 0.05)。与模型组比较,M-PMS组、H-PMS组可降低IL-1β、IL-6、TNF-α表达(P < 0.05)。与模型组比较,L-PMS组、M-PMS组、H-PMS组均可不同程度地降低VDAC1、TOM20与COX IV蛋白表达水平(P < 0.05)。与模型组比较,L-PMS组、M-PMS组、H-PMS组均可不同程度地使PINK1、Beclin1、Parkin与LC3蛋白表达上升(P < 0.05),P62蛋白表达下降(P < 0.05)。 结论 PMS可能通过促进PINK1/Parkin通路介导的线粒体自噬,缓解氧化应激相关的炎症损伤,在改善 DPN 小鼠神经损伤方面发挥作用。 -
关键词:
- 糖尿病周围神经病变 /
- 大车前苷 /
- 线粒体自噬 /
- PINK1/Parkin /
- 坐骨神经
Abstract:Objective To investigate the efficacy of plantamajoside (PMS) on diabetic peripheral neuropathy (DPN) and to explore its mechanism of action from mitochondrial autophagy. Methods Mice (C57BL/6J) were randomly divided into 6 groups(n = 10): normal group (Control), model group (Model), positive drug group (LA), and low (L-PMS), medium (M-PMS), and high (H-PMS) dosage groups. High-sugar and high-fat diet with intraperitoneal injection of streptozotocin was used to duplicate the DPN model. After successful model duplication, the intervention was administered by gavage for 4 weeks. Sciatic nerve was taken, and pathological changes were observed by HE and Nissl staining; oxidative stress indexes SOD, MDA, GSH-Px and inflammatory factors such as IL-1β, IL-6, TNF-αin sciatic nerve tissues were detected by kits, and the expression of VDAC1 , TOM20, COX IV, PINK1, Parkin, and autophagy proteins of Beclin1, LC3, P62 in mouse sciatic nerves was detected by Western blotting . Results PMS dose-dependently improved the behavioral indexes of DPN mice, reduced the pathological damage of sciatic nerve, and resulted in tightly arranged sciatic nerve fibers, clearly visible myelin structure, uniform coloration, and increased number of Schwann cells as well as Nissl bodies. Compared with the model group, both the M-PMS group and the H-PMS group increased the expression levels of SOD and GSH-Px (P < 0.05), while decreased the expression of MDA (P < 0.05); the M-PMS group and the H- PMS groups reduced the expression of IL-1β, IL-6, and TNF-α (P < 0.05); the L-PMS group, M-PMS group, and H-PMS group reduced the expression levels of VDAC1, TOM20, and COX IV proteins (P < 0.05); the L-PMS group, M-PMS group, and H-PMS group could differentially increase the expression of PINK1, Beclin1, Parkin, and LC3 proteins (P < 0.05), and decrease the expression of P62 proteins (P < 0.05). Conclusion PMS can play a role in ameliorating neurological injury in DPN mice by promoting PINK1/Parkin pathway-mediated mitochondrial autophagy and alleviating oxidative stress-related inflammatory injury. -
Key words:
- Diabetic peripheral neuropathy /
- Plantamajoside /
- Mitochondrial autophagy /
- PINK1/Parkin /
- Sciatic nerve
-
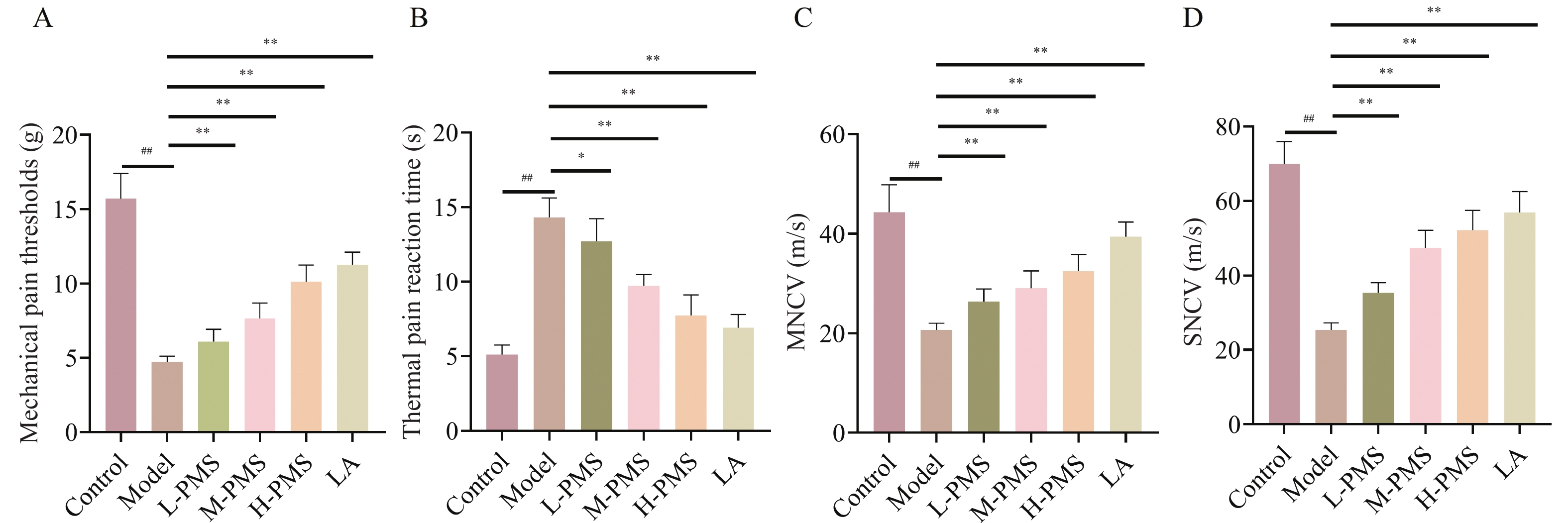
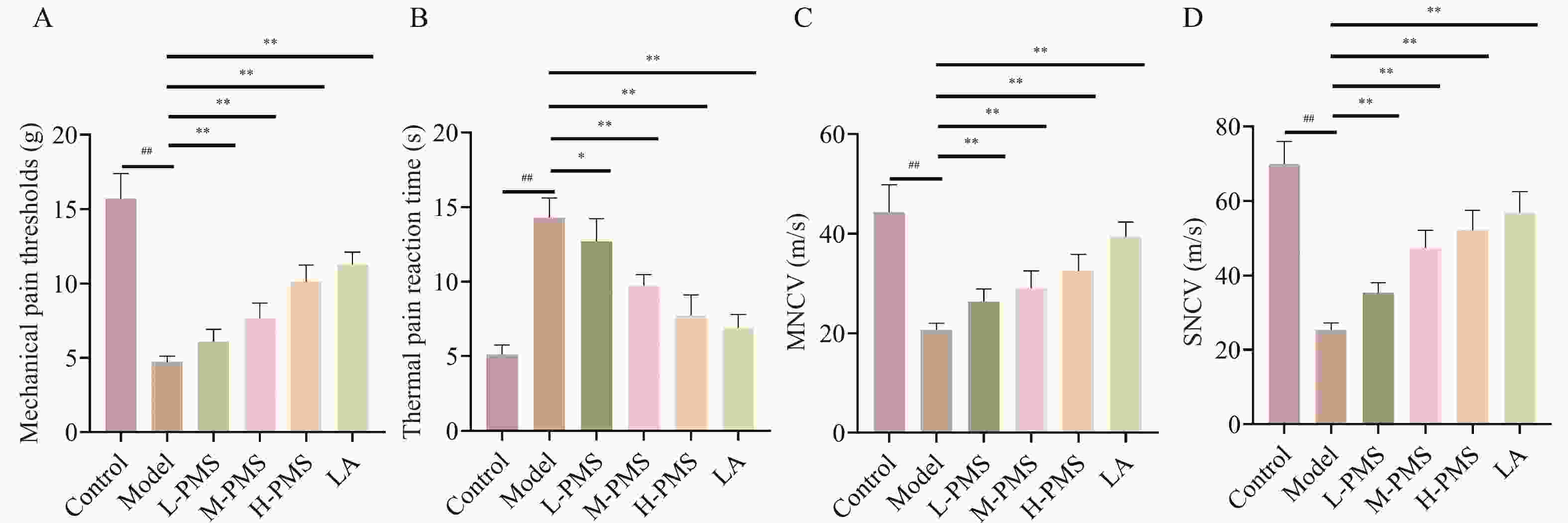
图 1 各组小鼠机械痛阈、热痛反应时间、运动神经传导速度、感觉神经传导速度比较[($\bar x \pm s $),n = 10]
A:各组小鼠机械痛阈统计学分析;B:各组小鼠热痛反应时间统计学分析;C:各组小鼠运动神经传导速度统计学分析;D:各组小鼠感觉神经传导速度统计学分析。与Control组比较,## P < 0.01;与Model组比较,* P < 0.05,** P < 0.01。
Figure 1. Comparison of mechanical pain threshold,thermal pain reaction time,motor nerve conduction velocity,and sensory nerve conduction velocity in the mice of each group[($\bar x \pm s $),n = 10]
图 3 各组小鼠MDA、SOD、GSH-Px、IL-1β、IL-6、TNF-α比较[($ \bar x \pm s$),n = 10]
A:小鼠血清SOD表达统计学分析;B:小鼠血清MDA表达统计学分析;C:小鼠血清GSH-Px表达统计学分析;D:小鼠血清IL-1β表达统计学分析;E:小鼠血清IL-6表达统计学分析;F:小鼠血清TNF-α表达统计学分析。注:与Control组比较,## P < 0.01;与Model组比较,* P < 0.05,** P < 0.01。
Figure 3. Comparison of IL-1β,IL-6,TNF-α,MDA,SOD,GSH-Px in the mice of each group[($\bar x \pm s $),n = 10]
图 4 各组小鼠线粒体形态结构蛋白表达水平的变化[($\bar x \pm s $),n = 3]
A:各组小鼠线粒体形态结构蛋白表达;B:各组小鼠VDAC1蛋白表达统计学分析;C:各组小鼠TOM20蛋白表达统计学分析;D:各组小鼠COX IV蛋白表达统计学分析。与Control组比较,## P < 0.01;与Model组比较,* P < 0.05,** P < 0.01。
Figure 4. Changes in the expression levels of mitochondrial morphological structure proteins in various groups of the mice[($\bar x \pm s $),n = 3]
图 5 各组小鼠PINK1/Parkin 介导线粒体自噬蛋白水平的变化[($\bar x \pm s $),n = 3]
A:各组小鼠线粒体自噬蛋白表达;B:各组小鼠PINK1蛋白表达统计学分析。C:各组小鼠Parkin蛋白表达统计学分析;D:各组小鼠Beclin1蛋白表达统计学分析;E:各组小鼠LC3蛋白表达统计学分析;F:各组小鼠P62蛋白表达统计学分析。注:与Control组比较,## P < 0.01;与Model组比较,* P < 0.05,** P < 0.01。
Figure 5. Changes in PINK1/Parkin-mediated mitochondrial autophagy protein levels in various groups of the mice[($\bar x \pm s $),n = 3]
-
[1] Shillo P, Sloan G, Greig M, et al. Painful and painless diabetic neuropathies: What is the difference?[J]. Curr Diab Rep, 2019, 19(6): 32-45. doi: 10.1007/s11892-019-1150-5 [2] 梁晓春. 糖尿病周围神经病变整合诊治进展[J]. 中国中西医结合杂志, 2021, 41(6): 656-659. [3] Chen W, Wu X, Li S, et al. Optical coherence tomography of the retina combined with color Doppler ultrasound of the tibial nerve in the diagnosis of diabetic peripheral neuropathy[J]. Front Endocrinol (Lausanne), 2022, 13(1): 938659-938670. [4] Ziegler D. Pathogenetic treatments for diabetic peripheral neuropathy[J]. Diabetes Res Clin Pract, 2023, 206(1): 110764-110768. [5] 郑宏庭, 瞿华. 线粒体动力学与糖尿病并发症发病机制[J]. 陆军军医大学学报, 2022, 44(1): 64-68. [6] Ye D, Fairchild T J, Vo L, et al. Painful diabetic peripheral neuropathy: Role of oxidative stress and central sensitisation[J]. Diabet Med, 2022, 39(1): e14729-e14744. doi: 10.1111/dme.14729 [7] Wu L, Wang X J, Luo X, et al. Diabetic peripheral neuropathy based on Schwann cell injury: Mechanisms of cell death regulation and therapeutic perspectives[J]. Front Endocrinol (Lausanne), 2024, 15(1): 1427679-1427691. [8] Li J, Yang D, Li Z, et al. PINK1/Parkin-mediated mitophagy in neurodegenerative diseases[J]. Ageing Res Rev, 2023, 22(1): 101817-101829. [9] 黄苏苏, 王雪茹, 袁久术, 等. 中医药基于自噬防治糖尿病周围神经病变的实验研究进展[J]. 中国中药杂志, 2023, 48(23): 6315-6323. [10] Hu T, Lu X Y, Shi J J, et al. Quercetin protects against diabetic encephalopathy via SIRT1/NLRP3 pathway in db/db mice[J]. J Cell Mol Med, 2020, 24(6): 3449-3459. doi: 10.1111/jcmm.15026 [11] 陈信朝, 董新刚. 氧化应激途径下中药单体对血管性痴呆的调控机制研究[J]. 中山大学学报(医学科学版), 2025, 46(2): 221-229. [12] Xu H, Yu H, Fu J, et al. Metabolites analysis of plantamajoside based on gut microbiota-drug interaction[J]. Phytomedicine, 2023, 116(1): 154841-154852. [13] Guo X, Chen L, Li J. Plantamajoside alleviates substantia nigra damage in Parkinson's disease mice by inhibiting HDAC2/MAPK signaling and reducing microglia polarization[J]. ACS Chem Neurosci, 2023, 14(6): 1119-1125. doi: 10.1021/acschemneuro.2c00668 [14] Hu H, Jian X. The protective mechanism of action of plantamajoside on a rat model of acute spinal cord injury[J]. Exp Ther Med, 2021, 12(4): 378-385. doi: 10.3892/etm.2021.9809 [15] 殷娟, 张明红, 杨红英. 2型糖尿病大鼠周围神经病变模型的建立及相关指标检测[J]. 国际检验医学杂志, 2013, 34(1): 1-2+5. doi: 10.3969/j.issn.1673-4130.2013.01.001 [16] 王璇, 徐斌. 2型糖尿病周围神经病变动物模型的建立及检测指标分析[J]. 中国医学科学院学报, 2020, 42(5): 658-666. [17] Abdelkader N F, Elbaset M A, Moustafa P E, et al. Empagliflozin mitigates type 2 diabetes-associated peripheral neuropathy: A glucose-independent effect through AMPK signaling[J]. Arch Pharm Res, 2022, 45(7): 475-493. doi: 10.1007/s12272-022-01391-5 [18] 杨海艳, 曾庆雅, 欧阳香, 等. 大车前苷对氧嗪酸钾诱导小鼠急性高尿酸血症模型的影响[J]. 中国药理学通报, 2021, 37(12): 1709-1715. [19] Goebel A, Krock E, Gentry C, et al. Passive transfer of fibromyalgia symptoms from patients to mice[J]. J Clin Invest, 2021, 98(13): e144201-e144214. doi: 10.1172/JCI144201 [20] Zhang L, Li M, Li X, et al. Characteristics of sensory innervation in synovium of rats within different knee osteoarthritis models and the correlation between synovial fibrosis and hyperalgesia[J]. J Adv Res, 2022, 12(1): 141-151. [21] Hossain M J, Kendig M D, Wild B M, et al. Evidence of altered peripheral nerve function in a rodent model of diet-induced prediabetes[J]. Biomedicines, 2020, 8(9): 313-328. doi: 10.3390/biomedicines8090313 [22] Kadar A, Wittmann G, Liposits Z, et al. Improved method for combination of immunocytochemistry and nissl staining[J]. J Neurosci Methods, 2009, 31(1): 115-118. doi: 10.1016/j.jneumeth.2009.07.010 [23] Qian C, Zhang Z, Zhao R, et al. Effect of acellular nerve scaffold containing human umbilical cord-derived mesenchymal stem cells on nerve repair and regeneration in rats with sciatic nerve defect[J]. Ann Transl Med, 2022, 10(8): 483-494. doi: 10.21037/atm-22-1578 [24] 韩霞, 薛坚. α-硫辛酸联合甲钴胺治疗糖尿病周围神经病变的临床研究[J]. 哈尔滨医药, 2024, 44(1): 76-78. doi: 10.3969/j.issn.1001-8131.2024.01.025 [25] Hsieh R Y, Huang I C, Chen C, et al. Effects of oral alpha-lipoic acid treatment on diabetic polyneuropathy: A meta-analysis and systematic review[J]. Nutrients, 2023, 15(16): 3634-3647. doi: 10.3390/nu15163634 [26] Shen B, Zhao C, Wang Y, et al. Aucubin inhibited lipid accumulation and oxidative stress via Nrf2/HO-1 and AMPK signalling pathways[J]. J Cell Mol Med, 2019, 23(6): 4063-4075. doi: 10.1111/jcmm.14293 [27] Huang K, Liang Y, Wang K, et al. Influence of circulating nesfatin-1, GSH and SOD on insulin secretion in the development of T2DM[J]. Front Public Health, 2022, 16(1): 882686-882697. [28] Wang J, Hu C, Ma X, et al. The role of oxidative stress biomarkers in the development of peri-implant disease: A systematic review and meta-analysis[J]. J Dent, 2024, 53(1): 105026-105039. [29] Lotfy M, Khattab A, Shata M, et al. Melatonin increases AKT and SOD gene and protein expressions in diabetic rats[J]. Heliyon, 2024, 10(7): e28639-e28654. doi: 10.1016/j.heliyon.2024.e28639 [30] Chen M, Wang Q, Wang Y, et al. Thiostrepton induces oxidative stress, mitochondrial dysfunction and ferroptosis in HaCaT cells[J]. Cell Signal, 2024, 36(1): 111285-111299. [31] Xue T, Zhang X, Xing Y, et al. Advances about immunoinflammatory pathogenesis and treatment in diabetic peripheral neuropathy[J]. Front Pharmacol, 2021, 12(1): 748193-748203. [32] Zeng G, An H, Fang D, et al. Plantamajoside protects H9c2 cells against hypoxia/reoxygenation-induced injury through regulating the akt/Nrf2/HO-1 and NF-κB signaling pathways[J]. J Recept Signal Transduct Res, 2022, 42(2): 125-132. doi: 10.1080/10799893.2020.1859534 [33] Xiao D, Yang R, Gong L, et al. Plantamajoside inhibits high glucose-induced oxidative stress, inflammation, and extracellular matrix accumulation in rat glomerular mesangial cells through the inactivation of Akt/NF-κB pathway[J]. J Recept Signal Transduct Res, 2021, 41(1): 45-52. doi: 10.1080/10799893.2020.1784939 [34] Marchi S, Guilbaud E, Tait S, et al. Mitochondrial control of inflammation[J]. Nat Rev Immunol, 2023, 23(3): 159-173. doi: 10.1038/s41577-022-00760-x [35] Lacombe A, Scorrano L. The interplay between mitochondrial dynamics and autophagy: From a key homeostatic mechanism to a driver of pathology[J]. Semin Cell Dev Biol, 2024, 161-162: 1-19. doi: 10.1016/j.semcdb.2024.02.001 [36] Li A, Gao M, Liu B, et al. Mitochondrial autophagy: Molecular mechanisms and implications for cardiovascular disease[J]. Cell Death Dis, 2022, 13(5): 444-459. doi: 10.1038/s41419-022-04906-6 [37] Hu H, Guo L, Overholser J, et al. Mitochondrial VDAC1: A potential therapeutic target of inflammation-related diseases and clinical opportunities[J]. Cells, 2022, 11(19): 3174-3200. doi: 10.3390/cells11193174 [38] Narendra D P, Youle R J. The role of PINK1-Parkin in mitochondrial quality control[J]. Nat Cell Biol, 2024, 26(10): 1639-1651. doi: 10.1038/s41556-024-01513-9 [39] Quinn P, Moreira P I, Ambrósio A F, et al. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation[J]. Acta Neuropathol Commun, 2020, 8(1): 189-209. doi: 10.1186/s40478-020-01062-w -





 下载:
下载: