|
[1]
|
Zhang Q,Madden N E,Wong A S T,et al. The role of endocrine G protein-coupled receptors in ovarian cancer progression[J]. Frontiers in Endocrinology,2017,8:66.
|
|
[2]
|
Hsien Lai S,Zervoudakis G,Chou J,et al. PDE4 subtypes in cancer[J]. Oncogene,2020,39(19):3791-3802. doi: 10.1038/s41388-020-1258-8
|
|
[3]
|
Fu C,Guan G,Wang H. The anticancer effect of sanguinarine:A review[J]. Current Pharmaceutical Design,2018,24(24):2760-2764. doi: 10.2174/1381612824666180829100601
|
|
[4]
|
Liu X, Ouyang S, Yu B, et al. Pharm Mapper server: a web server for potential drug target identification using pharmacophore mapping approach[J]. Nucleic Acids Research, 2010, 38 (Web Server issue): W609-614.
|
|
[5]
|
Rhodes D R,Yu J,Shanker K,et al. ONCOMINE:a cancer microarray database and integrated data-mining platform[J]. Neoplasia (New York,NY),2004,6(1):1-6. doi: 10.1016/S1476-5586(04)80047-2
|
|
[6]
|
Yu H,Touna A,Yin X,et. al. Identification of differentially expressed genes and biological pathways in sanguinarine-treated ovarian cancer by integrated bioinformatics analysis[J]. Pharmacognosy Magazine,2021,17(73):106-113. doi: 10.4103/pm.pm_111_20
|
|
[7]
|
李京辉,朱明,曲海,等. miR-490-3p调控SW1990胰腺癌细胞上皮间充质转化[J]. 昆明医科大学学报,2021,42(3):10-17. doi: 10.12259/j.issn.2095-610X.S20210305
|
|
[8]
|
Lin D C,Xu L,Ding L W,et al. Genomic and functional characterizations of phosphodiesterase subtype 4D in human cancers[J]. Proceedings of the National Academy of Sciences of the United States of America,2013,110(15):6109-6114. doi: 10.1073/pnas.1218206110
|
|
[9]
|
Pullamsetti S S,Banat G A,Schmall A,et al. Phosphodiesterase-4 promotes proliferation and angiogenesis of lung cancer by crosstalk with HIF[J]. Oncogene,2013,32(9):1121-1134. doi: 10.1038/onc.2012.136
|
|
[10]
|
Rahrmann E P,Collier L S,Knutson T P,et al. Identification of PDE4D as a proliferation promoting factor in prostate cancer using a Sleeping Beauty transposon-based somatic mutagenesis screen[J]. Cancer Research,2009,69(10):4388-4397. doi: 10.1158/0008-5472.CAN-08-3901
|
|
[11]
|
Liu F,Ma J,Wang K,et al. High expression of PDE4D correlates with poor prognosis and clinical progression in pancreaticductal adenocarcinoma[J]. Journal of Cancer,2019,10(25):6252-6260. doi: 10.7150/jca.35443
|
|
[12]
|
Cao B,Wang K,Liao J M,et al. Inactivation of oncogenic cAMP-specific phosphodiesterase 4D by miR-139-5p in response to p53 activation[J]. eLife,2016,5:e15978. doi: 10.7554/eLife.15978
|
|
[13]
|
Muntean D M,Sturza A,Pavel I Z,et al. Modulation of cancer metabolism by phytochemicals-a brief overview[J]. Anti-cancer Agents in Medicinal Chemistry,2018,18(5):684-692. doi: 10.2174/1871520617666171114102218
|
|
[14]
|
梁宏. 基于生物信息学挖掘卵巢癌顺铂耐药机制及潜在治疗药物 [D]. 昆明: 昆明医科大学硕士论文, 2016.
|
|
[15]
|
Yu Y,Luo Y,Fang Z,et al. Mechanism of sanguinarine in inhibiting macrophages to promote metastasis and proliferation of lung cancer via modulating the exosomes in A549 cells[J]. OncoTargets and Therapy,2020,13:8989-9003. doi: 10.2147/OTT.S261054
|
|
[16]
|
Rahman A,Pallichankandy S,Thayyullathil F,et al. Critical role of H(2)O(2) in mediating sanguinarine-induced apoptosis in prostate cancer cells via facilitating ceramide generation,ERK1/2 phosphorylation,and Par-4 cleavage[J]. Free Radical Biology & Medicine,2019,134:527-544.
|
|
[17]
|
Khan A Q,Mohamed E A N,Hakeem I,et al. Sanguinarine induces apoptosis in papillary thyroid cancer cells via generation of reactive oxygen species[J]. Molecules (Basel,Switzerland),2020,25(5):1229. doi: 10.3390/molecules25051229
|
|
[18]
|
Fan H N,Chen W,Peng S Q,et al. Sanguinarine inhibits the tumorigenesis of gastric cancer by regulating the TOX/DNA-PKcs/ KU70/80 pathway[J]. Pathology,Research and Practice,2019,215(11):152677.
|
|
[19]
|
Gong X,Chen Z,Han Q,et al. Sanguinarine triggers intrinsic apoptosis to suppress colorectal cancer growth through disassociation between STRAP and MELK[J]. BMC Cancer,2018,18(1):578. doi: 10.1186/s12885-018-4463-x
|
|
[20]
|
Ma Y,Yu W,Shrivastava A,et al. Sanguinarine inhibits pancreatic cancer stem cell characteristics by inducing oxidative stress and suppressing sonic hedgehog-Gli-Nanog pathway[J]. Carcinogenesis,2017,38(10):1047-1056. doi: 10.1093/carcin/bgx070
|
|
[21]
|
Sarkhosh-Inanlou R,Molaparast M,Mohammadzadeh A,et. al. Sanguinarine enhances cisplatin sensitivity via glutathione depletion in cisplatin-resistant ovarian cancer (A2780) cells[J]. Chemical Biology & Drug Design,2020,95(2):215-223.
|
|
[22]
|
Zhang S,Leng T,Zhang Q,et. al. Sanguinarine inhibits epithelial ovarian cancer development via regulating long non-coding RNA CASC2-EIF4A3 axis and/or inhibiting NF-κB signaling or PI3K/AKT/mTOR pathway[J]. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie,2018,102:302-308.
|
|
[23]
|
赵洪波,王妍妍,冷天艳,等. 血根碱对紫杉醇耐药卵巢癌A2780/taxol细胞生长及化疗敏感性影响的研究[J]. 实用妇产科杂志,2018,34(4):268-272.
|
|
[24]
|
Zhang H,Kong Q,Wang J,et al. Complex roles of cAMP-PKA-CREB signaling in cancer[J]. Experimental Hematology & Oncology,2020,9(1):32.
|
|
[25]
|
Huang H,Wang Y,Kandpal M,et al. FTO-dependent N (6)-methyladenosine modifications inhibit ovarian cancer stem cell self-renewal by blocking cAMP signaling[J]. Cancer Research,2020,80(16):3200-3214. doi: 10.1158/0008-5472.CAN-19-4044
|
|
[26]
|
Gong S,Chen Y,Meng F,et al. Roflumilast enhances cisplatin-sensitivity and reverses cisplatin-resistance of ovarian cancer cells via cAMP/PKA/CREB-FtMt signalling axis[J]. Cell Proliferation,2018,51(5):e12474. doi: 10.1111/cpr.12474
|





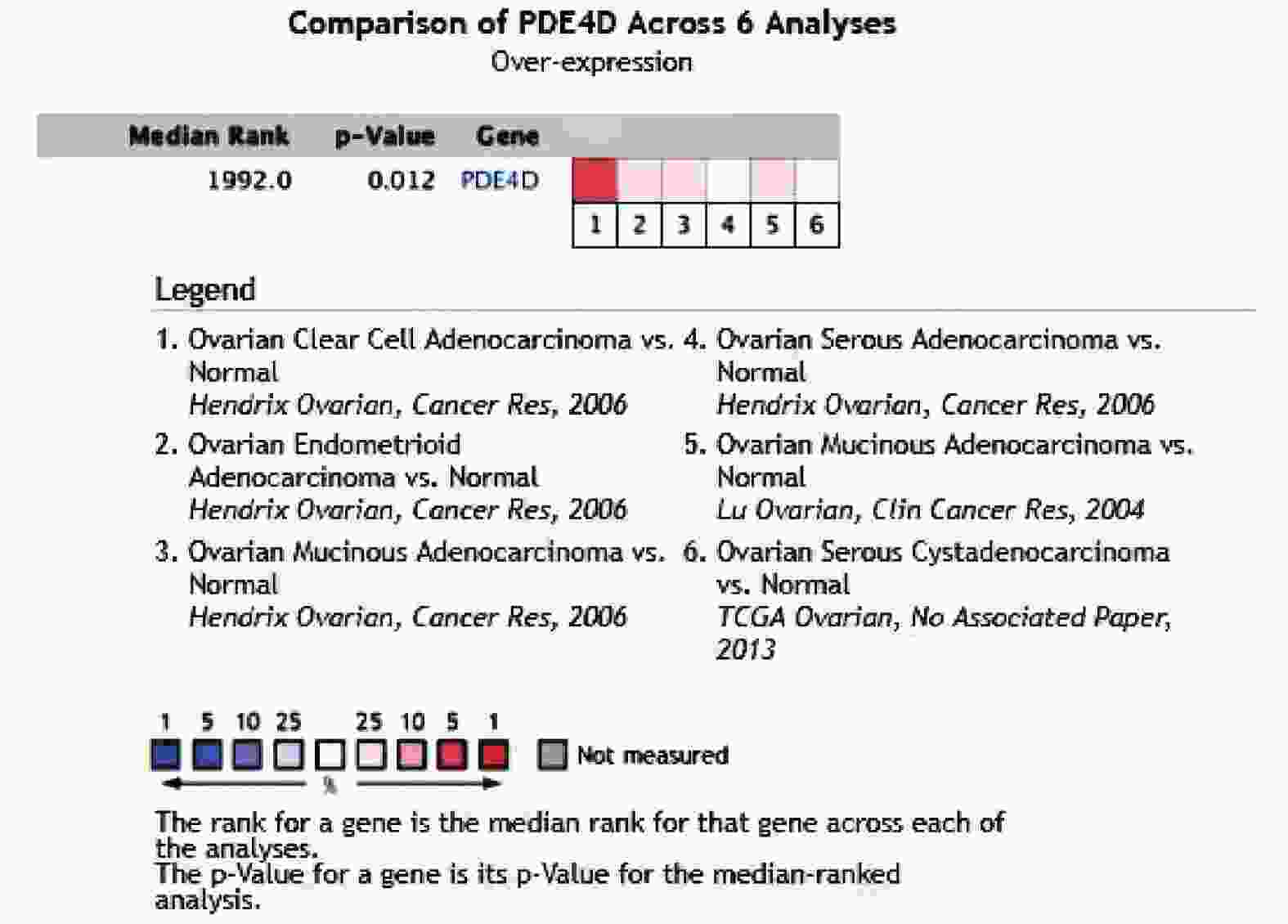
 下载:
下载: