The Role of HDAC8 Expression in Necrotizing Enterocolitis Through Ferroptosis and Inflammatory Response
-
摘要:
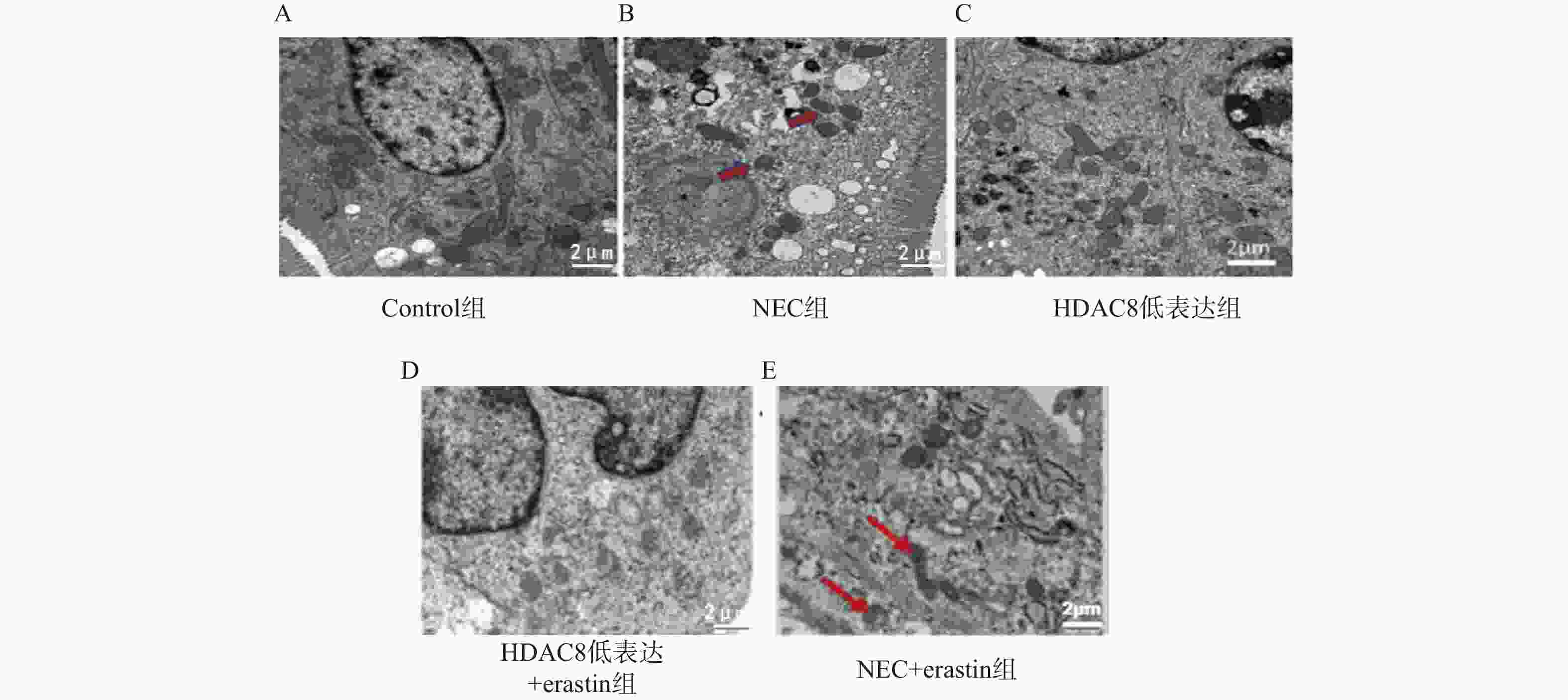
目的 探讨组蛋白去乙酰酶8(histone deacetylase 8,HDAC8)抑制剂PCI- 34051 对坏死性小肠结肠炎(necrotizing enterocolitis,NEC)小鼠肠道损伤的保护作用及其与铁死亡(ferroptosis)的关系,并通过体外实验进一步验证HDAC8在肠上皮细胞铁死亡中的作用机制。方法 选取40只出生8~11 d SPF级雄性C57BL/6小鼠,随机分为对照组、NEC组、HDAC8低表达组(NEC+PCI- 34051 )、HDAC8低表达+Erastin组(NEC+PCI-34051 +Erastin)及NEC+Erastin组(每组8只)。采用缺氧加冷应激法建立NEC模型,给予HDAC8抑制剂PCI-34051 (20 mg/kg)及/或铁死亡诱导剂Erastin(40 mg/kg)腹腔注射,每日1次,共14 d。检测肠道组织病理学变化、氧化应激及炎症指标、铁死亡相关蛋白(SLC7A11、GPX4、p53)表达及线粒体超微结构。同时采用小鼠肠上皮细胞系IEC-6进行体外实验,分为对照组、Erastin组、HDAC8低表达组(PCI-34051 )、HDAC8过表达组(ADV-HDAC8)及HDAC8低表达+SLC7A11敲低组。通过qRT-PCR与Western blot检测HDAC8、SLC7A11、GPX4及ACSL4的表达,并检测细胞活性变化。结果 与对照组相比,NEC组小鼠肠道结构紊乱,炎性细胞浸润明显,GSH下降(P < 0.001),MDA、ROS及Fe2+升高(P < 0.001),SLC7A11和GPX4表达下降(P < 0.001),p53升高(P < 0.001);HDAC8低表达组肠道组织损伤明显减轻,GSH表达增加,MDA、ROS及Fe2+降低,SLC7A11和GPX4表达增加,p53降低(P < 0.001)。Erastin干预可部分逆转PCI- 34051 的保护作用。体外实验结果一致:Erastin诱导IEC-6细胞铁死亡,HDAC8抑制上调SLC7A11和GPX4并提高细胞活性,而HDAC8过表达或SLC7A11敲低均增强铁死亡表型(P < 0.001)。结论 HDAC8抑制剂PCI- 34051 可通过上调SLC7A11/GPX4、抑制p53信号通路,减轻NEC小鼠及肠上皮细胞的氧化应激、炎症反应及线粒体损伤,其保护作用与抑制铁死亡密切相关。-
关键词:
- 坏死性小肠结肠炎 /
- 组蛋白去乙酰化酶8 /
- 铁死亡 /
- 溶质载体家族7成员11 /
- 谷胱甘肽过氧化物酶 4 /
- 炎性因子
Abstract:Objective To investigate the protective effect of histone deacetylase 8 (HDAC8) inhibitor PCI- 34051 on intestinal injury in a mouse model of necrotizing enterocolitis (NEC) and its relationship with ferroptosis, and to further validate the mechanism of HDAC8 in ferroptosis of intestinal epithelial cells through in vitro experiments.Methods Forty 8–11day-old male SPF-grade C57BL/6 mice were randomly divided into five groups (n = 8 per group): control group, NEC group, HDAC8 low-expression group (NEC+PCI- 34051 ), HDAC8 low-expression+Erastin group (NEC+PCI-34051 +Erastin), and NEC+Erastin group (8 mice per group). An NEC model was established by hypoxia-cold stress. The HDAC8 inhibitor PCI-34051 (20 mg/kg) and/or ferroptosis inducer Erastin (40 mg/kg) were administered intraperitoneally once daily for 14 days. Intestinal histopathological changes, oxidative stress and inflammatory markers, expression of ferroptosis-related proteins (SLC7A11, GPX4, p53), and mitochondrial ultrastructure were detected. In parallel, in vitro experiments were conducted using the mouse intestinal epithelial cell line IEC-6, divided into control group, Erastin group, HDAC8 low-expression group (PCI-34051 ), HDAC8 over-expression group (ADV-HDAC8), and HDAC8 low-expression + SLC7A11 knockdown group. The expression of HDAC8, SLC7A11, GPX4, and ACSL4 was detected by qRT-PCR and Western blot, and changes in cell viability were assessed.Results Compared with the control group, the NEC group exhibited intestinal structural disorder, significant inflammatory cell infiltration, decreased GSH (P < 0.001), elevated MDA, ROS, and Fe2+ (P < 0.001), reduced SLC7A11 and GPX4 expression (P < 0.001), and increased p53 (P < 0.001). The HDAC8 low-expression group showed significantly reduced intestinal tissue damage, increased GSH expression, decreased MDA, ROS, and Fe2+, increased SLC7A11 and GPX4 expression, and decreased p53 (P < 0.001). Erastin intervention partially reversed the protective effect of PCI-34051. Consistent in vitro results demonstrated that Erastin induced ferroptosis in IEC-6 cells, HDAC8 inhibited the upregulation of SLC7A11 and GPX4 and enhanced cell viability, while HDAC8 overexpression or SLC7A11 knockdown both enhanced the ferroptotic phenotype (P < 0.001). Conclusion The HDAC8 inhibitor PCI- 34051 alleviates oxidative stress, inflammatory response and mitochondrial damage in NEC mice and intestinal epithelial cells by upregulating SLC7A11/GPX4 and inhibiting the p53 signaling pathway. Its protective effect is closely related to the inhibition of ferroptosis.-
Key words:
- Necrotizing Enterocolitis /
- HDAC8 /
- Ferroptosis /
- SLC7A11 /
- GPX4 /
- Inflammatory Factors
-
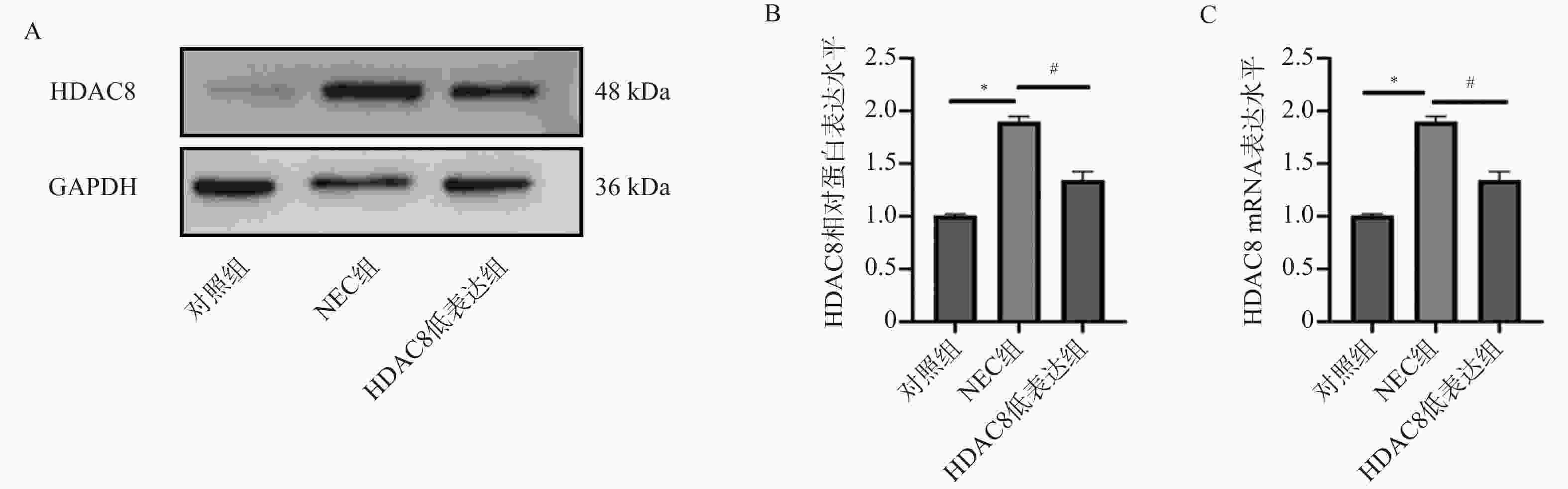
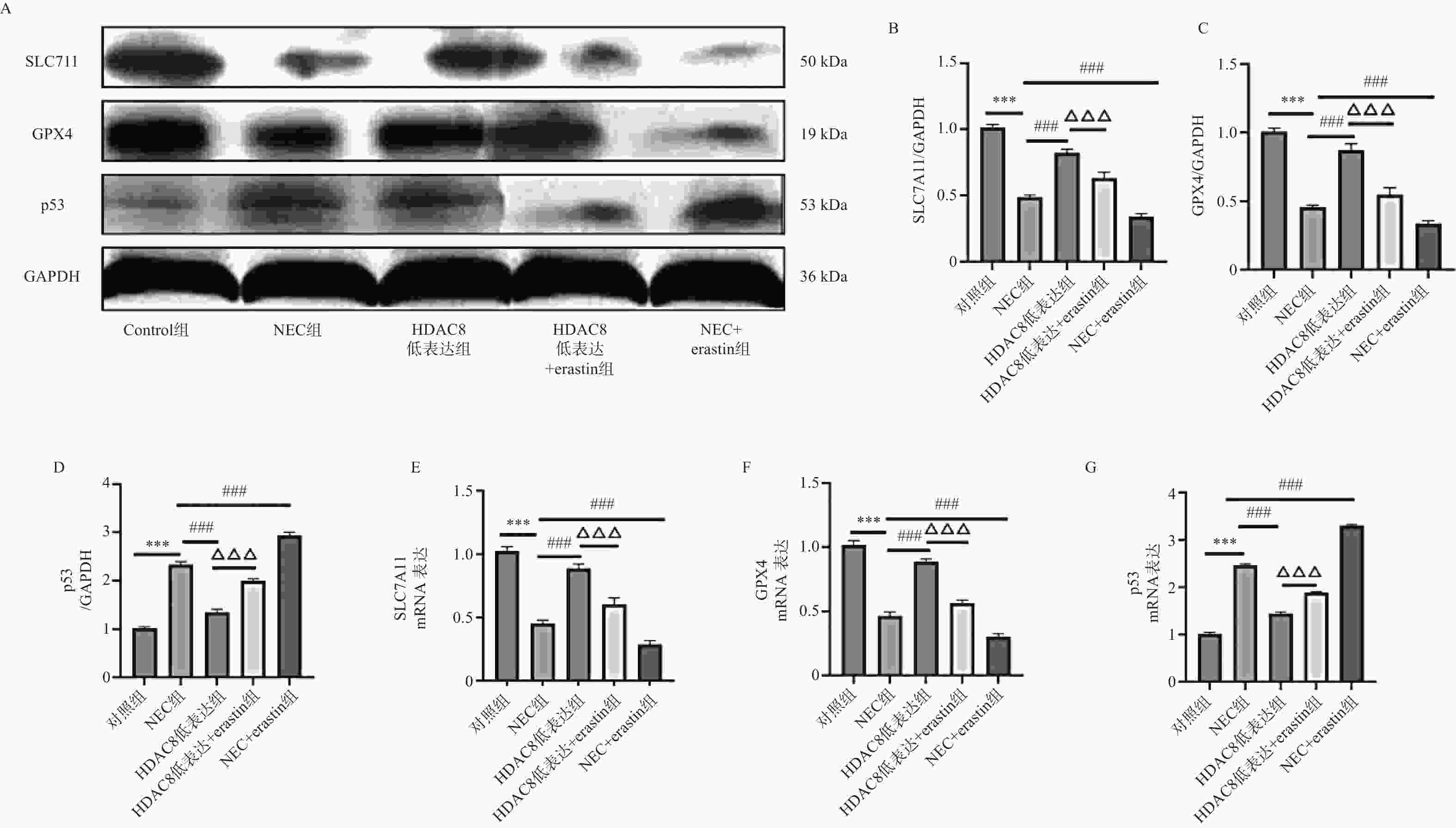
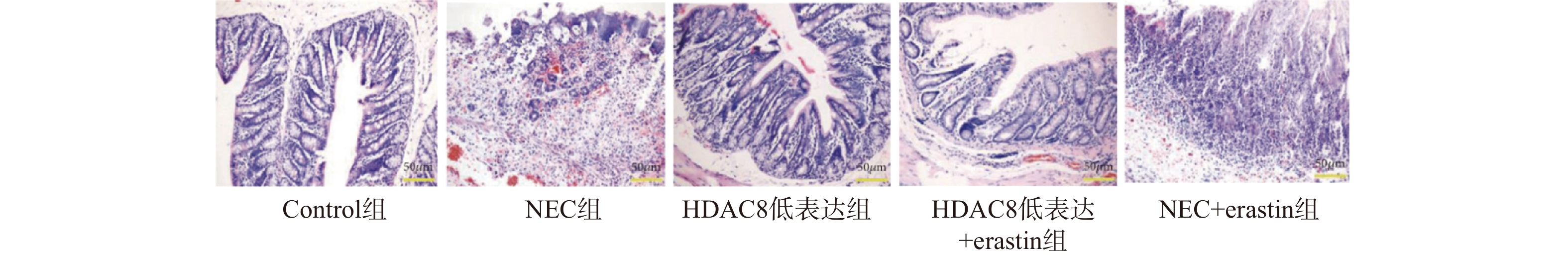
图 4 小鼠肠道组织SLC7A11、GPX4、p53 蛋白及mRNA水平($ \bar x \pm s $,n = 3)
A-D:Western blotting检测小鼠肠道组织中SLC7A11、GPX4、p53蛋白水平;E-G:RT-qPCR检测小鼠肠道组织SLC7A11、GPX4 、p53mRNA表达水平。注:与对照组比较,***P < 0.001;与NEC组比较,###P < 0.001;与HDAC8低表达组相比,△△△P < 0.001。
Figure 4. Protein and mRNA levels of SLC7A11,GPX4,and p53 in mouse intestinal tissues($ \bar x \pm s $,n = 3)
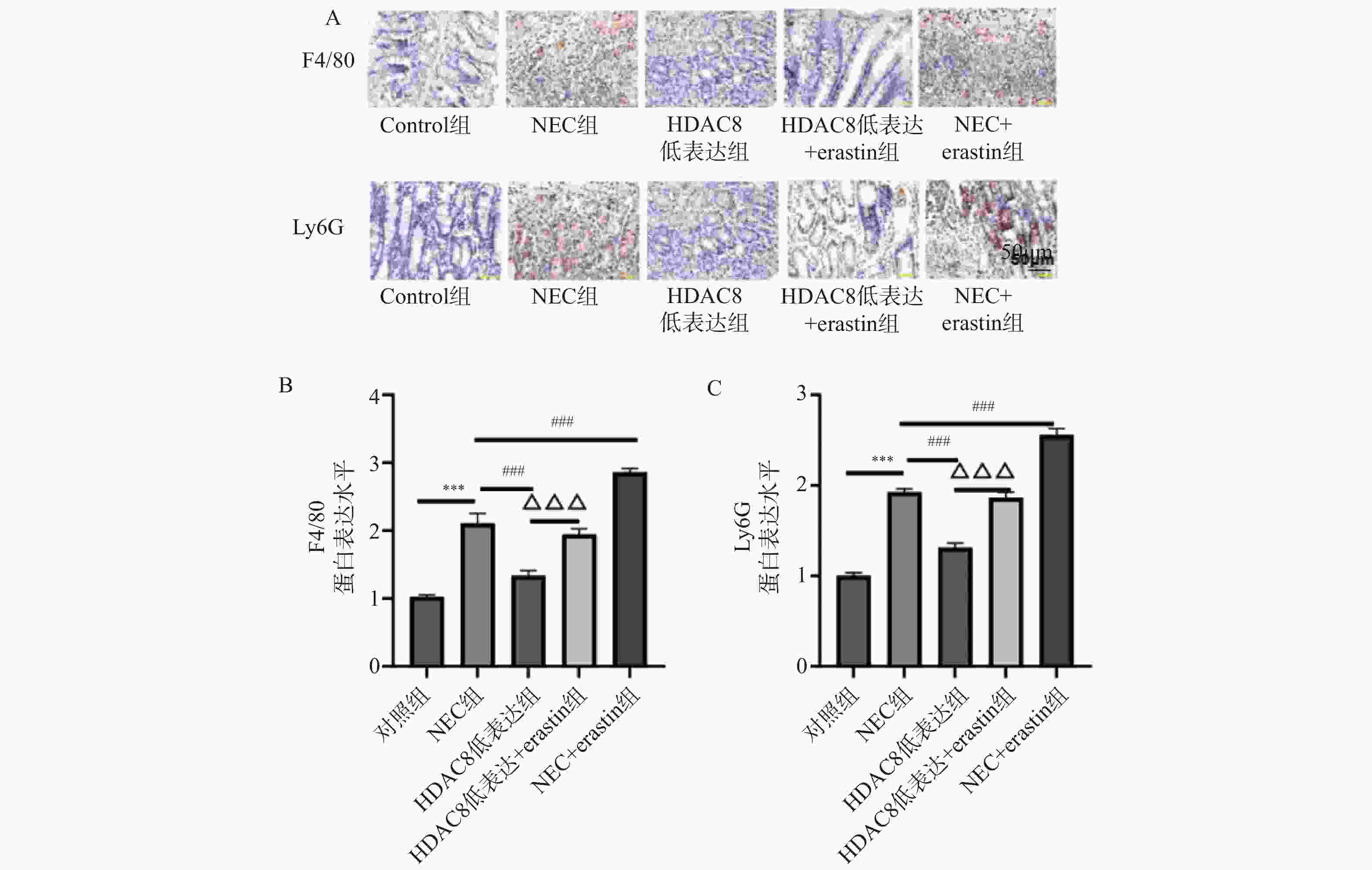
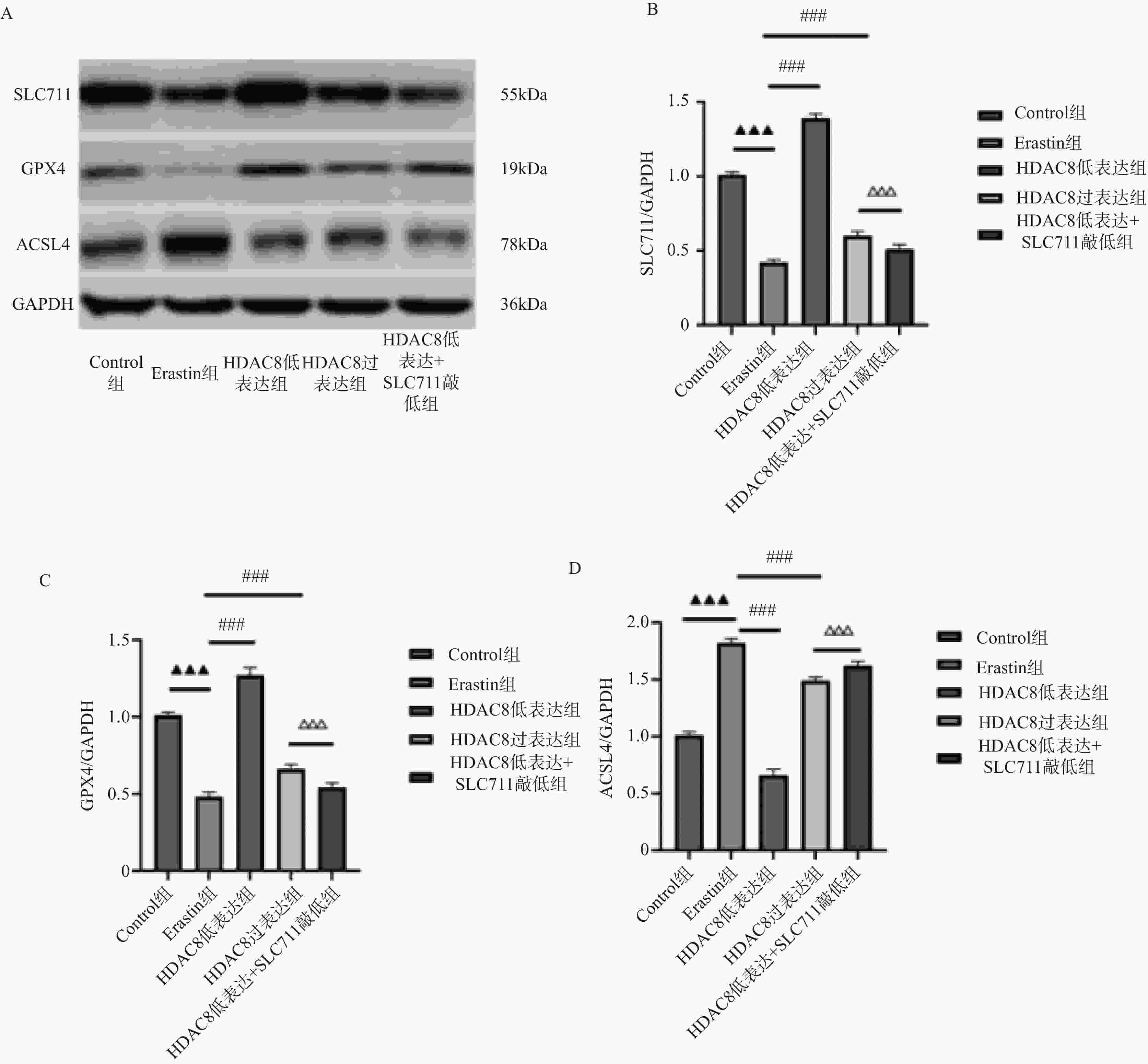
图 6 肠上皮细胞HDAC8、GPX4、ACSL4 蛋白表达比较($ \overline{\boldsymbol{x}} $ ± s,n = 3)
A:Western blotting检测肠上皮细胞SLC7A11、GPX4、p53蛋白印迹图;B:肠上皮细胞SLC7A11蛋白定量分析;C:肠上皮细胞GPX4蛋白定量分析;D:肠上皮细胞p53蛋白定量分析;***P<0.001;与对照组比较,▲▲▲P < 0.001;与Erastin组比较,###P < 0.001;与HDAC8低表达组相比,△△△P < 0.001。
Figure 6. Comparison of HDAC8,GPX4,and ACSL4 protein expression in intestinal epithelial cells
表 1 引物序列
Table 1. Primer sequences
基因 上游引物 下游引物 SLC7A11 5′-GGTGGAACTGCTCGTAAT-3′ 5′-GCTGACACTCGTGCTATT-3′ GPX4 5′-CCTCTGCTGCAAGAGCCTCCC-3′ 5′-CTTATCCAGGCAGACCATGTGC-3′ HDAC8 5′-ATCTCAATGATGCTGTCCTGG-3′ 5′-CATGATCTGGGATCTCAGAGG-3′ P53 5′-CCAGCCAAAGAAGAAACCAC-3′ 5′-CCTCATTCAGCTCTCGGAAC-3′ GAPDH 5′-ACTCCACTCACGGCAAATTC-3′ 5′-TCTCCATGGTGGTGAAGACA-3′ 表 2 小鼠肠道组织中GSH、MDA、4-HNE、ROS、Fe2+表达情况($ \bar x \pm s $,n = 3)
Table 2. Expression levels of GSH,MDA,4-HNE,ROS,and Fe2+in mouse intestinal tissues ($ \bar x \pm s $,n = 3)
组别 GSH(μmol/g) MDA(nmol/mg ) 4-HNE(ng/mL) ROS(U/mg) Fe2+(μmol/g) 对照组 31.44 ± 0.85 20.44 ± 0.99 10.68 ± 0.54 1.01 ± 0.03 1.00 ± 0.04 NEC组 24.74 ± 1.17▲▲▲ 14.74 ± 1.17▲▲▲ 18.72 ± 0.50▲▲▲ 2.15 ± 0.04▲▲▲ 2.14 ± 0.05▲▲▲ HDAC8低表达组 28.86 ± 0.56### 18.86 ± 0.56### 13.48 ± 0.96### 1.42 ± 0.03### 1.47 ± 0.05### HDAC8低表达+erastin组 26.87 ± 0.25△ 16.87 ± 0.25△ 16.77 ± 0.96△△△ 1.88 ± 0.04△△ 1.84 ± 0.05△△ NEC+erastin组 19.38 ± 0.84### 11.38 ± 1.22### 23.36 ± 1.03### 2.55 ± 0.09### 2.63 ± 0.04### F 99.209 44.905 103.325 423.381 523.911 P < 0.001*** < 0.001*** < 0.001*** < 0.001*** < 0.001*** ***P < 0.001;与对照组比较,▲▲▲P < 0.001;与NEC组比较,###P < 0.001;与HDAC8低表达组相比,△P < 0.05,△△P < 0.01,△△△P < 0.001。 表 3 小鼠的肠道组织中炎症因子表达水平($ \bar x \pm s $,n = 3)
Table 3. Expression levels of inflammatory factors in mouse intestinal tissues($ \bar x \pm s$,n = 3)
组别 TNF-α(pg/mL) IL-6(pg/mL) IL-1β(pg/mL) 对照组 13.44 ± 1.16 22.44 ± 1.16 16.57 ± 0.95 NEC组 22.07 ± 0.50▲▲▲ 31.74 ± 0.84▲▲▲ 23.74 ± 1.17▲▲▲ HDAC8低表达组 17.53 ± 0.74### 22.86 ± 0.95### 18.86 ± 0.56### HDAC8低表达+erastin组 20.20 ± 0.61△△ 27.87 ± 0.76△△△ 21.20 ± 0.61△△ NEC+erastin组 28.72 ± 0.75### 35.38 ± 1.07### 31.38 ± 0.78### F 156.794 101.210 137.340 P < 0.001*** < 0.001*** < 0.001*** ***P < 0.001;与对照组比较,▲▲▲P < 0.001;与NEC组比较,###P < 0.001;与HDAC8低表达组相比,△△P < 0.01,△△△P < 0.001。 表 4 肠上皮细胞HDAC8和SLC7A11 mRNA表达比较($ \bar x \pm s $,n = 3)
Table 4. Comparison of HDAC8 and SLC7A11 mRNA expression in intestinal epithelial cells($ \bar x \pm s $,n = 3)
组别 HDAC8 mRNA SLC7A11mRNA Control组 1.00 ± 0.02 1.00 ± 0.03 Erastin组 1.04 ± 0.04▲▲▲ 0.40 ± 0.02▲▲▲ HDAC8低表达组 0.33 ± 0.02### 1.48 ± 0.02### HDAC8过表达组 4.79 ± 0.07### 0.60 ± 0.02### HDAC8低表达 +
SLC7A11敲低组0.34 ± 0.02△△△ 0.38 ± 0.03△△△ F 8181.307 1355.329 P < 0.001*** < 0.001*** ***P < 0.001;与对照组比较,▲▲▲P < 0.001;与Erastin组比较,###P < 0.001;与HDAC8低表达组相比,△△△P < 0.001。 表 5 肠上皮细胞HDAC8和SLC7A11 mRNA表达比较($ \bar x \pm s $,n = 3)
Table 5. Comparison of HDAC8 and SLC7A11 mRNA expression in intestinal epithelial cells ($ \bar x \pm s $,n = 3)
组别 相对细胞活性 Control组 1.02 ± 0.03 Erastin组 0.55 ± 0.04▲▲▲ HDAC8低表达组 0.91 ± 0.03### HDAC8过表达组 0.66 ± 0.04### HDAC8低表达 + SLC7A11敲低组 0.58 ± 0.04△△△ F 124.092 P < 0.001*** ***P < 0.001;与对照组比较,▲▲▲P < 0.001;与Erastin组比较,###P < 0.001;与HDAC8低表达组相比,△△△P < 0.001。 -
[1] Meister A L, Doheny K K, Travagli R A. Necrotizing enterocolitis: It’s not all in the gut[J]. Exp Biol Med (Maywood), 2020, 245(2): 85-95. doi: 10.1177/1535370219891971 [2] Jiang X, Stockwell B R, Conrad M. Ferroptosis: Mechanisms, biology and role in disease[J]. Nat Rev Mol Cell Biol, 2021, 22(4): 266-282. [3] Gao W, Wang X, Zhou Y, et al. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy[J]. Signal Transduct Target Ther, 2022, 7(1): 196. [4] Dang D, Zhang C, Meng Z, et al. Integrative analysis links ferroptosis to necrotizing enterocolitis and reveals the role of ACSL4 in immune disorders[J]. iScience, 2022, 25(11): 105406. doi: 10.1016/j.isci.2022.105406 [5] Hackam D J, Sodhi C P. Bench to bedside - new insights into the pathogenesis of necrotizing enterocolitis[J]. Nat Rev Gastroenterol Hepatol, 2022, 19(7): 468-479. doi: 10.1038/s41575-022-00594-x [6] Kim J Y, Cho H, Yoo J, et al. Pathological role of HDAC8: Cancer and beyond[J]. Cells, 2022, 11(19): 3161. doi: 10.3390/cells11193161 [7] Guo T, Hu S, Xu W, et al. Elevated expression of histone deacetylase HDAC8 suppresses arginine-proline metabolism in necrotizing enterocolitis[J]. iScience, 2023, 26(6): 106882. doi: 10.1016/j.isci.2023.106882 [8] Pang Y, Li L, Yang Y, et al. LncRNA-ANAPC2 and lncRNA-NEFM positively regulates the inflammatory response via the miR-451/npr2/hdac8 axis in grass carp[J]. Fish Shellfish Immunol, 2022, 128: 1-6. doi: 10.1016/j.fsi.2022.07.014 [9] Zhang K, Zhang X, Lv A, et al. Saccharomyces boulardii modulates necrotizing enterocolitis in neonatal mice by regulating the sirtuin 1/NF-κB pathway and the intestinal microbiota[J]. Mol Med Rep, 2020, 22(2): 671-680. [10] 陈岚, 贾波, 邓怀涵, 等. 基于“逆流挽舟”法探索人参败毒散对溃疡性结肠炎模型小鼠胃肠功能的影响及作用机制[J]. 陕西中医, 2019, 40(3): 283-286. [11] Ganji N, Li B, Lee C, et al. Necrotizing enterocolitis: Recent advances in treatment with translational potential[J]. Pediatr Surg Int, 2023, 39(1): 205. doi: 10.1007/s00383-023-05476-0 [12] 李菲, 李盈. 新生儿坏死性小肠结肠炎血清 C 反应蛋白、降钙素原、血小板活化因子、肠型脂肪酸结合蛋白表达水平及预后相关性研究[J]. 陕西医学杂志, 2020, 49(10): 1228-1231+1236. doi: 10.3969/j.issn.1000-7377.2020.10.007 [13] Neu J, Pammi M. Necrotizing enterocolitis: The intestinal microbiome, metabolome and inflammatory mediators[J]. Semin Fetal Neonatal Med, 2018, 23(6): 400-405. [14] Duchon J, Barbian M E, Denning P W. Necrotizing enterocolitis[J]. Clin Perinatol, 2021, 48(2): 229-250. doi: 10.1016/j.clp.2021.03.002 [15] Fontana A, Cursaro I, Carullo G, et al. A therapeutic perspective of HDAC8 in different diseases: An overview of selective inhibitors[J]. Int J Mol Sci, 2022, 23(17): 10014. doi: 10.3390/ijms231710014 [16] Chen J, Cao L, Ma J, et al. HDAC8 promotes liver metastasis of colorectal cancer via inhibition of IRF1 and upregulation of SUCNR1[J]. Oxid Med Cell Longev, 2022, 2022: 2815187. doi: 10.1155/2022/2815187 [17] Saito S, Zhuang Y, Suzuki T, et al. HDAC8 inhibition ameliorates pulmonary fibrosis[J]. Am J Physiol Lung Cell Mol Physiol, 2019, 316(1): L175-L186. doi: 10.1152/ajplung.00551.2017 [18] 郭桓博, 马瑞雪, 朱正望, 等. 中药基于铁死亡机制防治肝癌研究进展[J]. 陕西中医, 2024, 45(2): 282-285. doi: 10.3969/j.issn.1000-7369.2024.02.030 [19] 张一楠, 任彩佩, 吴亚俐, 等. 白藜芦醇通过调节铁死亡通路抑制小鼠溃疡性结肠炎相关性结肠癌的实验研究[J]. 陕西医学杂志, 2023, 52(6): 671-675+682. [20] 韦巧珍, 韦冰梅, 黄清梅, 等. 新生儿坏死性小肠结肠炎中细胞死亡机制的研究进展[J]. 广西科学, 2023, 30(2): 355-362. doi: 10.13656/j.cnki.gxkx.20230529.015 [21] 党丹. 肠上皮细胞铁死亡在新生儿坏死性小肠结肠炎中的作用及机制研究 [D]. 吉林大学, 2022. [22] Dang D, Meng Z, Zhang C, et al. Heme induces intestinal epithelial cell ferroptosis via mitochondrial dysfunction in transfusion-associated necrotizing enterocolitis[J]. FASEB J, 2022, 36(12): e22649. [23] Zou D, Hu F, Zhou Q, et al. miRNA-301 as a molecule promoting necrotizing enterocolitis by inducing inflammation[J]. Acta Biochim Pol, 2023, 70(4): 905-910. doi: 10.18388/abp.2020_6806 [24] Zhang X, Zhang Y, He Y, et al. β-glucan protects against necrotizing enterocolitis in mice by inhibiting intestinal inflammation, improving the gut barrier, and modulating gut microbiota[J]. J Transl Med, 2023, 21(1): 14. [25] Yeramilli V, Cheddadi R, Benjamin H, et al. The impact of stress, microbial dysbiosis, and inflammation on necrotizing enterocolitis[J]. Microorganisms, 2023, 11(9): 2206. -





 下载:
下载: