Molecular Mechanisms of Osteogenesis Promoted by Bone Repair Materials
-
摘要: MAPK、PI3K/AKT、AMPK、TGF-β超家族、Wnt、Hippo、NF-κB、Notch、JAK/STAT、Hedgehog、整合素、OPG/RANKL/RANK、HIF等信号通路都与成骨分化相关,在骨修复材料促进成骨的过程中发挥着一定的调控作用,金属材料、无机材料、有机高分子材料和复合材料等骨修复材料可以通过激活一条或多条成骨相关信号通路促进骨组织再生。进一步了解骨修复材料促进成骨作用的相关分子机制将有助于骨修复材料更广泛地应用于骨组织工程和付诸临床实践,但目前关于骨修复材料促进成骨作用的具体分子机制还未彻底阐述清晰,未来仍需进一步研究。简要介绍现今与成骨分化相关的信号通路,综述骨修复材料在多种信号通路中的研究,为骨修复材料促成骨机制的深入研究提供借鉴。Abstract: Signal pathways including MAPK, PI3K/AKT, AMPK, TGF-β superfamily, Wnt, Hippo, NF-κB, Notch, JAK/STAT, Hedgehog, integrin, OPG/RANKL/RANK, HIF, etc. are all related to osteogenic differentiation and play a certain regulatory role in the process of bone regeneration promoted by bone repair materials. Bone repair materials such as metal materials, inorganic materials, organic polymer materials and composite materials can promote bone tissue regeneration by activating one or more signaling pathways related osteogenic. Further understanding of the molecular mechanisms underlying the promotion of osteogenesis by bone repair materials will help to broaden their application in bone tissue engineering and clinical practice. However, the specific molecular mechanisms by which bone repair materials promote osteogenesis have not yet been fully elucidated, and further research is still needed. This article briefly introduces the signal pathways related to osteogenic differentiation, summarizes the research on bone repair materials in various signal pathways, and provides a reference for the in-depth study of the mechanism of bone repair materials in promoting osteogenesis.
-
Key words:
- Bone repair materials /
- Osteogenesis /
- Bone regeneration /
- Signaling pathways
-
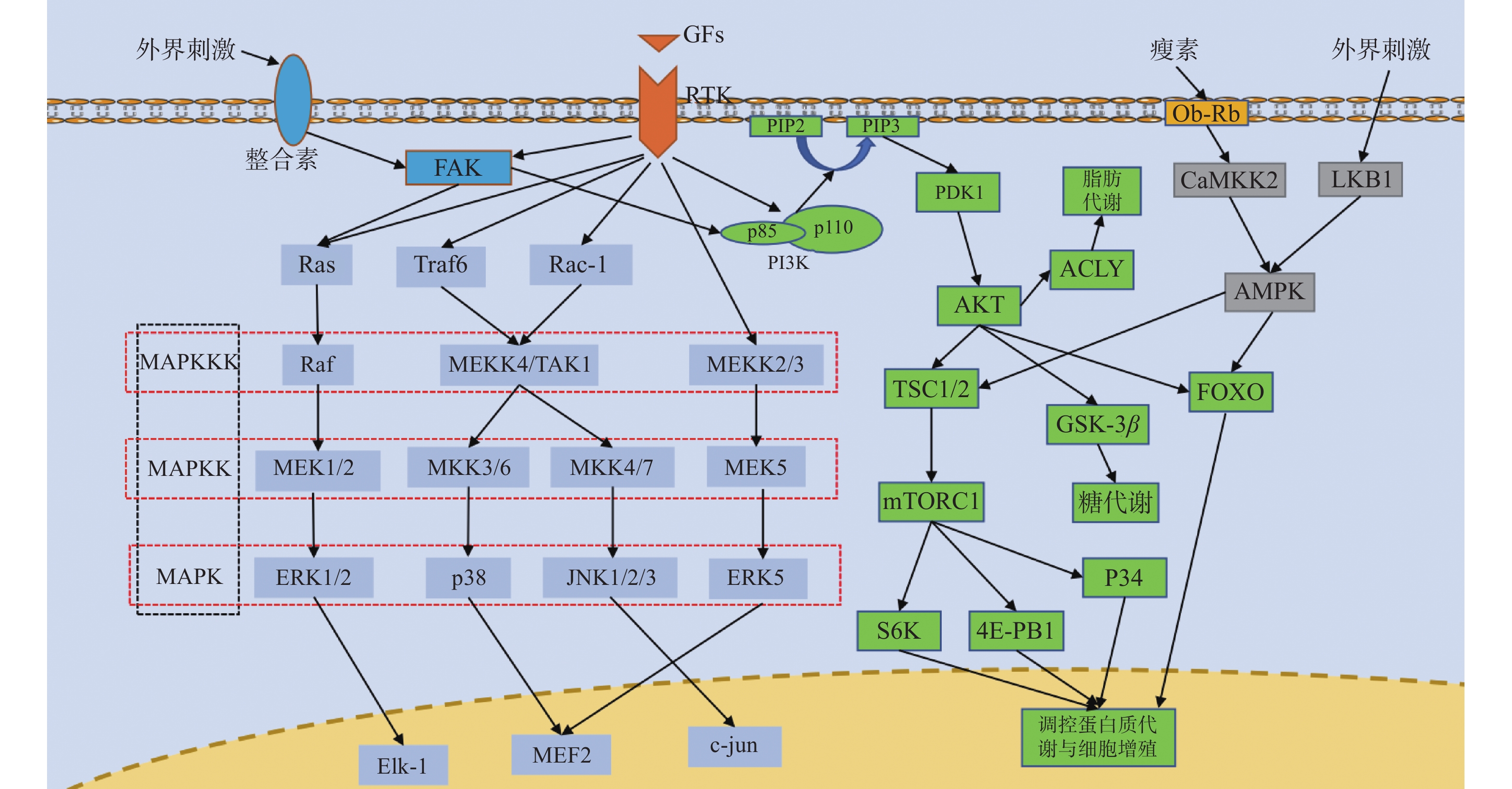
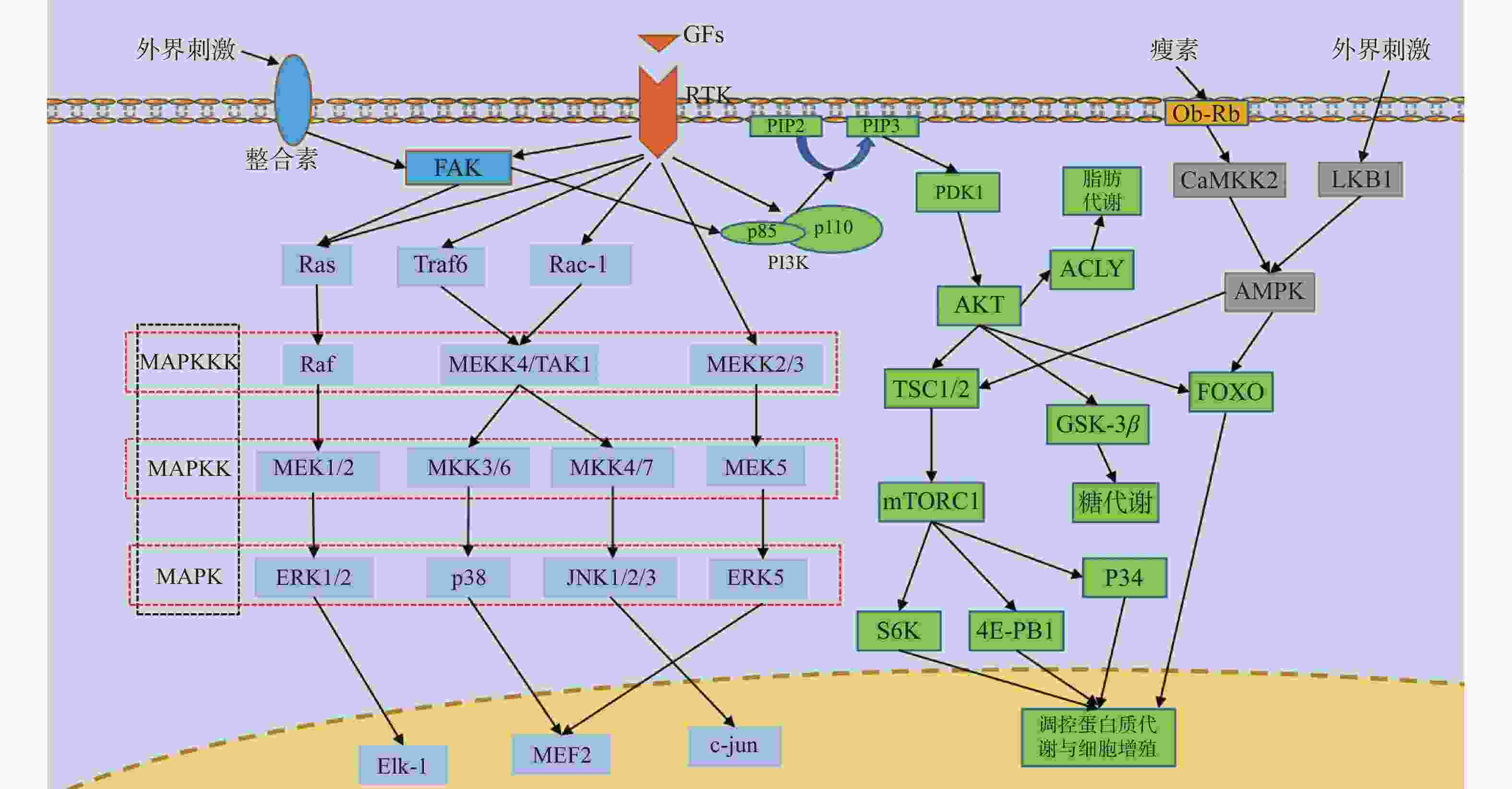
图 1 RTK通路、MAPK通路、PI3K/AKT通路、AMPK通路、整合素通路并行示意图
箭头仅表示作用方向,MAPK为丝裂原活化蛋白激酶,MAPKK为MAPK激酶,MAPKKK为MAPKK激酶,FAK为局部黏着斑激酶,Ras为GTP结合蛋白Ras,Raf为丝/苏氨酸蛋白激酶Raf,MEK1/2/5为MAPK激酶1/2/5,ERK1/2/5为细胞外调节激酶1/2/5,Elk-1:细胞转录因子Elk-1,Traf6为泛素连接酶,Rac-1为GTP结合蛋白Rac-1,MEKK2/3/4为MAPKK激酶2/3/4,TAK1为转化生长因子β激酶1,MKK3/4/6/7为MAPK激酶3/4/6/7,JNK1/2/3为应激活化蛋白激酶1/2/3,MEF2:细胞转录因子MEF2,c-jun:细胞转录因子c-jun,GFs为各类生长因子,RTK为受体酪氨酸激酶,PI3K为磷脂酰肌醇3激酶,PIP2为磷脂酰肌醇2磷酸,PIP3为磷脂酰肌醇3磷酸,PDK1为三磷酸肌醇依赖性蛋白激酶1,AKT为蛋白激酶B,TSC1/2为GTP结合蛋白TSC1/2,mTORC1为哺乳动物雷帕霉素靶蛋白1,S6K为蛋白激酶S6K,4E-PB1为转录结合蛋白4E-PB1,GSK-3β为糖原合成酶激酶3β,FOXO为细胞转录因子FOXO,ACLY为ATP柠檬酸裂解酶,Ob-Rb为瘦素受体,CaMKK2为钙调蛋白依赖性蛋白激酶激酶2,LKB1为丝/苏氨酸激酶LKB1,AMPK为AMP依赖的蛋白激酶。
Figure 1. Parallel diagram of RTK pathway,MAPK pathway,PI3K/AKT pathway,AMPK pathway and integrin pathway
表 1 复合材料成骨机制的研究
Table 1. Studies on osteogenic mechanism of composite materials
研究者 发表年份 材料 生物相容性 信号通路 LEE等[86] 2023 掺入了氧化锌/阿仑膦酸钠/BMP2纳米颗粒的细胞外基质/聚乳酸-羟基乙酸共聚物/改性后的氢氧化镁复合材料 良好 NO/cGMP和Wnt/β-catenin信号通路 SHI等[77] 2022 聚己内酯/壳聚糖复合材料 良好 p38/MAPK和Hippo信号通路 ZHANG等[78] 2022 甲基丙烯酰壳聚糖/β-磷酸三钙水凝胶 良好 Hippo信号通路 WANG等[79] 2022 还原性谷胱甘肽接枝甲基丙烯酸明胶所制备的抗氧化水凝胶 良好 PI3K/AKT信号通路 XUE等[80] 2022 壳聚糖季铵盐/氧化石墨烯/聚多巴胺复合材料 良好 BMP /Smads信号通路 HUANG等[16] 2022 钽/钛合金 良好 ILK/ERK1/2信号通路 JIA等[81] 2021 锌/锶合金 良好 Wnt/β-catenin、PI3K/AKT和MAPK/ERK信号通路 YANG等[82] 2021 包含L-精氨酸和Ca2+的骨膜模拟助骨剂 良好 NO/cGMP信号通路 CHENG等[83] 2021 聚乳酸-羟基乙酸共聚物 /β-磷酸三钙复合材料 良好 MAPK和PI3K/AKT信号通路 ZOU等[85] 2021 负载甲状旁腺激素和纳米羟基磷灰石的壳聚糖/海藻酸钠水凝胶 良好 Notch信号通路 FU等[84] 2019 硫化铋/羟基磷灰石薄膜包裹的钛 良好 Wnt/Ca2+信号通路 -
[1] Majidinia M,Sadeghpour A,Yousefi B. The roles of signaling pathways in bone repair and regeneration[J]. J Cell Physiol,2018,233(4):2937-2948. doi: 10.1002/jcp.26042 [2] Dong S,Zhang Y,Mei Y,et al. Researching progress on bio-reactive electrogenic materials with electrophysiological activity for enhanced bone regeneration[J]. Front Bioeng Biotechnol,2022,10:921284. doi: 10.3389/fbioe.2022.921284 [3] Gan Q,Pan H,Zhang W,et al. Fabrication and evaluation of a BMP-2/dexamethasone co-loaded gelatin sponge scaffold for rapid bone regeneration[J]. Regen Biomater,2022,9:rbac008. doi: 10.1093/rb/rbac008 [4] Chen S,Chen X,Geng Z,et al. The horizon of bone organoid: A perspective on construction and application[J]. Bioact Mater,2022,18:15-25. doi: 10.1016/j.bioactmat.2022.01.048 [5] Shao R,Dong Y,Zhang S,et al. State of the art of bone biomaterials and their interactions with stem cells: Current state and future directions[J]. Biotechnol J,2022,17(4):e2100074. doi: 10.1002/biot.202100074 [6] Cai M,Liu Y,Tian Y,et al. Osteogenic peptides in periodontal ligament stem cell-containing three-dimensional bioscaffolds promote bone healing[J]. Biomater Sci,2022,10(7):1765-1775. doi: 10.1039/D1BM01673C [7] Guo P,Liu X,Zhang P,et al. A single-cell transcriptome of mesenchymal stromal cells to fabricate bioactive hydroxyapatite materials for bone regeneration[J]. Bioact Mater,2022,9:281-298. doi: 10.1016/j.bioactmat.2021.08.009 [8] Bose S,Sarkar N,Banerjee D. Natural medicine delivery from biomedical devices to treat bone disorders: A review[J]. Acta Biomater,2021,126:63-91. doi: 10.1016/j.actbio.2021.02.034 [9] Zhu G,Zhang T,Chen M,et al. Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds[J]. Bioact Mater,2021,6(11):4110-4140. doi: 10.1016/j.bioactmat.2021.03.043 [10] Gupta A,Singh S. Multimodal potentials of gold nanoparticles for bone tissue engineering and regenerative medicine: Avenues and prospects[J]. Small,2022,18(29):e2201462. doi: 10.1002/smll.202201462 [11] Zhang B,Han F,Wang Y,et al. Cells-micropatterning biomaterials for immune activation and bone regeneration[J]. Adv Sci (Weinh),2022,9(18):e2200670. doi: 10.1002/advs.202200670 [12] Boller L A,Shiels S M,Florian D C,et al. Effects of nanocrystalline hydroxyapatite concentration and skeletal site on bone and cartilage formation in rats[J]. Acta Biomater,2021,130:485-496. doi: 10.1016/j.actbio.2021.05.056 [13] 王靖,刘昌胜. 材料生物学——骨修复材料的机遇与挑战[J]. 中国材料进展,2019,38(4):359-364. [14] 徐朱杰,陈敬华,邵伟,等. 硫酸乙酰肝素成骨作用及成骨机制的研究进展[J]. 中国修复重建外科杂志,2017,31(8):1016-1020. [15] Aimaijiang M,Liu Y,Zhang Z,et al. LIPUS as a potential strategy for periodontitis treatment: A review of the mechanisms[J]. Front Bioeng Biotechnol,2023,11:1018012. doi: 10.3389/fbioe.2023.1018012 [16] Huang G,Pan S T,Qiu J X. The osteogenic effects of porous Tantalum and Titanium alloy scaffolds with different unit cell structure[J]. Colloids Surf B Biointerfaces,2022,210:112229. doi: 10.1016/j.colsurfb.2021.112229 [17] Sun J L,Jiao K,Song Q,et al. Intrafibrillar silicified collagen scaffold promotes in-situ bone regeneration by activating the monocyte p38 signaling pathway[J]. Acta Biomater,2018,67:354-365. doi: 10.1016/j.actbio.2017.12.022 [18] Wang H,Lin C,Zhang X,et al. Mussel-inspired polydopamine coating: A general strategy to enhance osteogenic differentiation and osseointegration for diverse implants[J]. ACS Appl Mater Interfaces,2019,11(7):7615-7625. doi: 10.1021/acsami.8b21558 [19] Ma L,Ke W,Liao Z,et al. Small extracellular vesicles with nanomorphology memory promote osteogenesis[J]. Bioact Mater,2022,17:425-438. doi: 10.1016/j.bioactmat.2022.01.008 [20] Zheng Z,He Y,Long L,et al. Involvement of PI3K/Akt signaling pathway in promoting osteogenesis on titanium implant surfaces modified with novel non-thermal atmospheric plasma[J]. Front Bioeng Biotechnol,2022,10:975840. doi: 10.3389/fbioe.2022.975840 [21] Xie H,Lin Y,Fang F. Glycogen synthase kinase-3beta inhibitor promotes the migration and osteogenic differentiation of rat dental pulp stem cells via the beta-catenin/PI3K/Akt signaling pathway[J]. J Dent Sci,2022,17(2):802-810. doi: 10.1016/j.jds.2021.09.035 [22] 张玉敏,葛林虎,曾素娟. AMPK在组织工程骨促骨再生研究中的应用及进展[J]. 中国骨质疏松杂志,2021,27(11):1685-1689. doi: 10.3969/j.issn.1006-7108.2021.11.025 [23] Zhang T,Jiang M,Yin X,et al. Mechanism of exosomes involved in osteoimmunity promoting osseointegration around titanium implants with small-scale topography[J]. Front Bioeng Biotechnol,2021,9:682384. doi: 10.3389/fbioe.2021.682384 [24] Wang G, Luo J, Qiao Y, et al. AMPK/mTOR pathway is involved in autophagy induced by magnesium-incorporated TiO(2) surface to promote BMSC osteogenic differentiation [J]. J Funct Biomater, 2022, 13(4): 221. [25] Liu Z,Yu Z,Chang H,et al. Strontium-containing alpha-calcium sulfate hemihydrate promotes bone repair via the TGF-beta/Smad signaling pathway[J]. Mol Med Rep,2019,20(4):3555-3564. [26] Guillot-Ferriols M,Lanceros-Mendez S,Gomez Ribelles J L,et al. Electrical stimulation: Effective cue to direct osteogenic differentiation of mesenchymal stem cells?[J]. Biomater Adv,2022,138:212918. doi: 10.1016/j.bioadv.2022.212918 [27] You J,Zhang Y,Zhou Y. Strontium functionalized in biomaterials for bone tissue engineering: A prominent role in osteoimmunomodulation[J]. Front Bioeng Biotechnol,2022,10:928799. doi: 10.3389/fbioe.2022.928799 [28] Dai T,Ma J,Ni S,et al. Attapulgite-doped electrospun PCL scaffolds for enhanced bone regeneration in rat cranium defects[J]. Biomater Adv,2022,133:112656. doi: 10.1016/j.msec.2022.112656 [29] Nelson A L,Fontana G,Miclau E,et al. Therapeutic approaches to activate the canonical Wnt pathway for bone regeneration[J]. J Tissue Eng Regen Med,2022,16(11):961-976. doi: 10.1002/term.3349 [30] Wu M,Chen F,Liu H,et al. Bioinspired sandwich-like hybrid surface functionalized scaffold capable of regulating osteogenesis,angiogenesis,and osteoclastogenesis for robust bone regeneration[J]. Mater Today Bio,2022,17:100458. doi: 10.1016/j.mtbio.2022.100458 [31] Yu D,Wang J,Qian K J,et al. Effects of nanofibers on mesenchymal stem cells: environmental factors affecting cell adhesion and osteogenic differentiation and their mechanisms[J]. J Zhejiang Univ Sci B,2020,21(11):871-884. doi: 10.1631/jzus.B2000355 [32] Zhang N,Wang Y,Zhang J,et al. Controlled domain gels with a biomimetic gradient environment for osteochondral tissue regeneration[J]. Acta Biomater,2021,135:304-317. doi: 10.1016/j.actbio.2021.08.029 [33] 高倩,姚晓雨,隋磊. 微纳米分级形貌促进成骨细胞分化的分子机制研究进展[J]. 天津医科大学学报,2021,27(3):307-309,315. [34] Zhang Y,Fan Z,Xing Y,et al. Effect of microtopography on osseointegration of implantable biomaterials and its modification strategies[J]. Front Bioeng Biotechnol,2022,10:981062. doi: 10.3389/fbioe.2022.981062 [35] Wang H,Yu H,Huang T,et al. Hippo-YAP/TAZ signaling in osteogenesis and macrophage polarization: Therapeutic implications in bone defect repair[J]. Genes & Diseases,2023,10(6):2528-2539. [36] Chan Y H,Ho K N,Lee Y C,et al. Melatonin enhances osteogenic differentiation of dental pulp mesenchymal stem cells by regulating MAPK pathways and promotes the efficiency of bone regeneration in calvarial bone defects[J]. Stem Cell Res Ther,2022,13(1):73. doi: 10.1186/s13287-022-02744-z [37] Zhang Z,Fu X,Xu L,et al. Nanosized alumina particle and proteasome inhibitor bortezomib prevented inflammation and osteolysis induced by titanium particle via autophagy and NF-kappaB signaling[J]. Sci Rep,2020,10(1):5562. doi: 10.1038/s41598-020-62254-x [38] Deng Y,Li R,Wang H,et al. Biomaterial-mediated presentation of Jagged-1 mimetic ligand enhances cellular activation of Notch signaling and bone regeneration[J]. ACS Nano,2022,16(1):1051-1062. doi: 10.1021/acsnano.1c08728 [39] Du Z,Feng X,Cao G,et al. The effect of carbon nanotubes on osteogenic functions of adipose-derived mesenchymal stem cells in vitro and bone formation in vivo compared with that of nano-hydroxyapatite and the possible mechanism[J]. Bioact Mater,2021,6(2):333-345. doi: 10.1016/j.bioactmat.2020.08.015 [40] Rao P,Lou F,Luo D,et al. Decreased autophagy impairs osteogenic differentiation of adipose-derived stem cells via Notch signaling in diabetic osteoporosis mice[J]. Cell Signal,2021,87:110138. doi: 10.1016/j.cellsig.2021.110138 [41] Xu H,Zhou S,Qu R,et al. Icariin prevents oestrogen deficiency-induced alveolar bone loss through promoting osteogenesis via STAT3[J]. Cell Prolif,2020,53(2):e12743. doi: 10.1111/cpr.12743 [42] Gao A,Liao Q,Xie L,et al. Tuning the surface immunomodulatory functions of polyetheretherketone for enhanced osseointegration[J]. Biomaterials,2020,230:119642. doi: 10.1016/j.biomaterials.2019.119642 [43] Li F,Zhang R,Hu C,et al. Irradiation haematopoiesis recovery orchestrated by IL-12/IL-12Rbeta1/TYK2/STAT3-initiated osteogenic differentiation of mouse bone marrow-derived mesenchymal stem cells[J]. Front Cell Dev Biol,2021,9:729293. doi: 10.3389/fcell.2021.729293 [44] Lee C S,Hsu G C,Sono T,et al. Development of a biomaterial scaffold integrated with osteoinductive oxysterol liposomes to enhance Hedgehog signaling and bone repair[J]. Mol Pharm,2021,18(4):1677-1689. doi: 10.1021/acs.molpharmaceut.0c01136 [45] Hou H W,Xue P,Wang Y,et al. Liraglutide regulates proliferation,differentiation,and apoptosis of preosteoblasts through a signaling network of Notch/Wnt/Hedgehog signaling pathways[J]. Eur Rev Med Pharmacol Sci,2020,24(23):12408-12422. [46] Wang Q,Zhang W,Peng X,et al. GSK-3beta suppression upregulates Gli1 to alleviate osteogenesis inhibition in titanium nanoparticle-induced osteolysis[J]. J Nanobiotechnology,2022,20(1):148. doi: 10.1186/s12951-022-01351-7 [47] Han L,Guo Y,Jia L,et al. 3D magnetic nanocomposite scaffolds enhanced the osteogenic capacities of rat bone mesenchymal stem cells in vitro and in a rat calvarial bone defect model by promoting cell adhesion[J]. J Biomed Mater Res A,2021,109(9):1670-1680. doi: 10.1002/jbm.a.37162 [48] Zhang X,Wang X,Lee Y W,et al. Bioactive scaffold fabricated by 3D printing for enhancing osteoporotic bone regeneration[J]. Bioengineering (Basel),2022,9(10):525. doi: 10.3390/bioengineering9100525 [49] Tang Y,Luo K,Tan J,et al. Laminin alpha 4 promotes bone regeneration by facilitating cell adhesion and vascularization[J]. Acta Biomater,2021,126:183-198. doi: 10.1016/j.actbio.2021.03.011 [50] Tian Y,Zheng H,Zheng G,et al. Hierarchical microgroove/nanopore topography regulated cell adhesion to enhance osseointegration around intraosseous implants in vivo[J]. Biomater Sci,2022,10(2):560-580. doi: 10.1039/D1BM01657A [51] Liang C,Liu X,Liu C,et al. Integrin alpha10 regulates adhesion,migration,and osteogenic differentiation of alveolar bone marrow mesenchymal stem cells in type 2 diabetic patients who underwent dental implant surgery[J]. Bioengineered,2022,13(5):13252-13268. doi: 10.1080/21655979.2022.2079254 [52] Lin Y H,Lee A K,Ho C C,et al. The effects of a 3D-printed magnesium-/strontium-doped calcium silicate scaffold on regulation of bone regeneration via dual-stimulation of the AKT and WNT signaling pathways[J]. Biomater Adv,2022,133:112660. doi: 10.1016/j.msec.2022.112660 [53] Huang T B,Li Y Z,Yu K,et al. Effect of the Wnt signal-RANKL/OPG axis on the enhanced osteogenic integration of a lithium incorporated surface[J]. Biomater Sci,2019,7(3):1101-1116. doi: 10.1039/C8BM01411F [54] Bai H,Wang Y,Zhao Y,et al. HIF signaling: A new propellant in bone regeneration[J]. Biomater Adv,2022,138:212874. doi: 10.1016/j.bioadv.2022.212874 [55] Rehman M,Madni A,Webster T J. The era of biofunctional biomaterials in orthopedics: What does the future hold?[J]. Expert Rev Med Devices,2018,15(3):193-204. doi: 10.1080/17434440.2018.1430569 [56] 周岚曦,邵路,董士武,等. 医用金属材料促血管生成的分子机制[J]. 中国组织工程研究,2023,27(16):2616-2624. doi: 10.12307/2023.107 [57] Wu Q,Hu L,Yan R,et al. Strontium-incorporated bioceramic scaffolds for enhanced osteoporosis bone regeneration[J]. Bone Res,2022,10(1):55. doi: 10.1038/s41413-022-00224-x [58] Cui X,Zhang Y,Wang J,et al. Strontium modulates osteogenic activity of bone cement composed of bioactive borosilicate glass particles by activating Wnt/beta-catenin signaling pathway[J]. Bioact Mater,2020,5(2):334-347. doi: 10.1016/j.bioactmat.2020.02.016 [59] He Y,Li Z,Ding X,et al. Nanoporous titanium implant surface promotes osteogenesis by suppressing osteoclastogenesis via integrin beta1/FAKpY397/MAPK pathway[J]. Bioact Mater,2022,8:109-123. doi: 10.1016/j.bioactmat.2021.06.033 [60] Zhang G,Liu W,Wang R,et al. The role of tantalum nanoparticles in bone regeneration involves the BMP2/Smad4/Runx2 signaling pathway[J]. Int J Nanomedicine,2020,15:2419-2435. doi: 10.2147/IJN.S245174 [61] Ge Y W,Liu X L,Yu D G,et al. Graphene-modified CePO4 nanorods effectively treat breast cancer-induced bone metastases and regulate macrophage polarization to improve osteo-inductive ability[J]. J Nanobiotechnology,2021,19(1):11. doi: 10.1186/s12951-020-00753-9 [62] Xu Y,Wu L,Tang Y,et al. Immunology and bioinformatics analysis of injectable organic/inorganic microfluidic microspheres for promoting bone repair[J]. Biomaterials,2022,288:121685. doi: 10.1016/j.biomaterials.2022.121685 [63] Lu Q,Diao J,Wang Y,et al. 3D printed pore morphology mediates bone marrow stem cell behaviors via RhoA/ROCK2 signaling pathway for accelerating bone regeneration[J]. Bioact Mater,2023,26:413-424. doi: 10.1016/j.bioactmat.2023.02.025 [64] Wang X,Yu Y,Ji L,et al. Calcium phosphate-based materials regulate osteoclast-mediated osseointegration[J]. Bioact Mater,2021,6(12):4517-4530. doi: 10.1016/j.bioactmat.2021.05.003 [65] Peng Y,Wang J,Dai X,et al. Precisely tuning the pore-wall surface composition of bioceramic scaffolds facilitates angiogenesis and orbital bone defect repair[J]. ACS Appl Mater Interfaces,2022,14(38):43987-44001. doi: 10.1021/acsami.2c14909 [66] Yin C,Jia X,Miron R J,et al. Setd7 and its contribution to Boron-induced bone regeneration in Boron-mesoporous bioactive glass scaffolds[J]. Acta Biomaterialia,2018,73:522-530. doi: 10.1016/j.actbio.2018.04.033 [67] Mei P,Jiang S,Mao L,et al. In situ construction of flower-like nanostructured calcium silicate bioceramics for enhancing bone regeneration mediated via FAK/p38 signaling pathway[J]. J Nanobiotechnology,2022,20(1):162. doi: 10.1186/s12951-022-01361-5 [68] Yuan B,Zhang Y,Zhao R,et al. A unique biomimetic modification endows polyetherketoneketone scaffold with osteoinductivity by activating cAMP/PKA signaling pathway[J]. Sci Adv,2022,8(40):eabq7116. doi: 10.1126/sciadv.abq7116 [69] Soltani M,Alizadeh P. Aloe vera incorporated starch-64S bioactive glass-quail egg shell scaffold for promotion of bone regeneration[J]. Int J Biol Macromol,2022,217:203-218. doi: 10.1016/j.ijbiomac.2022.07.054 [70] Tang G,Liu Z,Liu Y,et al. Recent trends in the development of bone regenerative biomaterials[J]. Front Cell Dev Biol,2021,9:665813. doi: 10.3389/fcell.2021.665813 [71] Li Z,Li S,Yang J,et al. 3D bioprinted gelatin/gellan gum-based scaffold with double-crosslinking network for vascularized bone regeneration[J]. Carbohydr Polym,2022,290:119469. doi: 10.1016/j.carbpol.2022.119469 [72] Asparuhova M B,Chappuis V,Stahli A,et al. Role of hyaluronan in regulating self-renewal and osteogenic differentiation of mesenchymal stromal cells and pre-osteoblasts[J]. Clin Oral Investig,2020,24(11):3923-3937. doi: 10.1007/s00784-020-03259-8 [73] Li M,Bai J,Tao H,et al. Rational integration of defense and repair synergy on PEEK osteoimplants via biomimetic peptide clicking strategy[J]. Bioact Mater,2022,8:309-324. doi: 10.1016/j.bioactmat.2021.07.002 [74] Xue R,Qian Y,Li L,et al. Polycaprolactone nanofiber scaffold enhances the osteogenic differentiation potency of various human tissue-derived mesenchymal stem cells[J]. Stem Cell Res Ther,2017,8(1):148. doi: 10.1186/s13287-017-0588-0 [75] Sun X,Jiao X,Wang Z,et al. Polydopamine-coated 3D-printed beta-tricalcium phosphate scaffolds to promote the adhesion and osteogenesis of BMSCs for bone-defect repair: mRNA transcriptomic sequencing analysis[J]. J Mater Chem B,2023,11(8):1725-1738. doi: 10.1039/D2TB02280J [76] Abodunrin O D,El Mabrouk K,Bricha M. A review on borate bioactive glasses (BBG): effect of doping elements,degradation,and applications[J]. J Mater Chem B,2023,11(5):955-973. doi: 10.1039/D2TB02505A [77] Shi W,Zhang X,Bian L,et al. Alendronate crosslinked chitosan/polycaprolactone scaffold for bone defects repairing[J]. Int J Biol Macromol,2022,204:441-456. doi: 10.1016/j.ijbiomac.2022.02.007 [78] Zhang Y,Li Z,Wang Z,et al. Mechanically enhanced composite hydrogel scaffold for in situ bone repairs[J]. Biomater Adv,2022,134:112700. doi: 10.1016/j.msec.2022.112700 [79] Wang L, Shen M, Hou Q, et al. 3D printing of reduced glutathione grafted gelatine methacrylate hydrogel scaffold promotes diabetic bone regeneration by activating PI3K/Akt signaling pathway [J]. Int J Biol Macromol, 2022, 222(Pt A): 1175-1191. [80] Xue H,Zhang Z,Lin Z,et al. Enhanced tissue regeneration through immunomodulation of angiogenesis and osteogenesis with a multifaceted nanohybrid modified bioactive scaffold[J]. Bioactive Materials,2022,18:552-568. doi: 10.1016/j.bioactmat.2022.05.023 [81] Jia B,Yang H,Zhang Z,et al. Biodegradable Zn-Sr alloy for bone regeneration in rat femoral condyle defect model: In vitro and in vivo studies[J]. Bioact Mater,2021,6(6):1588-1604. doi: 10.1016/j.bioactmat.2020.11.007 [82] Yang Y,Xu T,Zhang Q,et al. Biomimetic,stiff,and adhesive periosteum with osteogenic-angiogenic coupling effect for bone regeneration[J]. Small,2021,17(14):e2006598. doi: 10.1002/smll.202006598 [83] Cheng W X,Liu Y Z,Meng X B,et al. PLGA/beta-TCP composite scaffold incorporating cucurbitacin B promotes bone regeneration by inducing angiogenesis[J]. J Orthop Translat,2021,31:41-51. doi: 10.1016/j.jot.2021.10.002 [84] Fu J,Liu X,Tan L,et al. Photoelectric-responsive extracellular matrix for bone engineering[J]. ACS Nano,2019,13(11):13581-13594. doi: 10.1021/acsnano.9b08115 [85] Zou Z,Wang L,Zhou Z,et al. Simultaneous incorporation of PTH(1-34) and nano-hydroxyapatite into Chitosan/Alginate Hydrogels for efficient bone regeneration[J]. Bioact Mater,2021,6(6):1839-1851. doi: 10.1016/j.bioactmat.2020.11.021 [86] Lee J K,Kim D S,Park S Y,et al. Nitric oxide-releasing bioinspired scaffold for exquisite regeneration of osteoporotic bone via regulation of homeostasis[J]. Adv Sci (Weinh),2023,10(6):e2205336. doi: 10.1002/advs.202205336 -





 下载:
下载: