Effect of AK4 on Proliferation and Migration of Intrahepatic Bile Duct Carcinoma Cell HUCCT1
-
摘要:
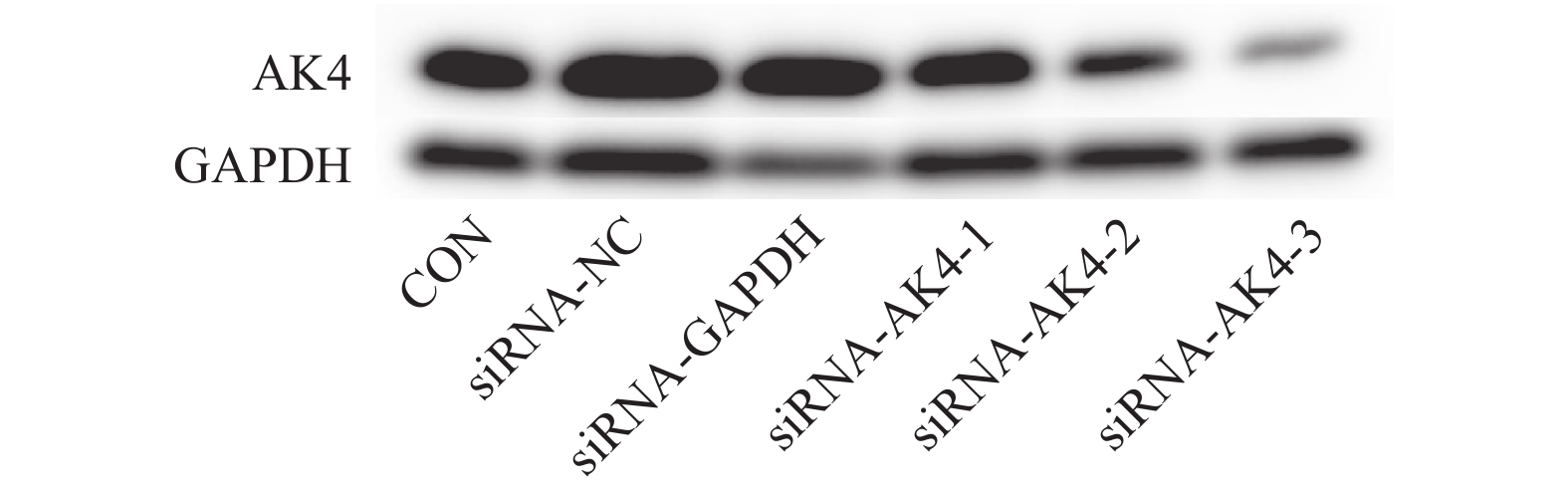
目的 探究AK4对肝内胆管癌细胞HUCCT1增殖、迁移能力的影响。 方法 采用小干扰RNA(siRNA)技术靶向沉默肝内胆管癌细胞HUCCT1中AK4的表达,采用免疫印迹法检测沉默效果以及筛选siRNA-AK4,通过EdU实验和细胞划痕实验检测该胆管癌细胞增殖、迁移能力。 结果 通过免疫印迹法检测出siRNA-AK4-3沉默效果最好,EdU实验显示沉默AK4后,细胞增殖能力下降(P < 0.05),细胞划痕实验显示沉默AK4后,细胞迁移能力下降(P < 0.01)。 结论 沉默肝内胆管癌细胞HUCCT1中AK4的表达后,其增殖、迁移能力受到抑制。 Abstract:Objective To investigate the effects of AK4 on the proliferation and migration of intrahepatic bile duct carcinoma cells HUCCT1. Methods Small interfering RNA (siRNA) technology was used to target the silencing of AK4 expression in HUCCT1 of intrahepatic bile duct cancer cells. Western blot was used to detect the transfection efficiency of siRNA and screen siRNA-AK4. The proliferation and migration ability of the bile duct cancer cells were detected by EdU assay and cell scar assay. Results EdU experiment showed that cell proliferation decreased after AK4 silencing (P < 0.05), the cell migration ability was decreased after AK4 silencing (P < 0.01). Conclusion AK4 can enhance the proliferation and migration of HUCCT1 in intrahepatic bile duct carcinoma cells. -
Key words:
- Intrahepatic cholangiocarcinoma /
- AK4 /
- Proliferation /
- Migration
-
表 1 si-RNA序列
Table 1. Sequence of si-RNA built by siRNA technology
组别 序列 Antisense Negative control 5′-UUCUCCGAACGUGUCACGUTT-3′ 5′-ACGUGACACGUUCGGAGAATT-3′ GAPDH Positive control 5′-UGACCUCAACUACAUGGUUTT-3′ 5′-AACCAUGUAGUUGAGGUCATT-3′ siRNA-AK4-1 5′-GCGGAAGGGUAUAUAACCUTT-3′ 5′-AGGUUAUAUACCCUUCCGCCT-3′ siRNA-AK4-2 5′-CAGGCUAAGACAGUACAAATT-3′ 5′-UUUGUACUGUCUUAGCCUGTT-3′ siRNA-AK4-3 5′-CACCUAUUCAGUCCAAAGATT-3′ 5′-UCUUUGGACUGAAUAGGUGTT-3′ 表 2 划痕面积比
Table 2. Scratch area ratio
项目 0 h 12 h 24 h 36 h siRNA-NC 0.39 ± 0.01 0.26 ± 0.017 0.12 ± 0.017 0.08 ± 0.026 siRNA-AK4-3 0.39 ± 0.005 0.33 ± 0.001 0.24 ± 0.024 0.24 ± 0.027 -
[1] El-diwany R,Pawlik T M,Ejaz A. Intrahepatic Cholangiocarcinoma[J]. Surgical oncology clinics of North America,2019,28(4):587-599. doi: 10.1016/j.soc.2019.06.002 [2] Kelley R K,Bridgewater J,Gores G J,et al. Systemic therapies for intrahepatic cholangiocarcinoma[J]. J Hepatol,2020,72(2):353-363. doi: 10.1016/j.jhep.2019.10.009 [3] Yoneda T,Sato M,Maeda M,et al. Identification of a novel adenylate kinase system in the brain:cloning of the fourth adenylate kinase[J]. Brain research Molecular brain research,1998,62(2):187-195. doi: 10.1016/S0169-328X(98)00249-6 [4] Yamamoto T,Kikkawa R,Yamada H,et al. Investigation of proteomic biomarkers in in vivo hepatotoxicity study of rat liver:toxicity differentiation in hepatotoxicants[J]. The Journal of toxicological sciences,2006,31(1):49-60. doi: 10.2131/jts.31.49 [5] Dzeja P,Terzic A. Adenylate kinase and AMP signaling networks:metabolic monitoring,signal communication and body energy sensing[J]. International journal of molecular sciences,2009,10(4):1729-1772. doi: 10.3390/ijms10041729 [6] Choi S B,Kim K S,Choi J Y,et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection:association of lymph node metastasis and lymph node dissection with survival[J]. Annals of surgical oncology,2009,16(11):3048-3056. doi: 10.1245/s10434-009-0631-1 [7] Endo I,Gonen M,Yopp A C,et al. Intrahepatic cholangiocarcinoma:rising frequency,improved survival,and determinants of outcome after resection[J]. Annals of surgery,2008,248(1):84-96. doi: 10.1097/SLA.0b013e318176c4d3 [8] Gray S, Lamarca A, Edeline J, et al. Targeted Therapies for Perihilar Cholangiocarcinoma [J]. Cancers, 2022, 14(7). [9] Wirth T C,Vogel A. Surveillance in cholangiocellular carcinoma[J]. Best practice & research Clinical gastroenterology,2016,30(6):987-99. [10] Weigt J,Malfertheiner P. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer[J]. Expert review of gastroenterology & hepatology,2010,4(4):395-397. [11] Kato A,Shimizu H,Ohtsuka M,et al. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer:a retrospective single-center study[J]. Annals of surgical oncology,2013,20(1):318-324. doi: 10.1245/s10434-012-2312-8 [12] Kato A, Shimizu H, Ohtsuka M, et al. Downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer patients treated with gemcitabine plus cisplatin combination therapy followed by radical surgery [J]. Annals of surgical oncology, 2015, 22 Suppl 3: S1093-9109. [13] Malka D,Cervera P,Foulon S,et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO):A randomised,open-label,non-comparative phase 2 trial[J]. The Lancet Oncology,2014,15(8):819-828. doi: 10.1016/S1470-2045(14)70212-8 [14] Lee J,Park S H,Chang H M,et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer:a multicentre,open-label,randomised,phase 3 study[J]. The Lancet Oncology,2012,13(2):181-188. doi: 10.1016/S1470-2045(11)70301-1 [15] Primrose J N,Fox R P,Palmer D H,et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP):a randomised,controlled,multicentre,phase 3 study[J]. The Lancet Oncology,2019,20(5):663-673. doi: 10.1016/S1470-2045(18)30915-X [16] Zhu A X,Macarulla T,Javle M M,et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation:The Phase 3 Randomized Clinical ClarIDHy Trial[J]. JAMA oncology,2021,7(11):1669-677. doi: 10.1001/jamaoncol.2021.3836 [17] Abou-alfa G K,Macarulla T,Javle M M,et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy):a multicentre,randomised,double-blind,placebo-controlled,phase 3 study[J]. The Lancet Oncology,2020,21(6):796-807. [18] Demols A,Borbath I,Van Den Eynde M,et al. Regorafenib after failure of gemcitabine and platinum-based chemotherapy for locally advanced/metastatic biliary tumors: REACHIN,a randomized,double-blind,phase II trial[J]. Annals of oncology:official journal of the European Society for Medical Oncology,2020,31(9):1169-1177. [19] Sirica A E,Gores G J,Groopman J D,et al. Intrahepatic Cholangiocarcinoma: Continuing Challenges and Translational Advances[J]. Hepatology,2019,69(4):1803-1815. [20] Kalckar H M. Spectroscopic microdetermination of muscle adenylic acid[J]. Science,1944,99(2563):131-132. [21] Amiri M,Conserva F,Panayiotou C,et al. The human adenylate kinase 9 is a nucleoside mono- and diphosphate kinase[J]. The international journal of biochemistry & cell biology,2013,45(5):925-931. [22] Miyoshi K,Akazawa Y,Horiguchi T,et al. Localization of adenylate kinase 4 in mouse tissues[J]. Acta histochemica et cytochemica,2009,42(2):55-64. [23] 田华,何小勇,王道猛,等. 腺苷酸激酶4在肺腺癌中的表达及其临床意义[J]. 中国癌症杂志,2021,31(2):108-113. [24] 李辰运,孙彤,卓娜,等. 胰腺导管腺癌中腺苷酸激酶4的表达及临床意义[J]. 天津医科大学学报,2018,24(1):43-46. [25] 李绍军,雷益,易文全,等. 食管鳞状细胞癌组织miR-199a-3p、AK4表达水平及临床意义[J]. 遵义医科大学学报,2021,44(6):756-761. [26] 夏林,王劲松. 胃癌组织中腺苷酸激酶4和HIF-1α的表达及其临床意义[J]. 临床与实验病理学杂志,2021,37(4):452-454+457. -






 下载:
下载: