Study on the Mechanism by Which GPX4 Expression Promotes Ferroptosis and Participates in Malignant Behavior of Endometrial Carcinoma
-
摘要:
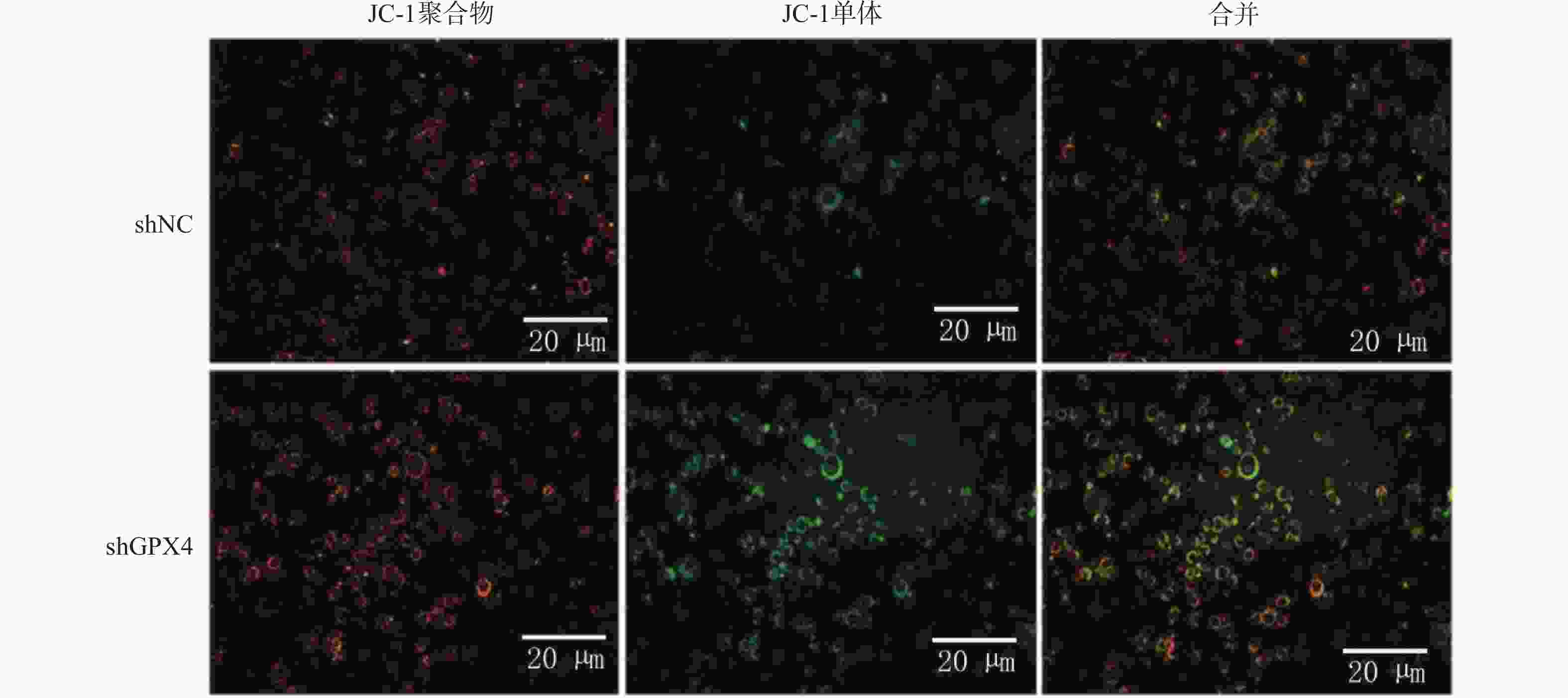
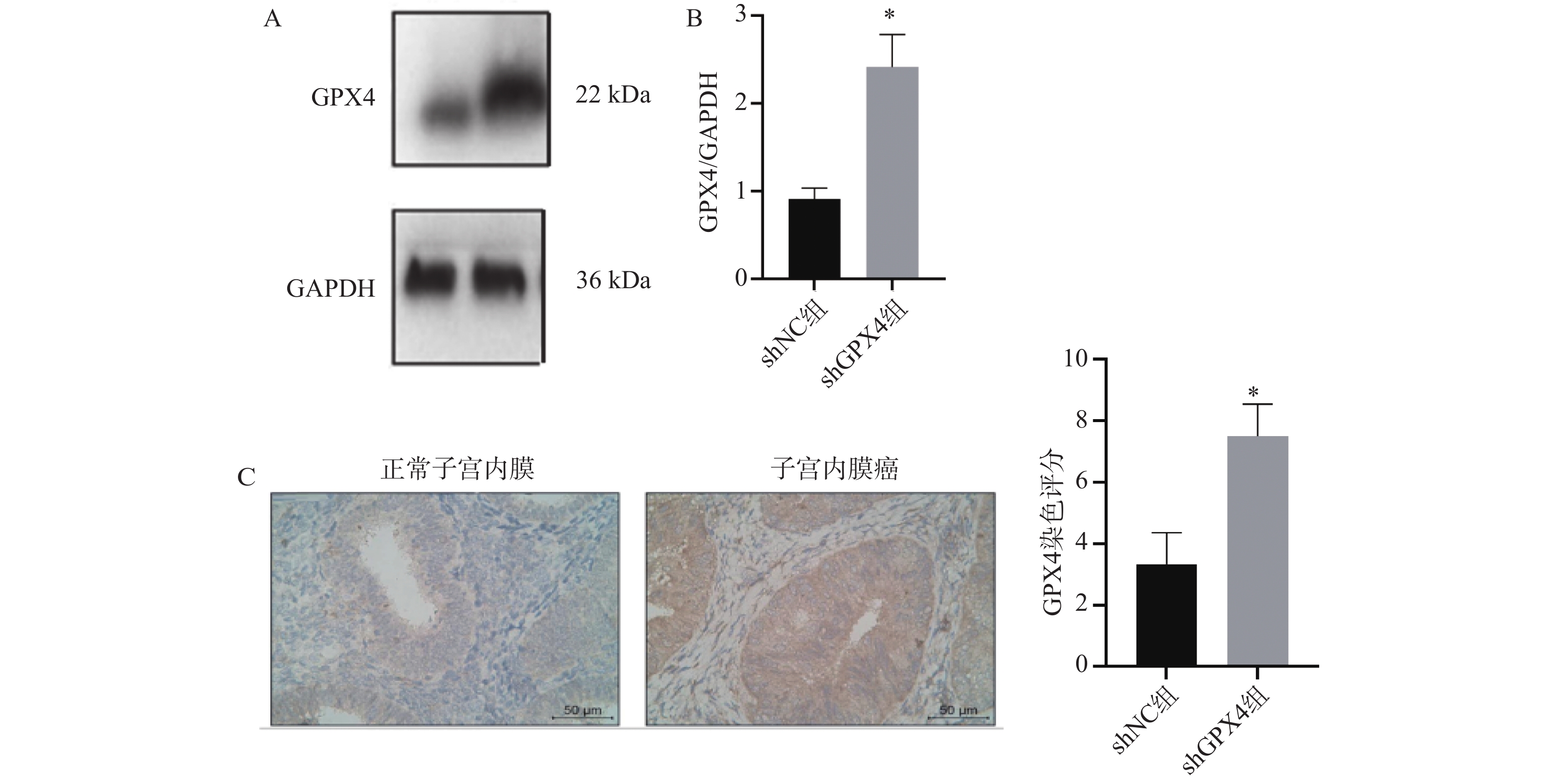
目的 探讨谷胱甘肽过氧化物酶4(glutathione peroxidase 4,GPX4)表达促进铁死亡参与子宫内膜癌(endometrial carcinoma,EC)恶性行为的机制。 方法 通过定量实时聚合酶链反应(quantitative polymerase chain reaction,qPCR)、 蛋白印迹和免疫组化(immunohistochemistry,IHC) 测定 EC 组织中 GPX4 的表达。培养KLE细胞,分为shNC组(n = 6)和shGPX4组(n = 6), Transwell、克隆形成测定和流式细胞研究了 GPX4 对 EC 细胞增殖、迁移、细胞凋亡的影响。检测细胞内 Fe2+、活性氧(reactive oxygen Species,ROS)和丙二醛(malondialdehyde,MDA)水平,JC染色检测线粒体膜电位(mitochondrial membrane Potential,MMP),GPX4 酶活性试剂盒检测酶活性,ELISA 法检测脂质过氧化物 4 - 羟基壬烯醛(4-Hydroxynonenal,4-HNE)含量,蛋白印迹检测铁死亡关键蛋白酰基辅酶A合成酶长链家族成员4(acyl-coa synthetase long chain family member 4,ACSL4)和溶质载体家族7成员11(solute Carrier family 7 member 11,SLC7A11)的表达;建立了裸鼠 EC 异种移植肿瘤模型,分为 shNC 组(n = 6)和 shGPX4 组(n = 6),通过检测其重量体积、重量、Fe2+和 MDA 水平评价 GPX4 敲低在体内的效果。 结果 GPX4 在 EC 组织及细胞系中高表达(EC 组织 GPX4 mRNA:118.1±6.92 vs 癌旁 62.72±5.20,t = 15.68,P < 0.001,Cohen's d=8.62);与 shNC 组相比,shGPX4 组 KLE 细胞增殖(克隆数:0.37±0.05 vs 0.89±0.07,t = 14.94,P < 0.001)、迁移能力显著降低,凋亡率升高(61.64±7.03% vs 12.60±2.48%,t = 16.12,P < 0.001),细胞内 Fe2+、脂质 ROS、MDA、4-HNE 水平升高,GSH 及 GPX4 酶活性降低,MMP 破坏,ACSL4 表达上调、SLC7A11 下调(均 P < 0.001);裸鼠模型中,shGPX4 组肿瘤体积(0.36±0.07 vs 0.87±0.12 cm3,t = 9.07,P < 0.001)、重量显著减小,肿瘤组织铁含量及 MDA 水平升高(均 P < 0.001)。 结论 研究表明GPX4 沉默能诱导铁死亡的发生,进而降低子宫内膜癌细胞的增殖和侵袭,为研究子宫内膜癌的潜在治疗策略提供了证据。 Abstract:Objective To investigate the mechanism by which Glutathione Peroxidase 4 (GPX4) expression promotes ferroptosis and participates in the malignant behavior of Endometrial Carcinoma (EC). Methods The expression of GPX4 in EC tissues was determined by quantitative real-time PCR (qPCR), Western blot, and immunohistochemistry (IHC). KLE cells were cultured and divided into an shNC group (n = 6) and an shGPX4 group (n = 6). The effects of GPX4 on EC cell proliferation, migration, and apoptosis were investigated using Transwell assay, colony formation assay, and flow cytometry. Intracellular Fe2+, reactive oxygen species (ROS), and malondialdehyde (MDA) levels were detected. Mitochondrial membrane potential (MMP) was measured by JC-1 staining. GPX4 enzyme activity was assessed with a commercial kit. The content of lipid peroxide 4-hydroxynonenal (4-HNE) was determined by ELISA; and the expression of key ferroptosis-related proteins, Acyl-CoA Synthetase Long Chain Family Member 4 (ACSL4) and Solute Carrier Family 7 Member 11 (SLC7A11), was analyzed by Western blot. A EC xenograft tumor model in nude mice was established and divided into an shNC group (n = 6) and an shGPX4 group (n = 6). The in vivo effect of GPX4 knockdown was evaluated by measuring tumor volume, weight, and levels of Fe2+ and MDA. Results GPX4 was highly expressed in EC tissues and cell lines (GPX4 mRNA in EC tissues: 118.1±6.92 vs. 62.72±5.20 in adjacent normal tissues, t = 15.68, P < 0.001, Cohen’s d=8.62).Compared with the shNC group, the shGPX4 group showed significantly decreased KLE cell proliferation (colony number: 0.37±0.05 vs. 0.89±0.07, t = 14.94, P < 0.001) and migration capacity, along with an increased apoptosis rate (61.64±7.03% vs. 12.60±2.48%, t = 16.12, P < 0.001). Additionally, in the shGPX4 group, intracellular levels of Fe2+, lipid ROS, MDA, and 4-HNE were increased; GSH level and GPX4 enzyme activity were decreased; MMP was impaired; and the expression of ACSL4 was up-regulated while that of SLC7A11 was down-regulated (all P < 0.001).In the nude mouse model, the shGPX4 group showed significantly reduced tumor volume (0.36±0.07 vs. 0.87±0.12 cm3, t = 9.07, P < 0.001) and weight, along with increased iron content and MDA levels in tumor tissues (both P < 0.001). Conclusion The study demonstrates that GPX4 silencing can significantly induce ferroptosis, thereby reducing the proliferation and invasion of endometrial cancer cells, providing evidence for the study of potential treatment strategies for endometrial cancer. -
Key words:
- GPX4 /
- ferroptosis /
- Endometrial cancer /
- Vicious behavior.
-
表 1 引物对
Table 1. Primer Pairs
基因名称 引物方向 引物序列 (5' → 3') GPX4 上游 Forward GAGGCAAGACCGAAGAGAAACTAC 下游 Reverse CCGAACTGGTTACACGGGAA GAPDH 上游 Forward GAGTCAACGGATTTGGTCGT 下游 Reverse GACAAGCTTCCCGTTCTCAG 表 2 GPX4对EC细胞中表达
Table 2. GPX4 expression in endometrial carcinoma cells
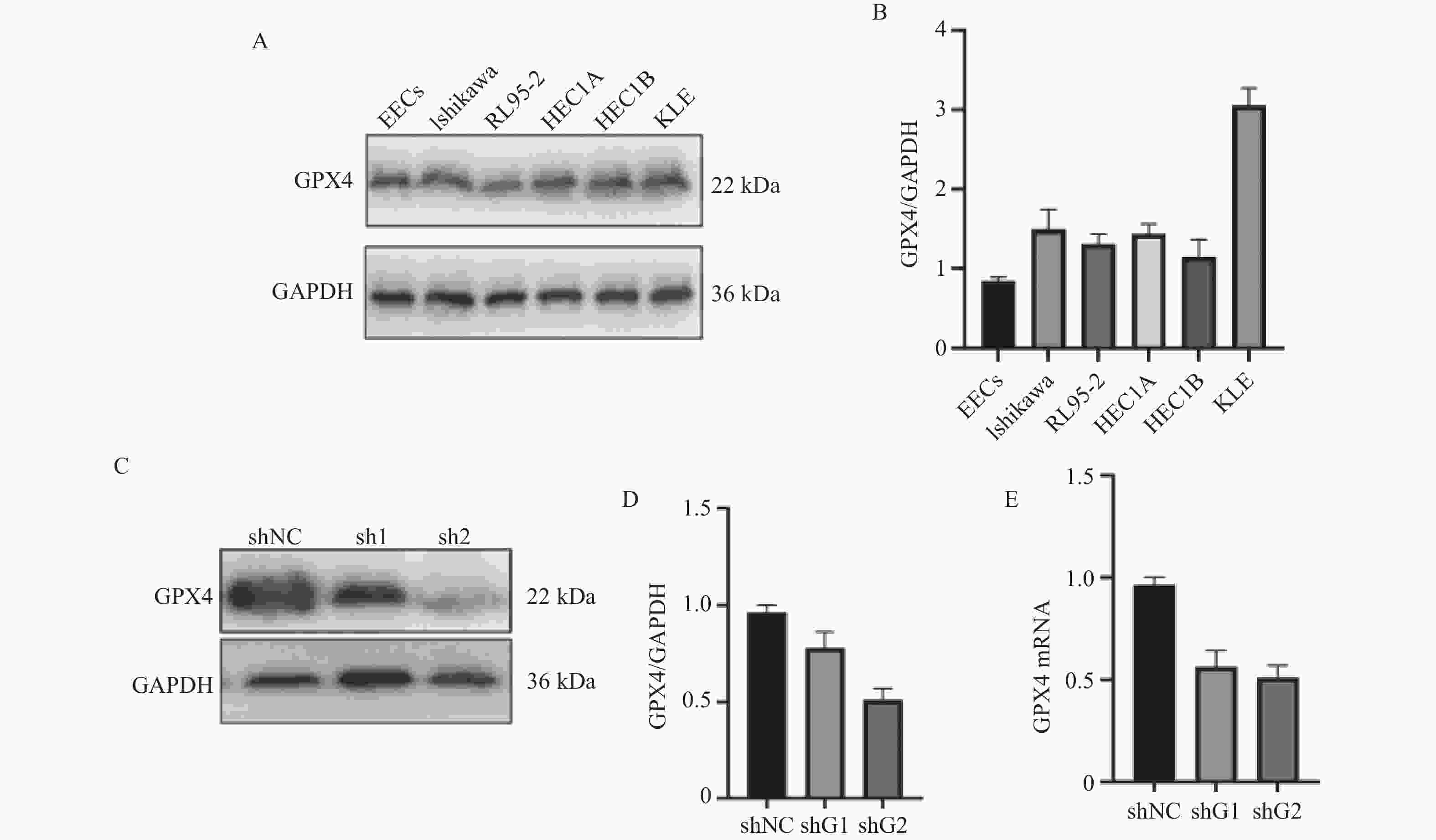
项目 EECs Ishikawa RL95-2 HEC1A HEC1B KLE F P η2 GPX4 mRNA 1.09±0.07 5.05±0.59 6.54±0.61 6.98±1.32 7.59±1.32 8.41±1.34 32.72 <0.001 0.84 多组比较,One-way ANOVA,事后检验Tukey HSD。 表 3 GPX4对EC细胞增殖和迁移的影响
Table 3. Effects of GPX4 on EC cell proliferation and migration
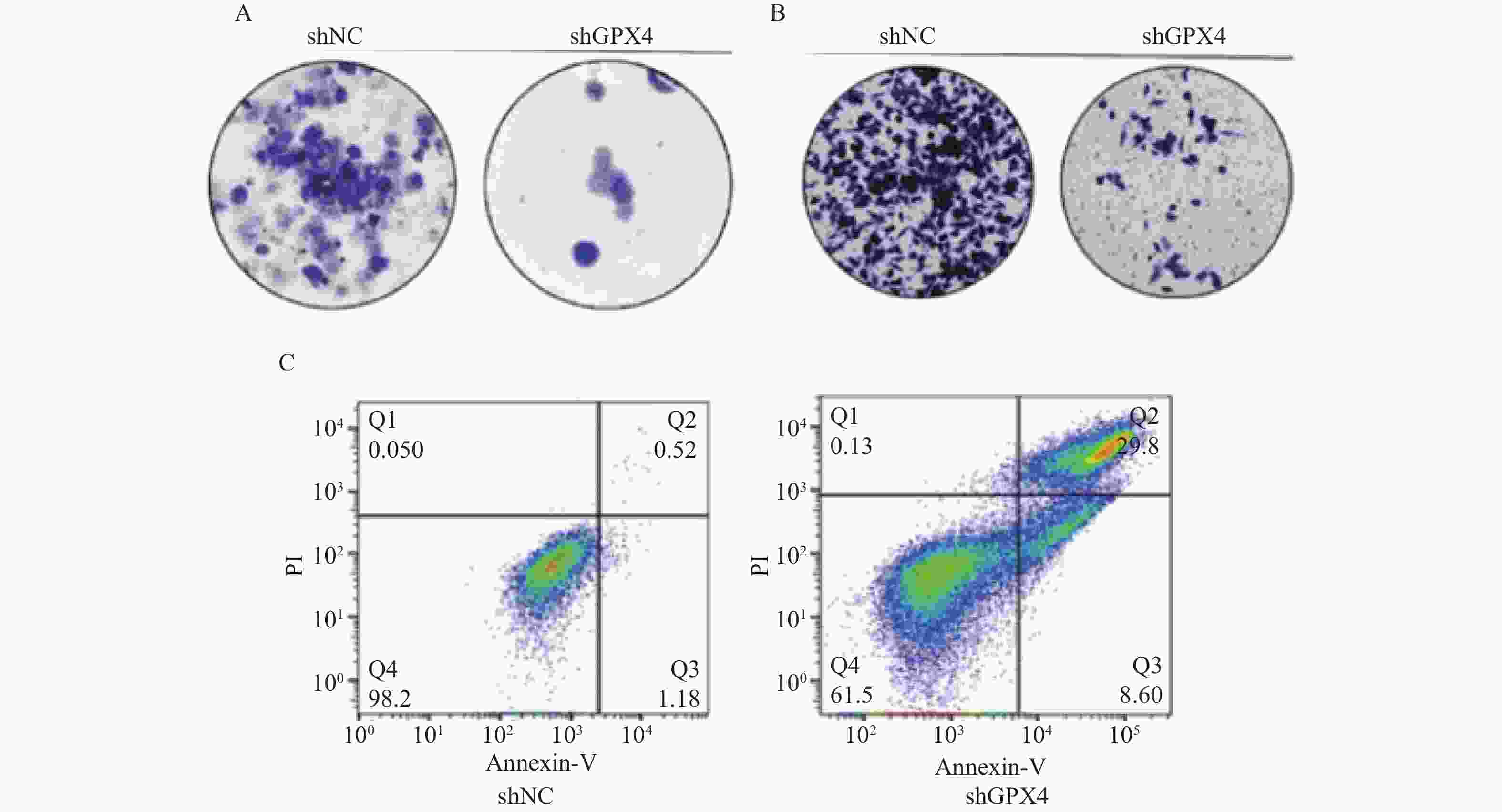
项目 shNC组(6) shGPX4组(6) t P df Cohen’s d 克隆的相对(折叠)数 0.89±0.07 0.37±0.05 14.94 <0.001 10 8.21 细胞的相对(折叠)数 0.99±0.07 0.33±0.06 16.72 <0.001 10 9.24 细胞凋亡率(%) 12.60±2.48 61.64±7.03 16.12 <0.001 10 8.91 (独立样本t检验) 表 4 GPX4 敲低诱导 EC 细胞铁死亡
Table 4. GPX4 knockdown induces ferroptosis in EC cells
项目 shNC组(6) shGPX4组(6) t P df Cohen’s d 细胞内亚铁水平(uM/g) 9.77±1.04 17.46±1.64 9.68 <0.001 10 5.34 脂质 ROS 水平 1.33±0.11 2.04±0.19 8.15 <0.001 10 4.51 MDA 水平(nmol/mg) 3.33±1.03 8.17±1.17 7.59 <0.001 10 4.19 (独立样本t检验) 表 5 GPX4 敲低对 EC 细胞脂质过氧化物及铁死亡关键蛋白的影响
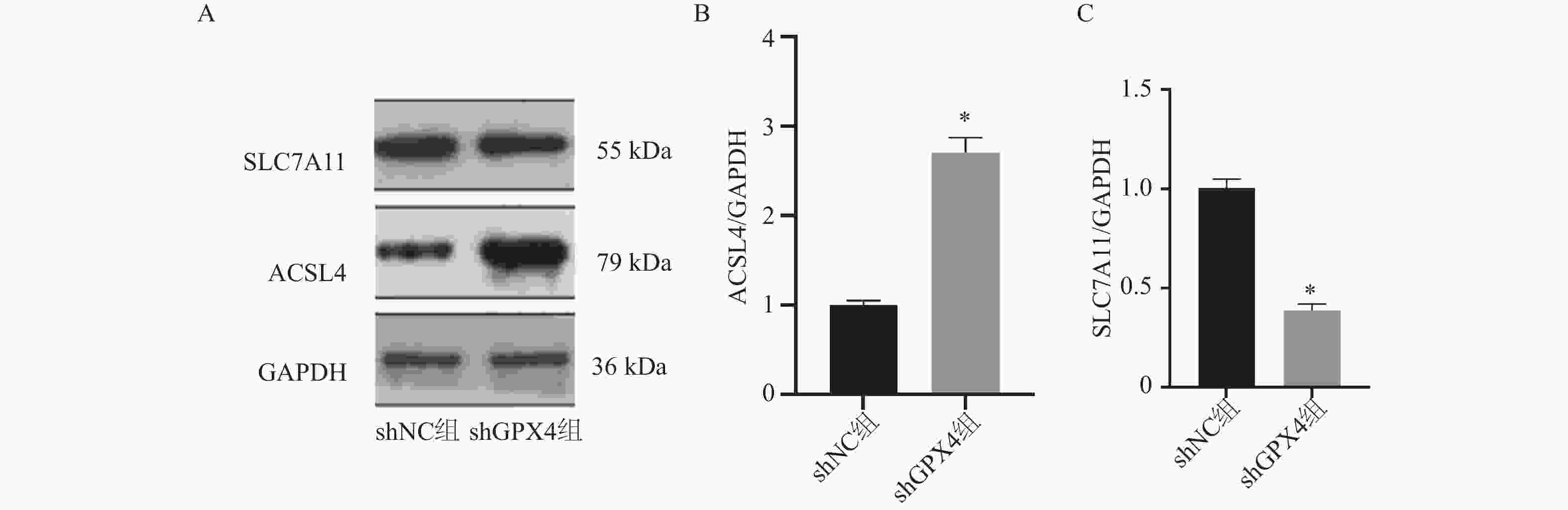
Table 5. Effects of GPX4 Knockdown on Lipid Peroxide and Expression of Key Ferroptosis Proteins in EC Cells.
项目 shNC组(6) shGPX4组(6) t P df Cohen’s d 细胞内 GSH 水平(nmol/mg prot) 28.64±3.12 12.37±2.05 11.82 <0.001 10 6.53 GPX4 酶活性(U/mg prot) 15.26±1.89 5.73±1.08 10.95 <0.001 10 6.06 4-HNE(ng/mL) 18.25±2.31 56.78±4.92 16.03 <0.001 10 8.86 (独立样本t检验) 表 6 GPX4 敲低增强体内铁死亡活性
Table 6. GPX4 knockdown enhances ferroptosis activity in vivo
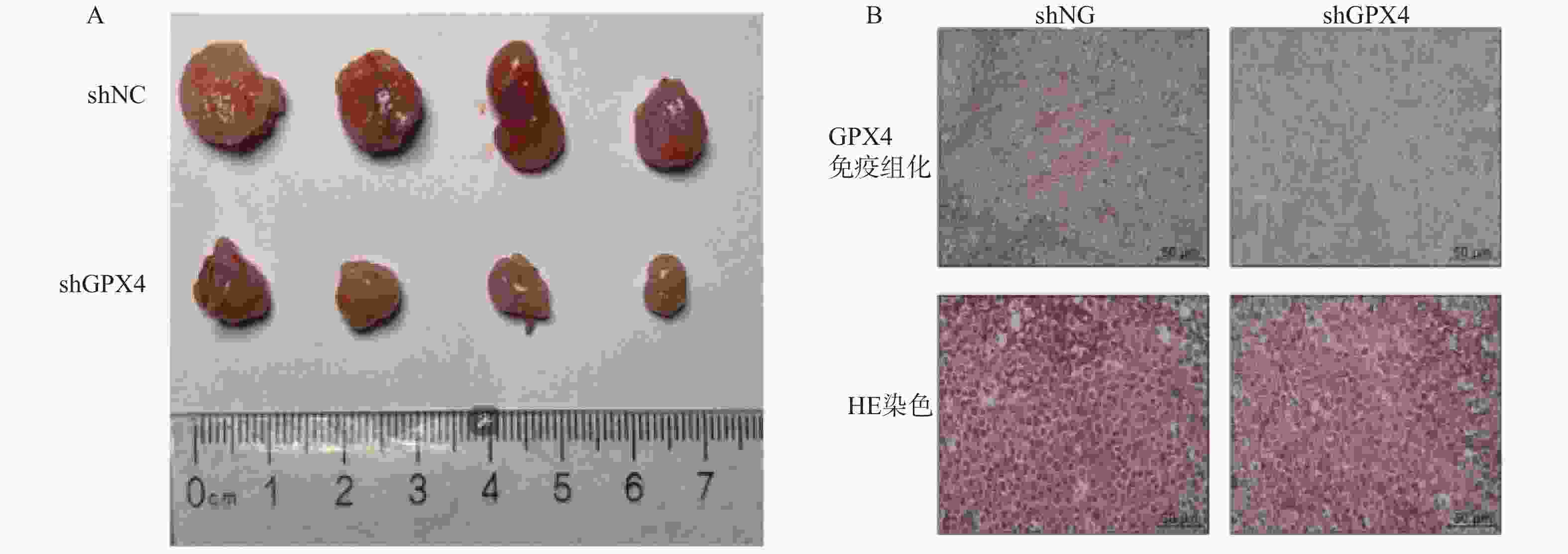
项目 shNC组(6) shGPX4组(6) t P df Cohen’s d 肿瘤体积(cm3) 0.87±0.12 0.36±0.07 9.07 <0.001 10 5.01 肿瘤重量(g) 0.73±0.07 0.32±0.06 10.53 <0.001 10 5.81 细胞铁含量(uM/g) 4.02±0.80 7.89±1.75 4.94 <0.001 10 2.73 MDA 水平(nmol/mg) 23.35±3.71 42.02±5.78 6.67 <0.001 10 3.68 (独立样本t检验) -
[1] Crosbie E J, Kitson S J, McAlpine J N, et al. Endometrial cancer[J]. Lancet, 2022, 399(10333): 1412-1428. doi: 10.1016/S0140-6736(22)00323-3 [2] Nees L K, Heublein S, Steinmacher S, et al. Endometrial hyperplasia as a risk factor of endometrial cancer[J]. Arch Gynecol Obstet, 2022, 306(2): 407-421. doi: 10.1007/s00404-021-06380-5 [3] Bogani G, Monk B J, Powell M A, et al. Adding immunotherapy to first-line treatment of advanced and metastatic endometrial cancer[J]. Ann Oncol, 2024, 35(5): 414-428. doi: 10.1016/j.annonc.2024.02.006 [4] Tronconi F, Nero C, Giudice E, et al. Advanced and recurrent endometrial cancer: State of the art and future perspectives[J]. Crit Rev Oncol Hematol, 2022, 180: 103851. doi: 10.1016/j.critrevonc.2022.103851 [5] Liu J, Kang R, Tang D. Signaling pathways and defense mechanisms of ferroptosis[J]. Febs J, 2022, 289(22): 7038-7050. doi: 10.1111/febs.16059 [6] Li P, Jiang M, Li K, et al. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity[J]. Nat Immunol, 2021, 22(9): 1107-1117. doi: 10.1038/s41590-021-00993-3 [7] Xie Y, Kang R, Klionsky D J, et al. GPX4 in cell death, autophagy, and disease[J]. Autophagy, 2023, 19(10): 2621-2638. doi: 10.1080/15548627.2023.2218764 [8] Sekhar K R, Hanna D N, Cyr S, et al. Glutathione peroxidase 4 inhibition induces ferroptosis and mTOR pathway suppression in thyroid cancer[J]. Sci Rep, 2022, 12(1): 19396. doi: 10.1038/s41598-022-23906-2 [9] Xue Q, Yan D, Chen X, et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis[J]. Autophagy, 2023, 19(7): 1982-1996. doi: 10.1080/15548627.2023.2165323 [10] Allred D C. Prognostic and predictive factors in breast cancer by immunohistochemical analysis[J]. Mod Pathol, 1998, 11(2): 155-168. [11] Liang D, Feng Y, Zandkarimi F, et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones[J]. Cell, 2023, 186(13): 2748-2764. e22. [12] Žalytė E. Ferroptosis, metabolic rewiring, and endometrial cancer[J]. Int J Mol Sci, 2023, 25(1): 75. doi: 10.3390/ijms25010075 [13] Cheng L, He Q, Liu B, et al. SGK2 promotes prostate cancer metastasis by inhibiting ferroptosis via upregulating GPX4[J]. Cell Death Dis, 2023, 14(1): 74. doi: 10.1038/s41419-023-05614-5 [14] Liu Y, Wan Y, Jiang Y, et al. GPX4: The hub of lipid oxidation, ferroptosis, disease and treatment[J]. Biochim Biophys Acta BBA Rev Cancer, 2023, 1878(3): 188890. [15] Wang Z, Shu W, Zhao R, et al. Sodium butyrate induces ferroptosis in endometrial cancer cells via the RBM3/SLC7A11 axis[J]. Apoptosis, 2023, 28(7-8): 1168-1183. doi: 10.1007/s10495-023-01850-4 -





 下载:
下载: