Effect of Material Structure on Angiogenesis in Bone Regeneration
-
摘要: 骨修复再生目前仍是临床治疗中常见的棘手问题,而骨组织工程近年来发展成为一种可能的骨修复再生替代方案。但骨作为高度血管化的组织,其发育、成熟、重塑和再生与修复再生过程中的血管生成息息相关。既往研究多倾向于材料结构特性对成骨的直接影响,关于材料结构所形成的物理线索对骨再生中血管生成的影响关注不足,而材料结构对于骨再生中血管生成的影响也是一个重要的环节,关系到最终的骨修复成效。阐述了材料结构对骨再生中血管生成的影响,简要介绍了骨再生中血管生成的过程及作用,着重讨论了现今骨组织工程中材料基地刚度、表面形貌、孔结构、空间结构四类主要结构因素对于骨再生中血管生成的影响作用,以期为将来骨修复材料的设计与构建提供借鉴。Abstract: Bone repair and regeneration is still a common and difficult problem in clinical treatment, and bone tissue engineering has been developed as a possible alternative to bone repair and regeneration in recent years. However, bone is a highly vascularized tissue, and its development, maturation, remodeling and regeneration are closely related to angiogenesis in the process of repair and regeneration. Previous studies tended to focus on the direct influence of material structure characteristics on osteogenesis, and insufficient attention was paid to the influence of physical cues formed by material structure on angiogenesis in bone regeneration. However, the influence of material structure on angiogenesis in bone regeneration is also an important link, which is related to the final effect of bone repair. Therefore, this paper expounds the influence of material structure on angiogenesis in bone regeneration, briefly introduces the process and role of angiogenesis in bone regeneration, and focuses on discussing the influence of the four main structural factors of material base stiffness, surface morphology, pore structure, and spatial structure in current bone tissue engineering on angiogenesis in bone regeneration. In the hope of providing reference for the design and construction of bone repair materials in the future.
-
Key words:
- Material /
- Structure /
- Morphology /
- Bone regeneration /
- Angiogenesis /
- Tissue engineering
-
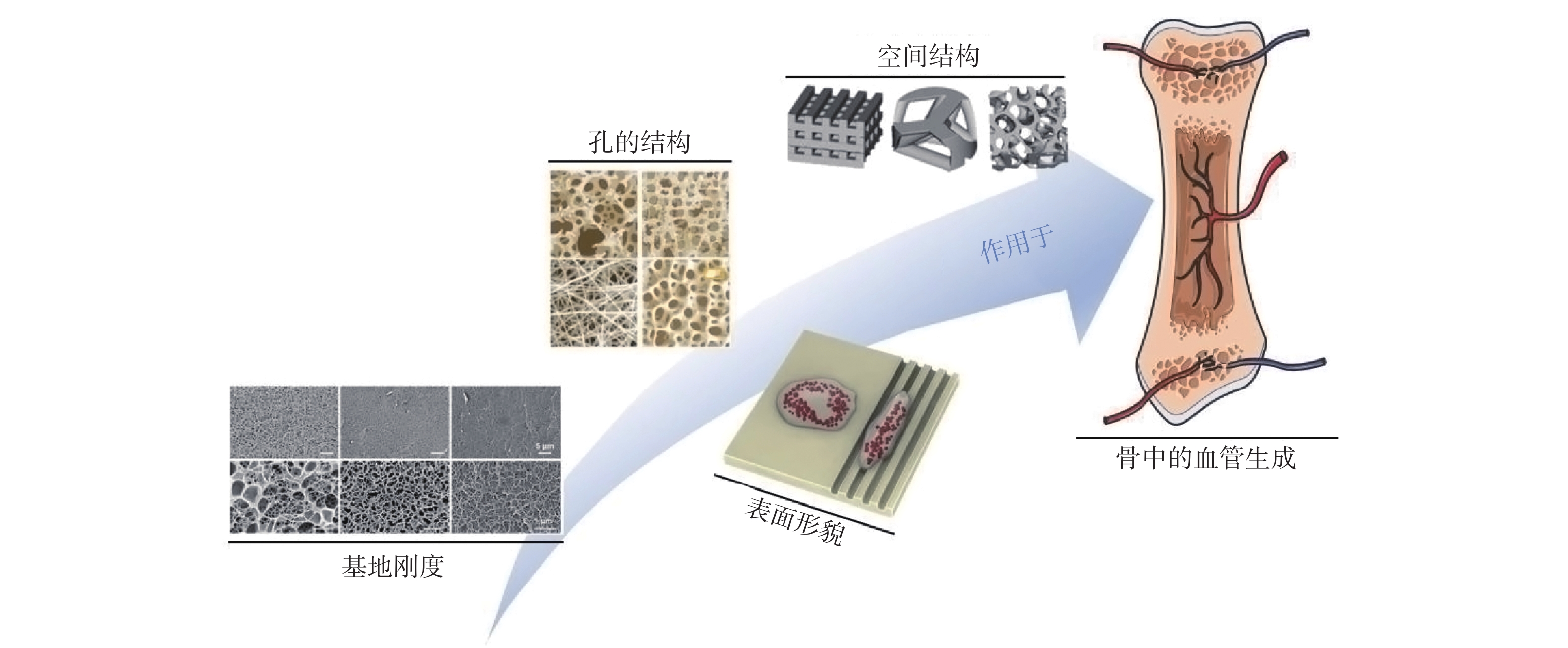
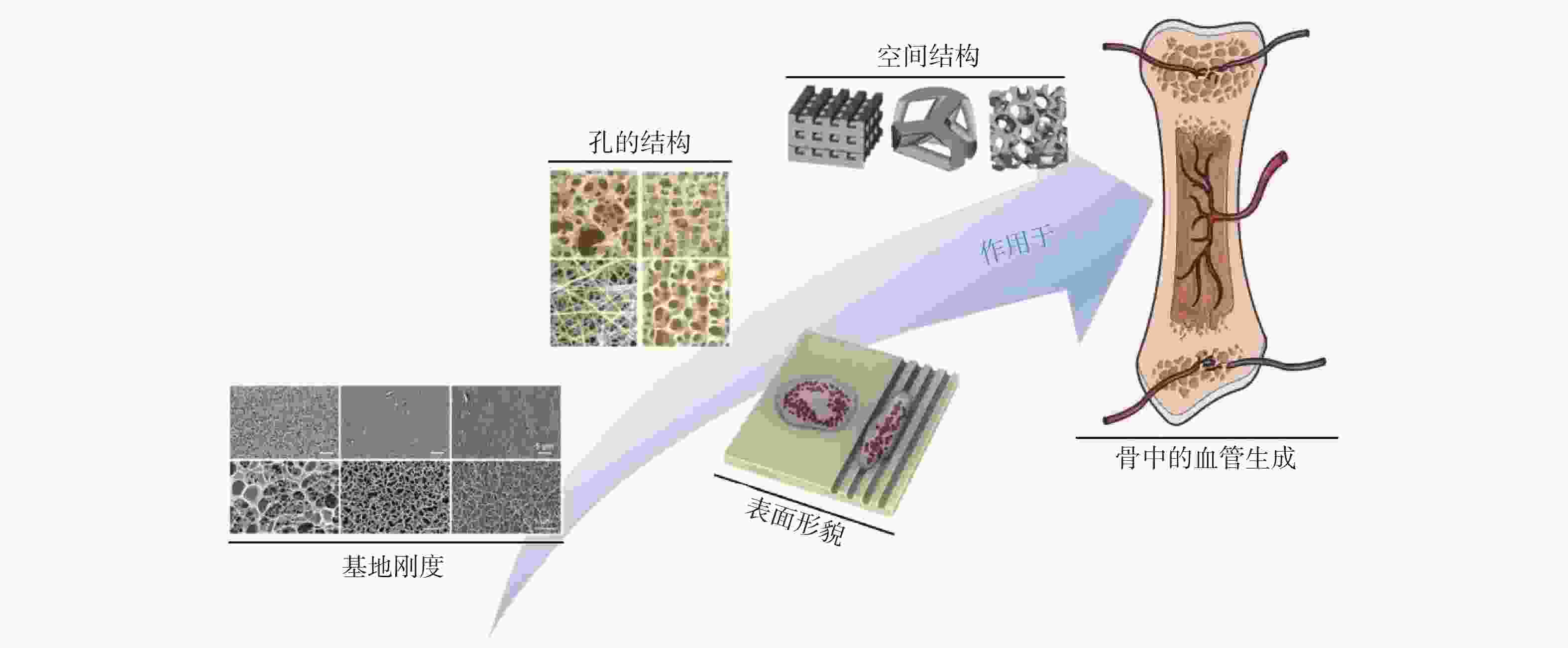
表 1 材料结构对血管生成的影响
Table 1. Effect of material structure on angiogenesis
材料结构 对血管生成的影响 孔隙率 较高的孔隙率有利于血管细胞的长入 亲水性 材料的亲水性越强越有利于血管生长 表面能 材料的表面能越高越有利于血管生长 粗造度 较粗糙表面比较光滑表面有利于血管生长 孔径大小 一定范围内,增加孔径有利于血管生长 基地刚度 相较于较高基地刚度,较低基地刚度有利于血管生长 微观粒径 亚微米级较微米级有利于血管生长 排列方式 有序结构较无序结构有利于血管生长 空间结构 对天然组织的仿生程度越高越有利于血管生长 孔的互连性 孔的互连性越高越有利于血管生长 -
[1] 卢承印, 罗志强, 简功辉, 等. H型血管促进成血管-成骨耦联在骨折愈合中作用机制的研究进展[J]. 华中科技大学学报(医学版), 2024, 53(1): 133-139. [2] Kong X, Zheng T, Wang Z, et al. Remote actuation and on-demand activation of biomaterials pre-incorporated with physical cues for bone repair[J]. Theranostics, 2024, 14(11): 4438-4461. [3] Tsai Y H, Tseng C C, Lin Y C, et al. Novel artificial tricalcium phosphate and magnesium composite graft facilitates angiogenesis in bone healing[J]. Biomedical Journal, 2024, 48(2): 100750. [4] Santoro A, Voto A, Fortino L, et al. Bone defect treatment in regenerative medicine: Exploring natural and synthetic bone substitutes[J]. International Journal of Molecular Sciences, 2025, 26(7): 3085. [5] Dasari A, Xue J, Deb S. Magnetic nanoparticles in bone tissue engineering[J]. Nanomaterials, 2022, 12(5): 757. doi: 10.3390/nano12050757 [6] Jin H, Zhu X, Liu H, et al. Type-I collagen polypeptide-based composite nanofiber membranes for fast and efficient bone regeneration[J]. ACS Biomaterials Science & Engineering, 2024, 10(9): 5632-5640. [7] Chen K, Wang Y, Tang H, et al. Fabrication of a nanoscale Magnesium/Copper metal-organic framework on Zn-based guided bone generation membranes for enhancing osteogenesis, angiogenesis, and bacteriostasis properties[J]. ACS Applied Materials & Interfaces, 2024, 16(5): 5648-5665. [8] 谢庆晟, 于海洋, 马涛, 等. 外泌体在成血管成骨偶联中作用的研究[J]. 中国骨质疏松杂志, 2024, 30(12): 1810-1814. [9] Lv N, Zhou Z, Hou M, et al. Research progress of vascularization strategies of tissue-engineered bone[J]. Frontiers in Bioengineering and Biotechnology, 2024, 11(9): 1291969. doi: 10.3389/fbioe.2023.1291969 [10] Jin S, Wen J, Zhang Y, et al. M2 macrophage-derived exosome-functionalized topological scaffolds regulate the foreign body response and the coupling of angio/osteoclasto/osteogenesis[J]. Acta Biomaterialia, 2024, 177(5): 91-106. doi: 10.1016/j.actbio.2024.01.043 [11] Liu C, Zhou Y, Sun M, et al. Light-induced cell alignment and harvest for anisotropic cell sheet technology[J]. ACS Applied Materials & Interfaces, 2017, 9(42): 36513-36524. [12] Nambiar J, Jana S, Nandi S K. Strategies for enhancing vascularization of biomaterial-based scaffold in bone regeneration[J]. The Chemical Record, 2022, 22(6): e202200008. doi: 10.1002/tcr.202200008 [13] Luo Y, Zheng Y, Chen Z, et al. Proangiogenic effect and underlying mechanism of holmium oxide nanoparticles: A new biomaterial for tissue engineering[J]. Journal of Nanobiotechnology, 2024, 22(1): 357. doi: 10.1186/s12951-024-02642-x [14] Wang M, Chen J, Luo Y, et al. Design strategies and application potential of multifunctional hydrogels for promoting angiogenesis[J]. International Journal of Nanomedicine, 2024, 19(11): 12719-12742. doi: 10.2147/IJN.S495971 [15] 王嘉旎, 陈俊宇. 金属离子促血管生成机制及在骨组织工程中的应用[J]. 中国组织工程研究, 2024, 28(5): 804-812. [16] Mahapatra C, Kumar P, Paul M K, et al. Angiogenic stimulation strategies in bone tissue regeneration[J]. Tissue and Cell, 2022, 79(12): 101908. doi: 10.1016/j.tice.2022.101908 [17] Shi J, Dai W, Gupta A, et al. Frontiers of hydroxyapatite composites in bionic bone tissue engineering[J]. Materials, 2022, 15(23): 8475. doi: 10.3390/ma15238475 [18] Hayashi K, Munar M L, Ishikawa K. Effects of macropore size in carbonate apatite honeycomb scaffolds on bone regeneration[J]. Materials Science and Engineering: C, 2020, 111(6): 110848. doi: 10.1016/j.msec.2020.110848 [19] Ortiz R, Aurrekoetxea-Rodrguez I, Rommel M, et al. Laser surface microstructuring of a bio-resorbable polymer to anchor stem cells, control adipocyte morphology, and promote osteogenesis[J]. Polymers, 2018, 10(12): 1337. doi: 10.3390/polym10121337 [20] 谢小蔓, 付钰澳, 王忠林, 等. 生物力学在调控成骨细胞生物特性机制中的研究进展[J]. 湖北医药学院学报, 2024, 43(6): 702-708. [21] Choisez A, Ishihara S, Ishii T, et al. Matrix stiffness regulates the triad communication of adipocytes/macrophages/endothelial cells through CXCL13[J]. Journal of Lipid Research, 2024, 65(9): 100620. doi: 10.1016/j.jlr.2024.100620 [22] Schunk C T, Wang W, Sabo L N, T et al. Matrix stiffness increases energy efficiency of endothelial cells[J]. Matrix Biology, 2024, 133(11): 77-85. doi: 10.1016/j.matbio.2024.08.004 [23] Chen W, Tian B, Liang J, et al. Matrix stiffness regulates the interactions between endothelial cells and monocytes[J]. Biomaterials, 2019, 221(11): 119362. doi: 10.1016/j.biomaterials.2019.119362 [24] Zhang Y, Dai J, Hang R, et al. Tailoring surface stiffness to modulate senescent macrophage immunomodulation: Implications for osteo-/angio-genesis in aged bone regeneration[J]. Biomaterials Advances, 2024, 165(12): 214010. doi: 10.1016/j.bioadv.2024.214010 [25] Shayan M, Huang M S, Navarro R, et al. Elastin-like protein hydrogels with controllable stress relaxation rate and stiffness modulate endothelial cell function[J]. Journal of Biomedical Materials Research Part A, 2023, 111(7): 896-909. doi: 10.1002/jbm.a.37520 [26] Li Y B, Zhang H Q, Lu Y P, et al. Construction of magnesium phosphate chemical conversion coatings with different microstructures on titanium to enhance osteogenesis and angiogenesis[J]. ACS Applied Materials & Interfaces, 2024, 16(17): 21672-21688. [27] Wei X, Zhou W, Tang Z, et al. Magnesium surface-activated 3D printed porous PEEK scaffolds for in vivo osseointegration by promoting angiogenesis and osteogenesis[J]. Bioactive Materials, 2023, 20(5): 16-28. doi: 10.1016/j.bioactmat.2022.05.011 [28] Liu C, Lou Y, Sun Z, et al. 4D printing of personalized-tunable biomimetic periosteum with anisotropic microstructure for accelerated vascularization and bone healing[J]. Advanced Healthcare Materials, 2023, 12(22): e2202868. doi: 10.1002/adhm.202202868 [29] Kim T H, Kim S H, Leong K W, et al. Nanografted substrata and triculture of human pericytes, fibroblasts, and endothelial cells for studying the effects on angiogenesis[J]. Tissue Engineering Part A, 2016, 22(7-8): 698-706. doi: 10.1089/ten.tea.2015.0461 [30] Duan R, Zhang Y, van Dijk L, et al. Coupling between macrophage phenotype, angiogenesis and bone formation by calcium phosphates[J]. Materials Science and Engineering: C, 2021, 122(5): 111948. doi: 10.1016/j.msec.2021.111948 [31] Jin S, Yang R, Chu C, et al. Topological structure of electrospun membrane regulates immune response, angiogenesis and bone regeneration[J]. Acta Biomaterialia, 2021, 129(7): 148-158. doi: 10.1016/j.actbio.2021.05.042 [32] Patel K D, Kim T H, Mandakhbayar N, et al. Coating biopolymer nanofibers with carbon nanotubes accelerates tissue healing and bone regeneration through orchestrated cell-and tissue-regulatory responses[J]. Acta Biomaterialia, 2020, 108(5): 97-110. doi: 10.1016/j.actbio.2020.03.012 [33] Chen Y, Chen S, Kawazoe N, et al. Promoted angiogenesis and osteogenesis by dexamethasone-loaded calcium phosphate nanoparticles/collagen composite scaffolds with microgroove networks[J]. Scientific Reports, 2018, 8(1): 14143. doi: 10.1038/s41598-018-32495-y [34] Tian T, Zhang T, Lin Y, et al. Vascularization in craniofacial bone tissue engineering[J]. Journal of Dental Research, 2018, 97(9): 969-976. doi: 10.1177/0022034518767120 [35] Huang J, Han Q, Cai M, et al. Effect of angiogenesis in bone tissue engineering[J]. Annals of Biomedical Engineering, 2022, 50(8): 898-913. doi: 10.1007/s10439-022-02970-9 [36] Gu J, Zhang Q, Geng M, et al. Construction of nanofibrous scaffolds with interconnected perfusable microchannel networks for engineering of vascularized bone tissue[J]. Bioactive Materials, 2021, 6(10): 3254-3268. doi: 10.1016/j.bioactmat.2021.02.033 [37] Zhang M, Huang Z, Wang X, et al. Personalized PLGA/BCL scaffold with hierarchical porous structure resembling periosteum-bone complex enables efficient repair of bone defect[J]. Advanced Science, 2024, 11(35): e2401589. [38] Bobbert F S L, Zadpoor A A. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone[J]. Journal of Materials Chemistry B, 2017, 5(31): 6175-6192. doi: 10.1039/C7TB00741H [39] Druecke D, Langer S, Lamme E, et al. Neovascularization of poly(ether ester) block-copolymer scaffolds in vivo: Long-term investigations using intravital fluorescent microscopy[J]. Journal of Biomedical Materials Research Part A, 2003, 68A(1): 10-18. [40] Feng B, Jinkang Z, Zhen W, et al. The effect of pore size on tissue ingrowth and neovascularization in porous bioceramics of controlled architecturein vivo[J]. Biomedical Materials, 2011, 6(1): 015007. doi: 10.1088/1748-6041/6/1/015007 [41] Walthers C M, Nazemi A K, Patel S L, et al. The effect of scaffold macroporosity on angiogenesis and cell survival in tissue-engineered smooth muscle[J]. Biomaterials, 2014, 35(19): 5129-5137. doi: 10.1016/j.biomaterials.2014.03.025 [42] Huang G J, Yu H P, Wang X L, et al. Highly porous and elastic aerogel based on ultralong hydroxyapatite nanowires for high-performance bone regeneration and neovascularization[J]. Journal of Materials Chemistry B, 2021, 9(5): 1277-1287. doi: 10.1039/D0TB02288H [43] Somo S I, Akar B, Bayrak E S, et al. Pore interconnectivity influences growth factor-mediated vascularization in sphere-templated hydrogels[J]. Tissue Engineering Part C: Methods, 2015, 21(8): 773-785. doi: 10.1089/ten.tec.2014.0454 [44] Lian M, Sun B, Han Y, et al. A low-temperature-printed hierarchical porous sponge-like scaffold that promotes cell-material interaction and modulates paracrine activity of MSCs for vascularized bone regeneration[J]. Biomaterials, 2021, 274(7): 120841. doi: 10.1016/j.biomaterials.2021.120841 [45] Doyle S E, Pannella M, Onofrillo C, et al. NEST3D printed bone-mimicking scaffolds: Assessment of the effect of geometrical design on stiffness and angiogenic potential[J]. Frontiers in Cell and Developmental Biology, 2024, 12(5): 1353154. doi: 10.3389/fcell.2024.1353154 [46] Cai Z, Qu C, Song W, et al. Hierarchical chiral calcium silicate hydrate films promote vascularization for tendon-to-bone healing[J]. Advanced Materials, 2024, 36(31): e2404842. doi: 10.1002/adma.202404842 [47] 廖红兵, 麦昱颖. 骨增量材料中血管新生性能的作用及认识[J]. 中国口腔种植学杂志, 2024, 29(2): 150-158. -





 下载:
下载: