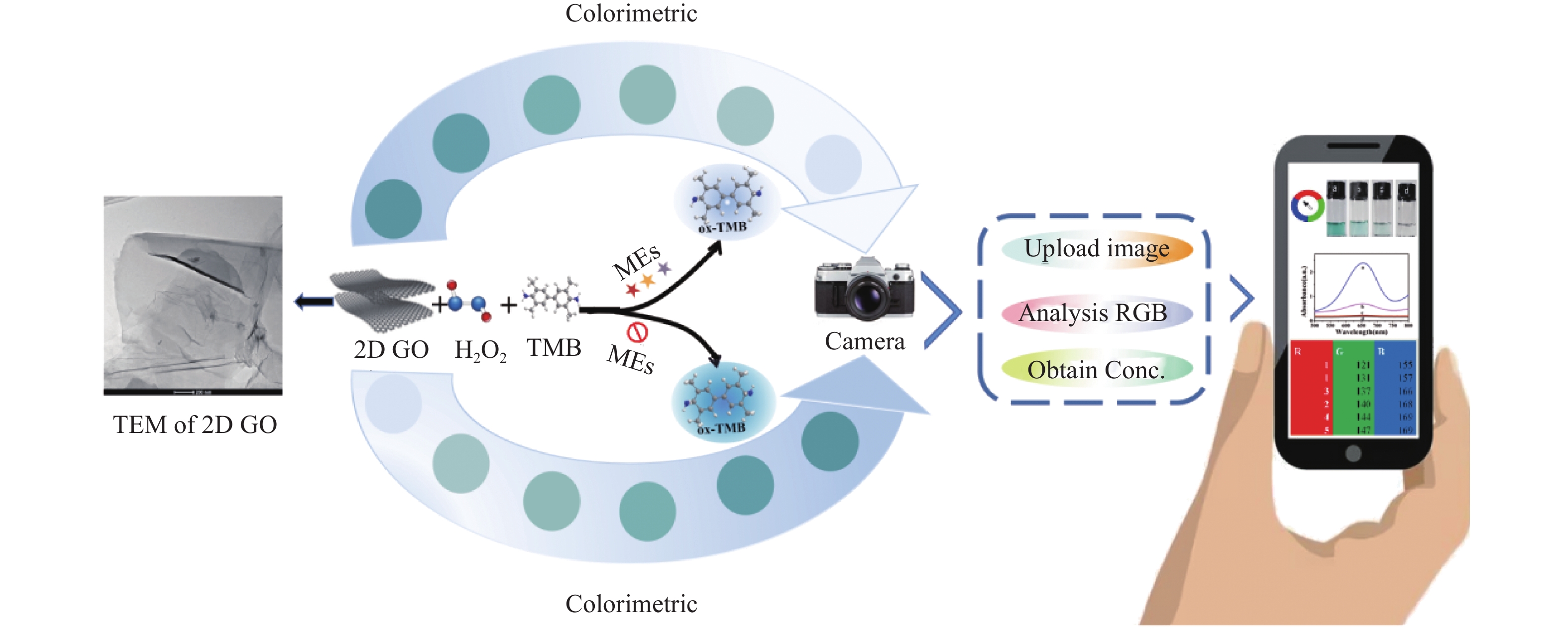
Preparation of Graphene Oxide Nanosheets and Their Portable Colorimetric Determination of Biothiols
-
摘要:
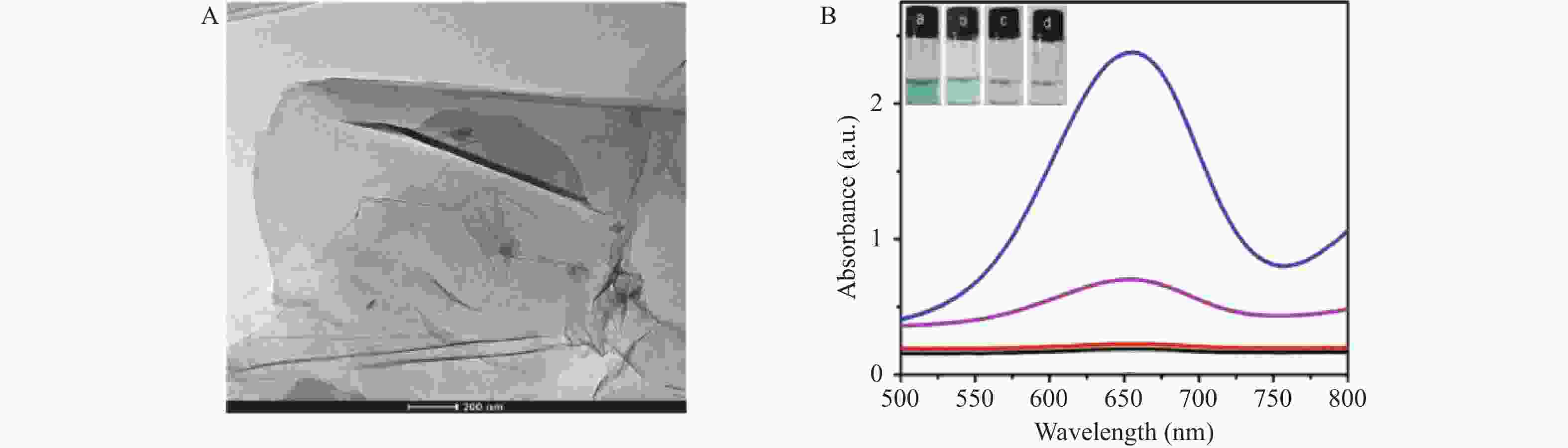
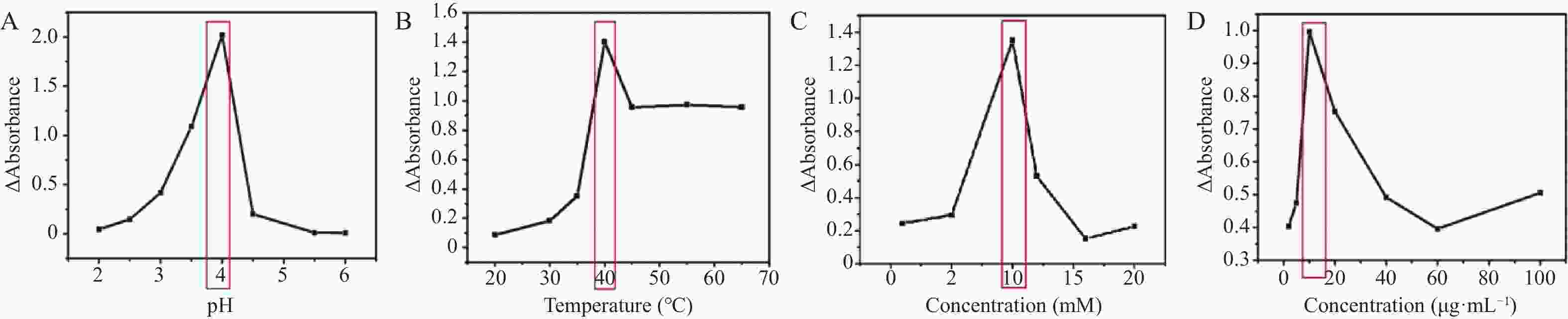
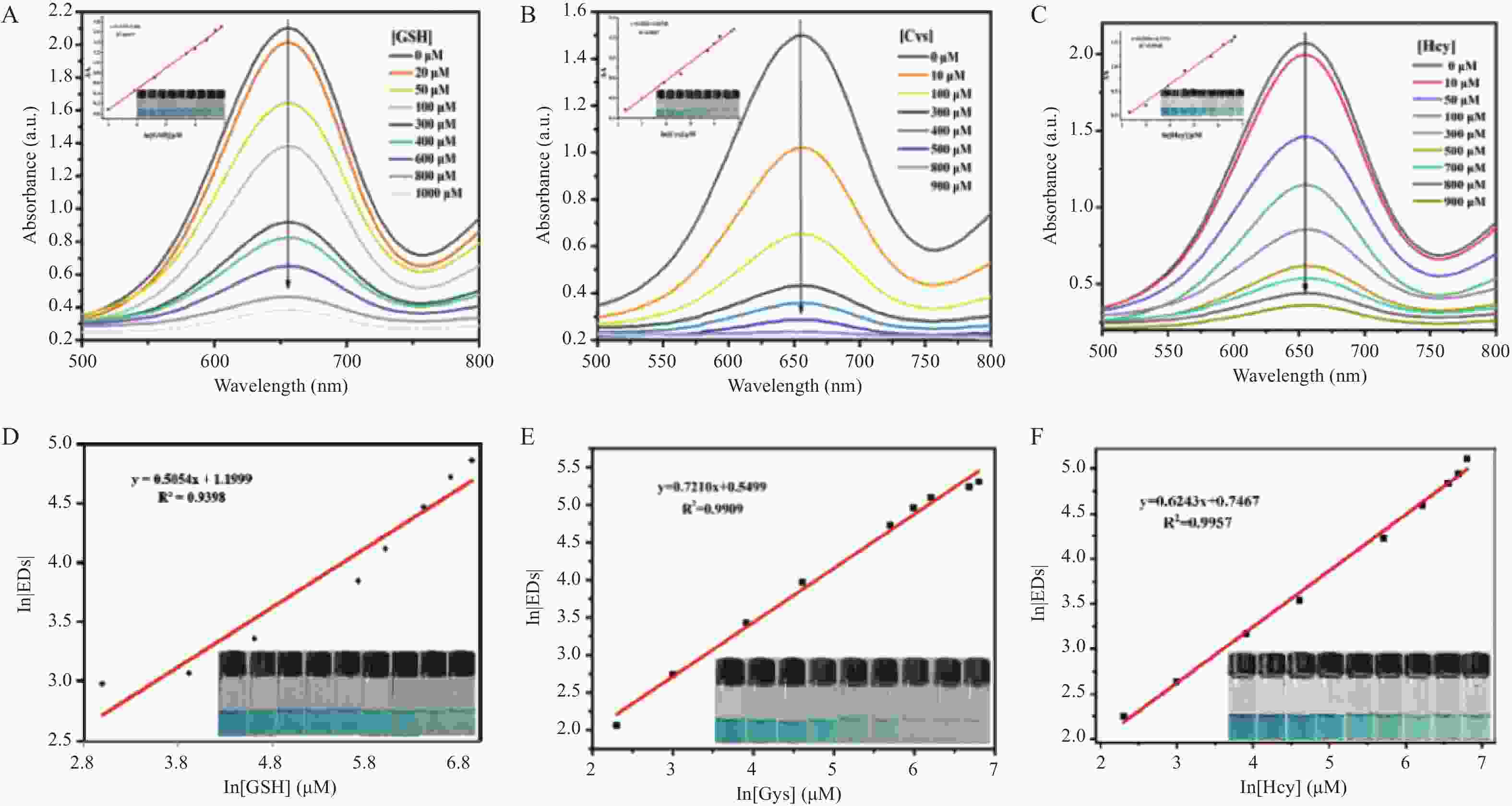
目的 开发一种能够实时、快速、可视化检测生物硫醇(MEs)的方法,为疾病的早期诊断和评估提供有力手段。 方法 通过改良的Hummers方法制备具有优异的类过氧化物酶活性(POD-like)的片状氧化石墨烯(2D GO),并将比色法与智能手机集成构建快速可视化检测MEs的传感策略。 结果 2D GO能够催化过氧化氢(H2O2)分解产生具有强氧化性的羟基自由基(·OH),进而将无色3,3',5,5'-四甲基联苯胺(3,3',5,5'-tetramethylbenzidine,TMB)氧化为蓝色的ox-TMB,结合MEs的强还原作用对“2D GO+TMB+H2O2”比色传感体系显色效果的抑制作用实现了对MEs的快速可视化检测。 结论 基于2D GO所构建的比色平台具有较宽的线性检测范围(10~ 1000 μmol/L)以及良好的检测限(LOD < 7 μM),并成功用于测定胎牛血清样品中的MEs,回收率表现良好。Abstract:Objective To develop a method capable of real-time, rapid, and visual detection of MEs , providing a powerful tool for early diagnosis and assessment of diseases. Methods In this study, flake graphene oxide (2D GO) with excellent peroxidase-like activity (POD-like) was prepared by a modified Hummers' method, and a sensing strategy for the rapid visual detection of MEs was constructed by integrating colorimetry with a smartphone. Results 2D GO can catalyse the decomposition of hydrogen peroxide (H2O2) to produce highly oxidative hydroxyl radicals (·OH), which oxidize the colourless 3, 3', 5, 5'-tetramethylbenzidine (TMB) into blue ox-TMB. The strong reducing effect of MEs on the "2D GO + TMB + H2O2" colorimetric sensing system enables rapid visual detection of MEs. Conclusion The colorimetric platform constructed based on 2D GO has a wide linear detection range (10- 1000 μmol/L) and a good detection limit (LOD < 7 μM), and was successfully used for the determination of MEs in foetal bovine serum samples, with satisfactory recovery rates.-
Key words:
- Graphene oxide /
- Biothiols /
- Colorimetric sensing /
- Peroxide mimics enzymes
-
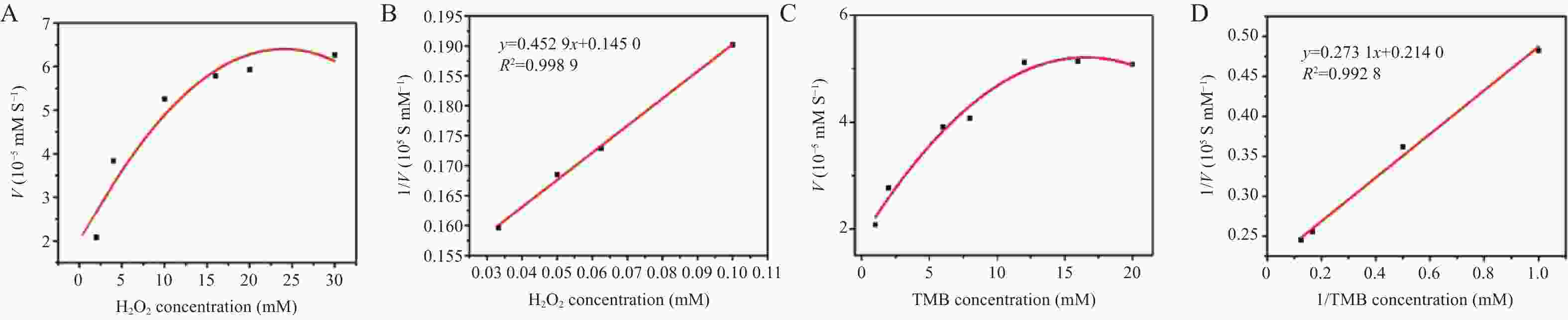
表 1 米氏常数(Km)和最大反应速率(Vmax)的比较
Table 1. Comparison of Michaelis constants ( Km ) and maximum reaction rates ( Vmax )
表 2 不同方法检测MEs的比较
Table 2. Comparison of different methods for detecting MEs
催化剂 方法 物质 线性范围 检出限 参考文献 mBrB 毛细管电泳 GSH 7.5~100 µmol/L 1.41 μmol/L [24] TCNQ 和 GO协同 电化学分析法 GSH 0.25~124.3 μmol/L,
124.3 μmol/L~1.67 mmol/L0.15 μmol/L [25] MNPG 荧光法 GSH 0.2~20 μmol/L 0.05 μmol/L [26] 7 氟苯并-2-氧杂 -1,
3-二唑-4-磺酸铵高效液相色谱 Cys、Hcy、
CysGly、GSH0.5 ~15 μmol/L 0.10 μmol/L [27] BrDMC 质谱法 GSH 1.0~100.0 μmol/L 0.4 μmol/L [28] 2D GO 比色法 Hcy 10~ 1000 μmol/L0.66 μmol/L 本工作 表 3 胎牛血清加标实验中MEs检测结果
Table 3. Detection results of MEs in fetal bovine serum spiking assays
样品 加标量 (μmol/L) 紫外-可见光谱法 手机可视化比色法 检测(μmol/L) 回收率(%) RSD(n=3,%) 检测(μmol/L) 回收率(%) RSD(n=3,%) GSH 0 55.20 − 4.3 7.80 − 4.9 50 101.90 96.90 7.0 60.99 105.5 2.6 400 458.74 100.8 3.3 411.82 101.0 0.5 800 851.42 99.6 6.5 871.99 107.9 1.0 Cys 0 13.55 − 4.3 11.15 − 4.9 50 60.20 94.20 7.9 65.90 107.8 4.3 400 411.47 99.50 7.7 403.83 98.20 1.4 800 817.99 100.5 6.3 716.78 88.40 0.5 Hcy 0 10.40 − 7.2 11.50 − 6.2 50 61.22 99.50 2.0 62.27 100.9 5.6 400 408.90 99.50 1.7 411 99.90 2.1 800 813.00 100.3 0.4 811.4 100.1 1.1 -
[1] Dai J,Ma C G,Zhang P,et al. Recent progress in the development of fluorescent probes for detection of biothiols[J]. Dyes Pigm,2020,177(3):108321. [2] Yin C X,Xiong K M,Huo F J,et al. Fluorescent probes with multiple binding sites for the discrimination of Cys,Hcy,and GSH[J]. Angew Chem Int Ed Engl,2017,56(43):13188-13198. doi: 10.1002/anie.201704084 [3] Chen X,Zhou Y,Peng X,et al. Fluorescent and colorimetric probes for detection of thiols[J]. Chem Soc Rev,2010,39(6):2120-2135. doi: 10.1039/b925092a [4] Jung H S,Chen X,Kim J S,et al. Recent progress in luminescent and colorimetric chemosensors for detection of thiols[J]. Chem Soc Rev,2013,42(14):6019-6031. doi: 10.1039/c3cs60024f [5] Yue Y,Huo F,Ning P,et al. Dual-site fluorescent probe for visualizing the metabolism of Cys in living cells[J]. J Am Chem Soc,2017,139(8):3181-3185. doi: 10.1021/jacs.6b12845 [6] Xu T,Zhao S J,Wu X L,et al. β-Cyclodextrin-promoted colorimetric and fluorescence turn-on probe for discriminating highly toxic thiophenol from biothiols[J]. ACS Sustain Chem Eng,2020,8(16):6413-6421. doi: 10.1021/acssuschemeng.0c00766 [7] Chen H,Tang Y G,Lin W Y,et al. Recent progress in the fluorescent probes for the specific imaging of small molecular weight thiols in living cells[J]. Trends Analyt Chem,2016,76:166-181. doi: 10.1016/j.trac.2015.11.014 [8] Liu X R,Qian M P,Zhang C X,et al. Bis-cyclometalated Ir(III) complex-based electrogenerated chemiluminescence sensor array for discriminating three biothiols[J]. JOAT,2020,4(2):114-121. [9] Li Z H,Li Z H,Sun S G,et al. Design and characterization of methyl mercaptan biosensor using alcohol oxidase[J]. Sens Actuators B Chem,2014,192:680-684. doi: 10.1016/j.snb.2013.10.100 [10] Shen Y,Yue J,Shi W,et al. Target-triggered hot spot dispersion for cellular biothiol detection via background-free surface-enhanced Raman scattering tags[J]. Biosens Bioelectron,2020,151:111957. doi: 10.1016/j.bios.2019.111957 [11] Tu F Q,Zhang L Y,Guo X F,et al. Dual labeling for simultaneous determination of nitric oxide,glutathione and cysteine in macrophage RAW264.7 cells by microchip electrophoresis with fluorescence detection[J]. J Chromatogr A,2014,1359:309-316. doi: 10.1016/j.chroma.2014.07.026 [12] Duan W,Qiu Z,Cao S,et al. Pd-Fe3O4 Janus nanozyme with rational design for ultrasensitive colorimetric detection of biothiols[J]. Biosens Bioelectron,2022,196:113724. doi: 10.1016/j.bios.2021.113724 [13] Wang X Y,Hua Y H,Wei H,et al. Nanozymes in bionanotechnology: From sensing to therapeutics and beyond[J]. Inorg Chem Front,2016,3(1):41-60. doi: 10.1039/C5QI00240K [14] Lin S,Zhang Y,Cao W,et al. Nucleobase-mediated synthesis of nitrogen-doped carbon nanozymes as efficient peroxidase mimics[J]. Dalton Trans,2019,5,48(6):1993-1999. [15] Gao L Z,Zhuang J,Nie L,et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles[J]. Nat Nanotechnol,2007,2(9):577-583. doi: 10.1038/nnano.2007.260 [16] Guo S J,Wang E K. Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors[J]. Nano Today,2011,6(3):240-264. doi: 10.1016/j.nantod.2011.04.007 [17] Vashist S K,Luong J H T. Recent advances in electrochemical biosensing schemes using graphene and graphene-based nanocomposites[J]. Carbon Lett,2015,84:519-550. doi: 10.1016/j.carbon.2014.12.052 [18] Reina G,González-Domínguez J M,Criado A,et al. Promises,facts and challenges for graphene in biomedical applications[J]. Chem Soc Rev,2017,46(15):4400-4416. doi: 10.1039/C7CS00363C [19] Saleh B D,Abdulwahhab G H,Ahmed S M R. Preparation and characterization of graphene oxide nanoparticles derived from wheat straw[J]. Mater Today,2023,80(2):860-869. [20] Wu S,Tian J,Xie N,et al. A sensitive,accurate,and high-throughput gluco-oligosaccharide oxidase-based HRP colorimetric method for assaying lytic polysaccharide monooxygenase activity[J]. Biofuel Bioprod Biorefin,2022,15(1):1-15. [21] Song Y J,Qu K G,Zhao C,et al. Graphene Oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection[J]. Adv Mater,2010,22(19):2206-2210. doi: 10.1002/adma.200903783 [22] Liu Y,Jin H,Zou W,et al. Protein-mediated sponge-like copper sulfide as an ingenious and efficient peroxidase mimic for colorimetric glucose sensing[J]. RSC Adv,2020,10(48):28819-28826. doi: 10.1039/D0RA05496H [23] Guo Y J,Deng L,L J,et al. Hemin-graphene hybrid nanosheets with intrinsic peroxidase-like activity for label-free colorimetric detection of single-nucleotide polymorphism. ACS Nano,2011,5(2): 1282-1290. [24] Pérez-Rama M,Abalde J,Herrero C,et al. A capillary zone electrophoresis for determination of thiolic peptides in biological samples[J]. J Sep Sci,2009,32(12):2152-2158. doi: 10.1002/jssc.200900104 [25] Yuan B Q,Xu C Y,Zhang R C,et al. Glassy carbon electrode modified with 7,7,8,8-tetracyanoquinodimethane and graphene oxide triggered a synergistic effect: Low-potential amperometric detection of reduced glutathione[J]. Biosens Bioelectron,2017,96:1-7. doi: 10.1016/j.bios.2017.04.026 [26] Zhang H J,Chen J,Yang Y L,et al. Discriminative detection of glutathione in cell lysates based on oxidase-like activity of magnetic nanoporous graphene[J]. Anal Chem,2019,91(8):5004-5010. doi: 10.1021/acs.analchem.8b04779 [27] Ferin R,Pavão M L,Baptista J. Methodology for a rapid and simultaneous determination of total cysteine,homocysteine,cysteinylglycine and glutathione in plasma by isocratic RP-HPLC[J]. J Chromatogr B,2012,911:15-20. [28] Feng C H,Huang H Y,Lu C Y. Quantitation of the glutathione in human peripheral blood by matrix-assisted laser desorption ionization time-of-flight mass spectrometry coupled with micro-scale derivatization[J]. Anal Chim Acta,2011,690(2):209-214. doi: 10.1016/j.aca.2011.02.015 [29] Shi R,Yang J,Cheng S Q,et al. Colorimetric determination of biothiols with AuNPs@MoS2 NSs as peroxidase mimetic enzyme[J]. New J Chem,2022,46(39):18718-18723. doi: 10.1039/D2NJ03052G -






 下载:
下载: