Construction of Stable Transfected Cell Line A549 of Non-small Cell Lung Cancer by Overexpressing and Knocking Down LncRNA RP11-521C20.3
-
摘要:
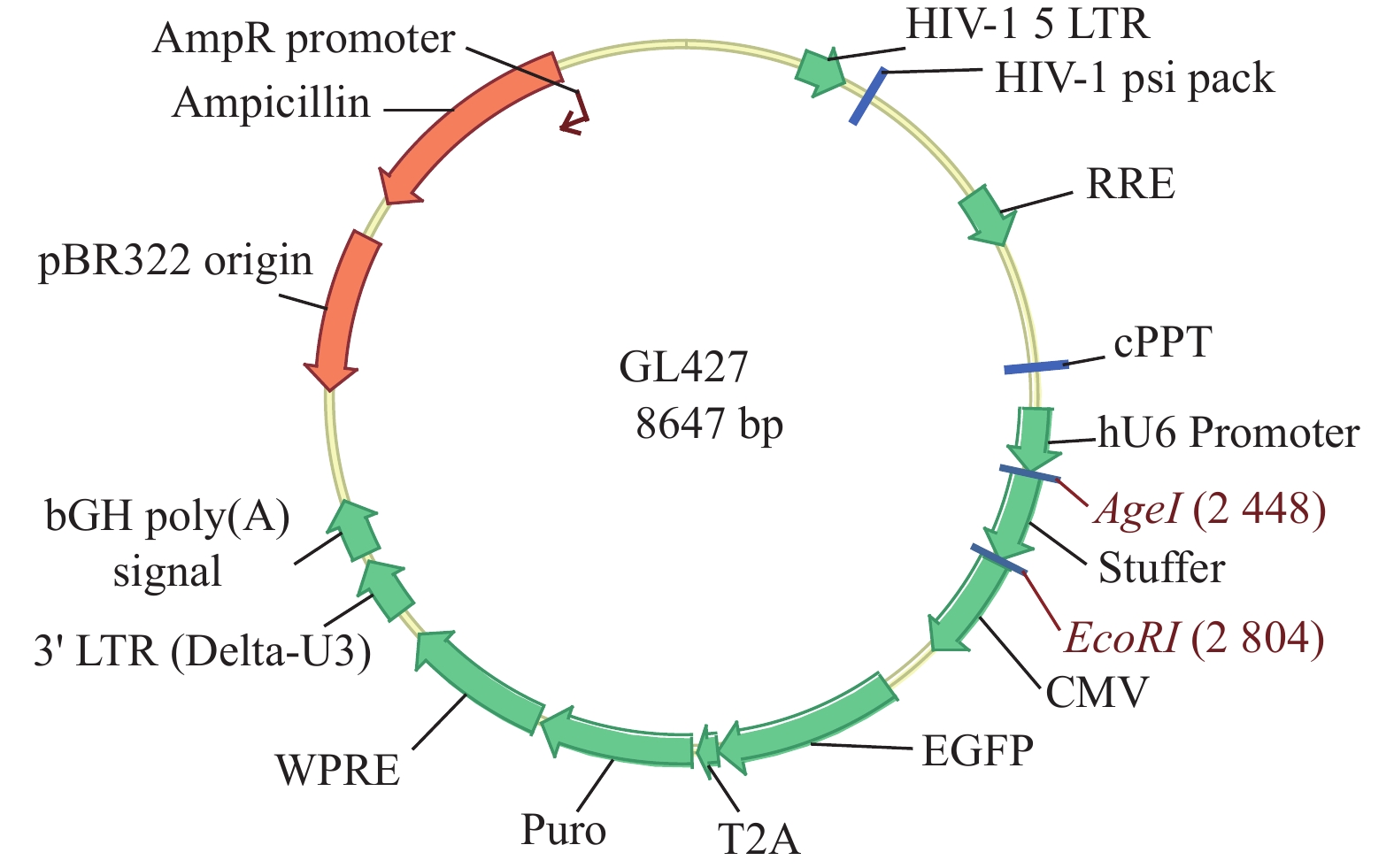
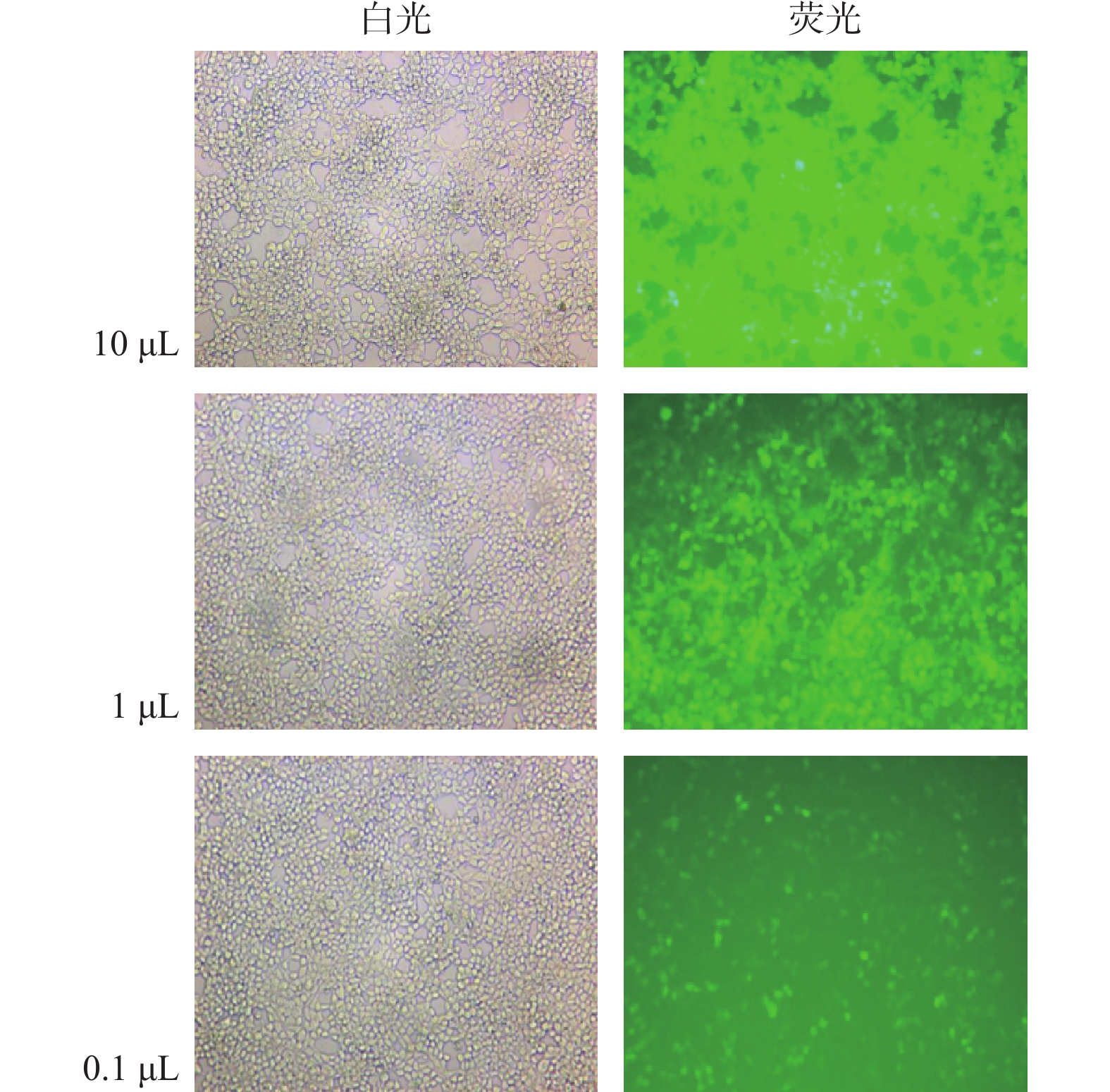
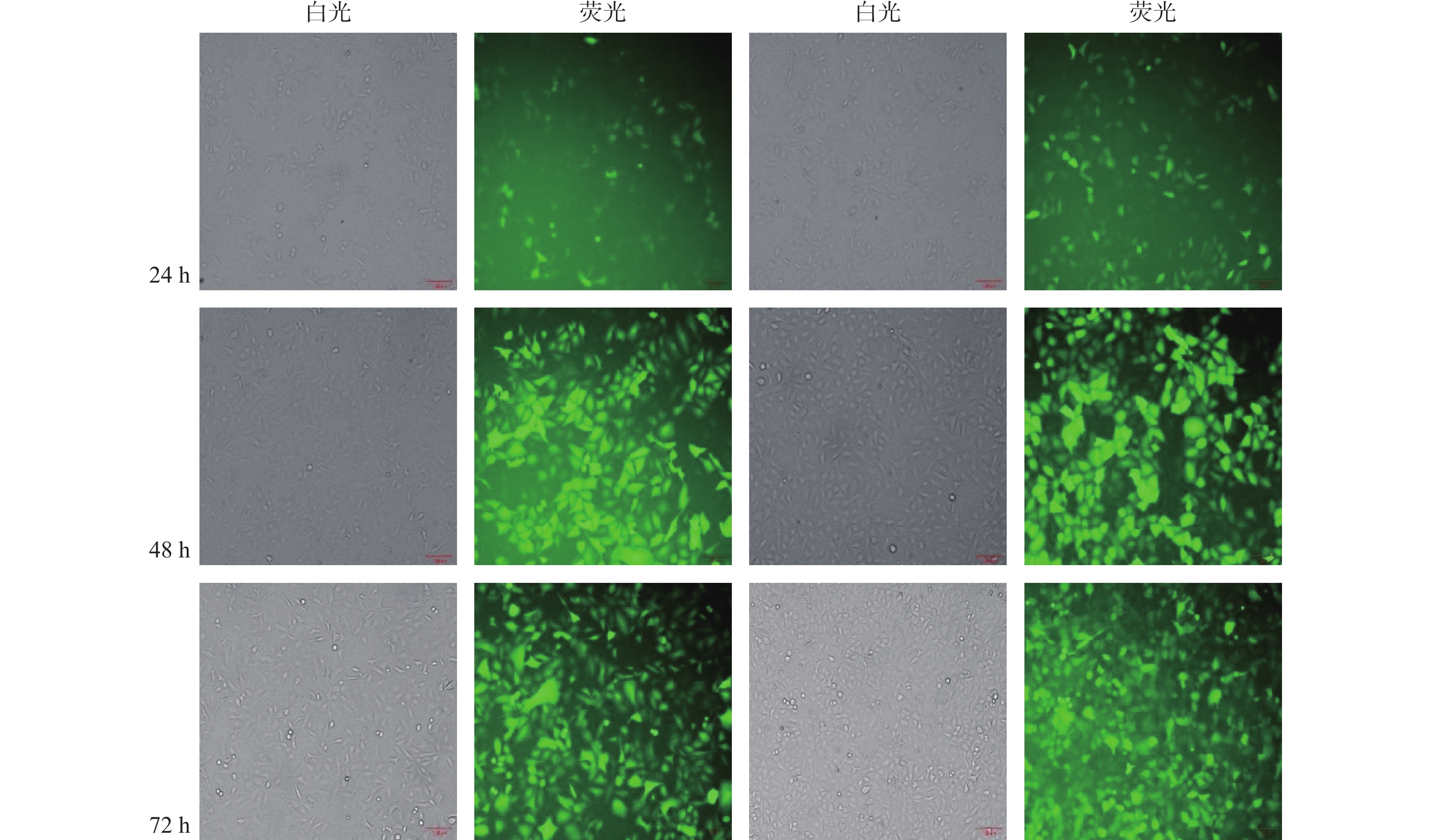
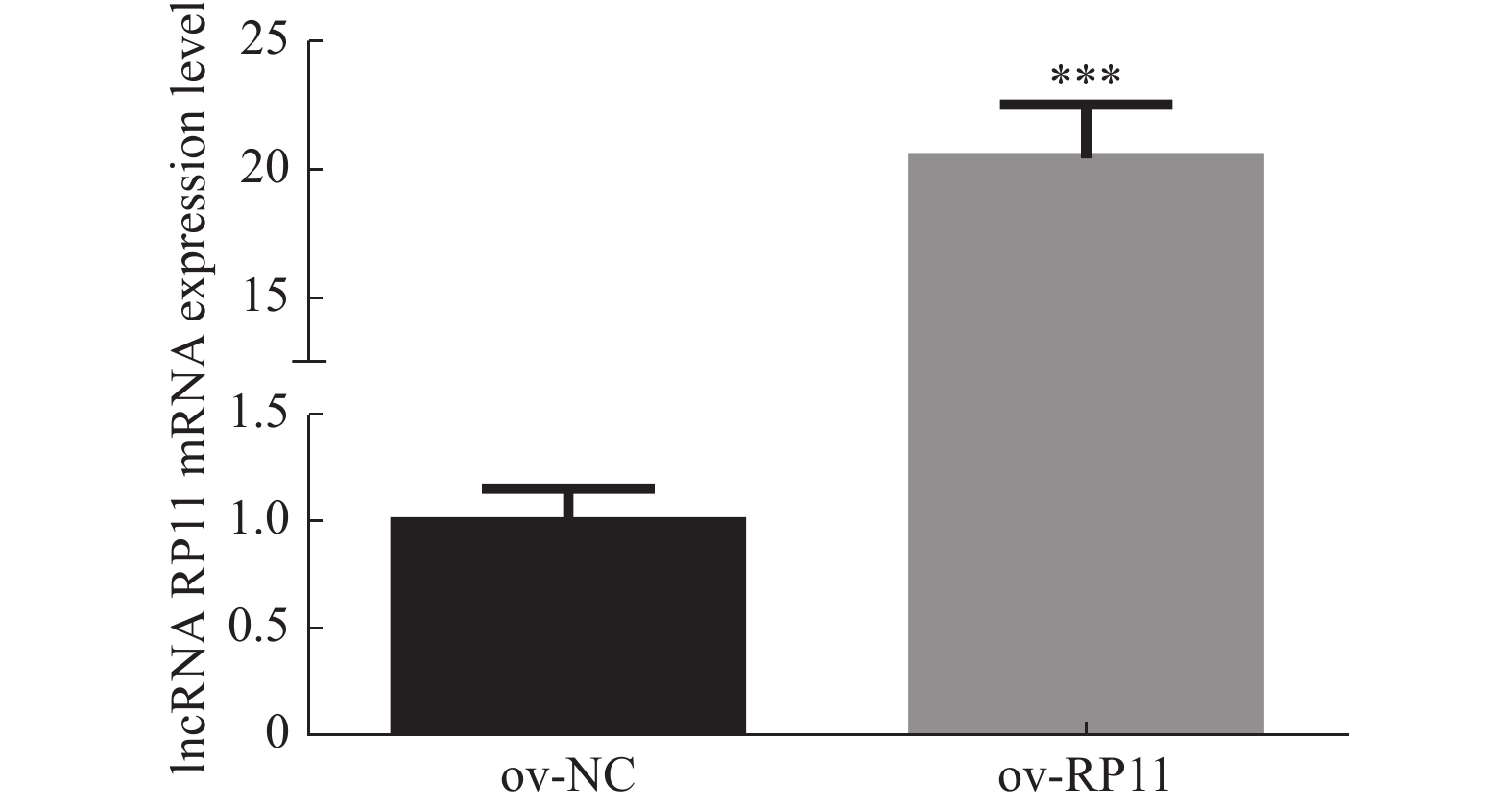
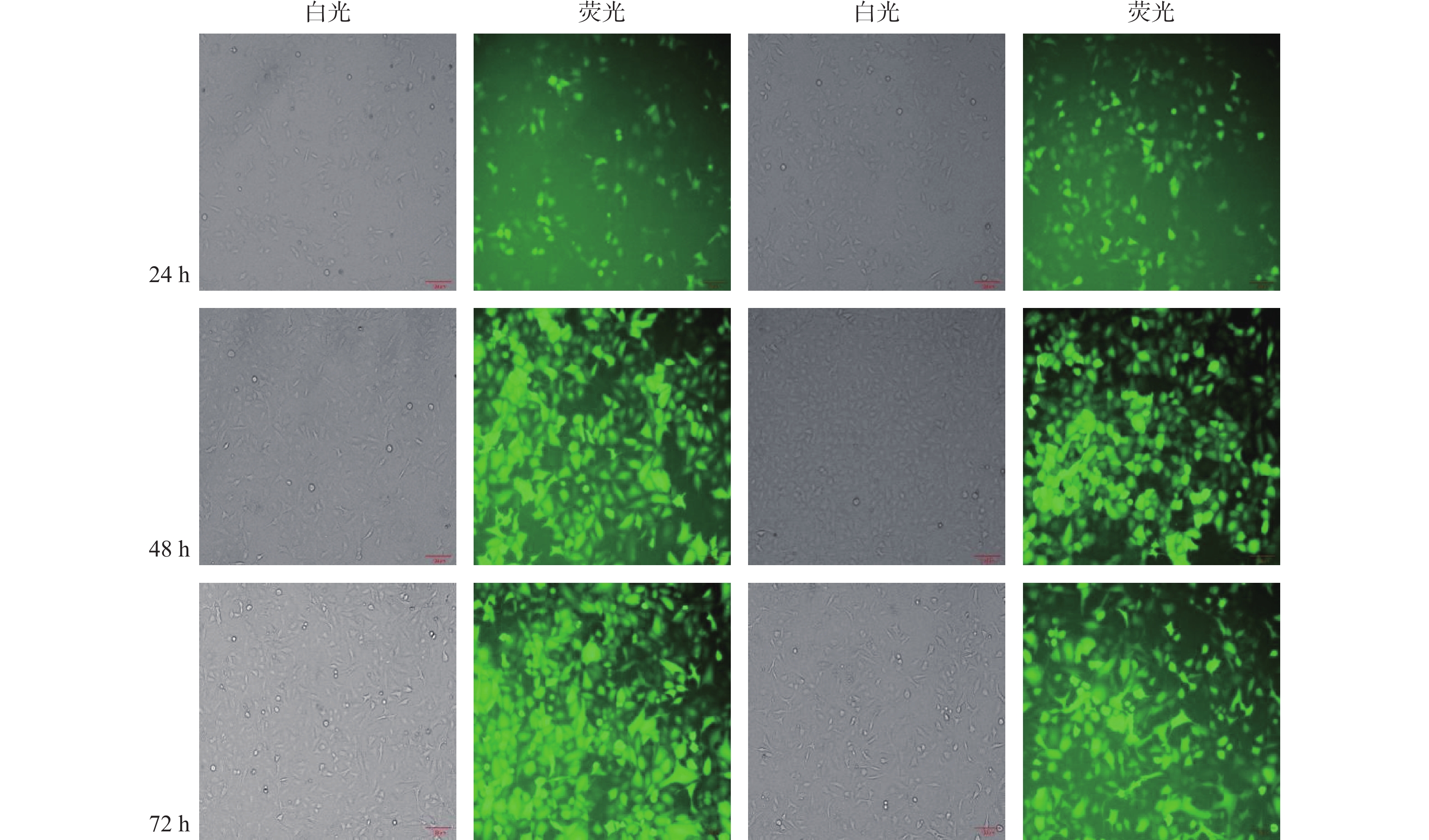
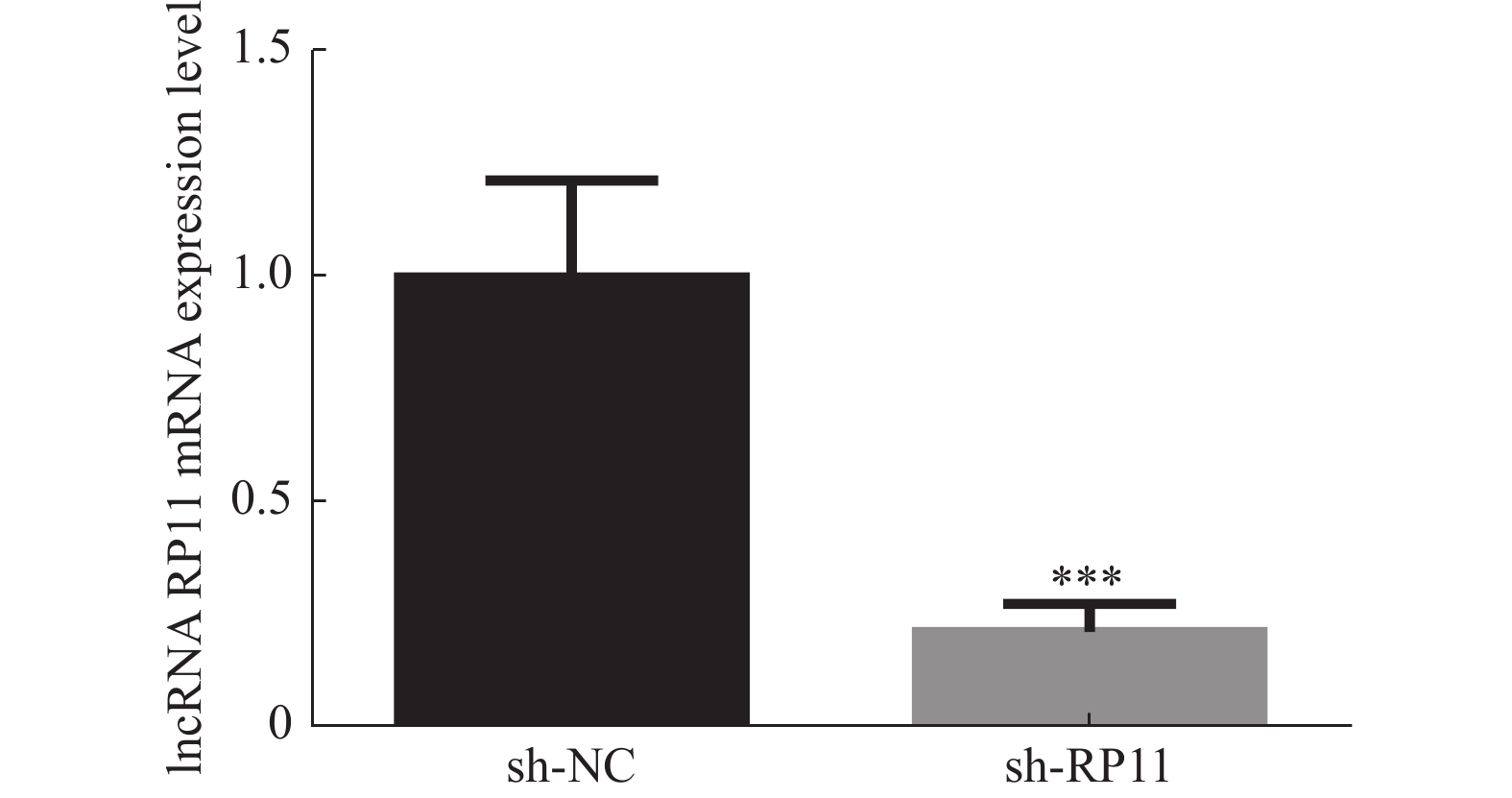
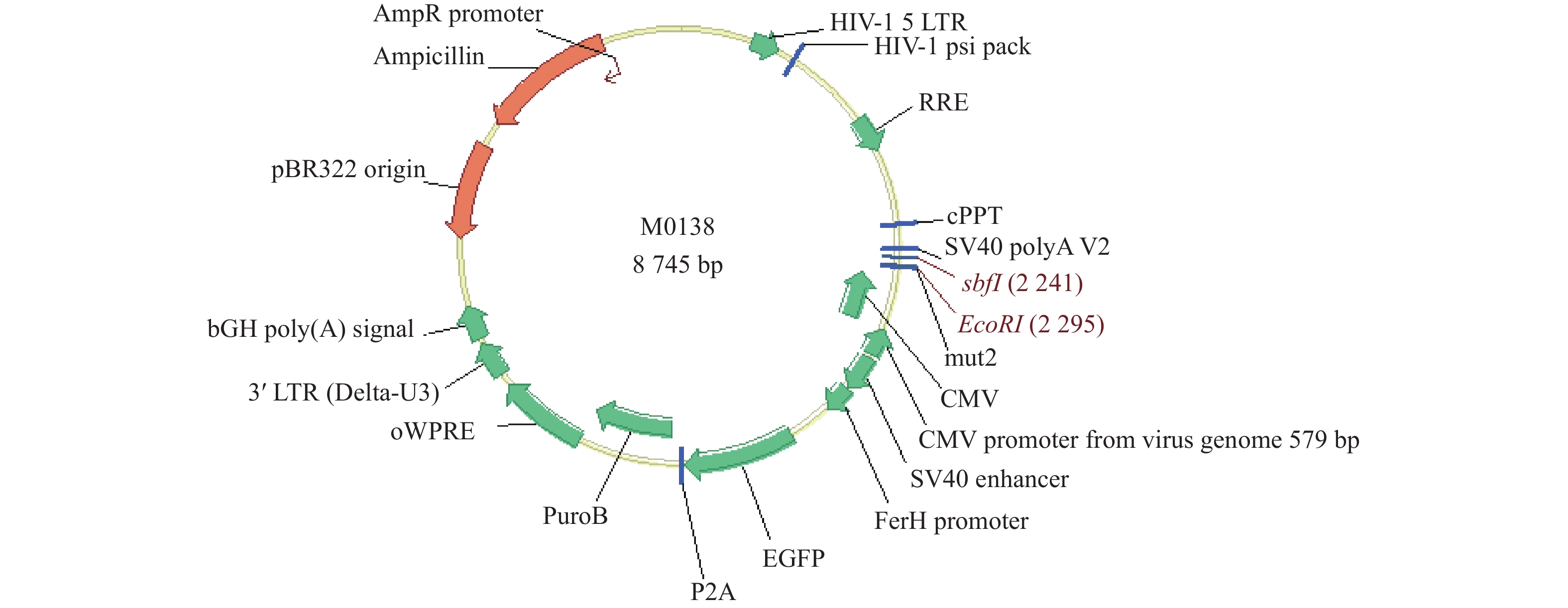
目的 构建过表达及敲减LncRNA RP11-521C20.3的非小细胞肺癌A549稳转细胞株。 方法 根据lncRNA RP11-521C20.3基因序列,设计合成引物并进行扩增。将目的基因与Sbf I和EcoRI酶切的载体连接,构建pcSLenti-pA-RP11-521C20.3-CMV-SFH-EGFP-P2A-Puro-WPRE重组质粒,转染293T细胞,包装含lncRNA RP11-521C20.3质粒重组体的慢病毒。构建shRNA(RP11-521C20.3),连接到AgeI和EcoRI双酶切后的pSLenti-U6-shRNA-CMV-EGFP-F2A-Puro-WPRE载体上,经鉴定后转染293T细胞。再利用慢病毒介导,构建重组质粒pcSLenti-pA-RP11-521C20.3-CMV-SFH-EGFP-P2A-Puro-WPRE和pSLenti-U6-shRNA(RP11-521C20.3)-CMV-EGFP-F2A-Puro-WPRE,导入A549细胞。最后采用RT-qPCR技术在基因水平检测lncRNA RP11-521C20.3的表达。 结果 OV-lncRNA RP11-521C20.3组(过表达组)的lncRNA RP11-521C20.3 mRNA表达量高于NC-lncRNA RP11-521C20.3组(过表达对照组)(P < 0.001),差异倍数为(20.43±0.69)。sh-lncRNA RP11-521C20.3组(敲减组)的lncRNA RP11-521C20.3mRNA表达量低于sh-NC组(敲减对照组)(P < 0.001),差异倍数为(0.21±0.08)。 结论 成功构建了lncRNA RP11-521C20.3过表达及敲减稳转细胞株。为后续研究lncRNA RP11-521C20.3在慢性阻塞性肺疾病(COPD)发病机制中的作用奠定重要基础。 -
关键词:
- lncRNA RP11-521C20.3 /
- 非小细胞肺癌 /
- A549 /
- 慢病毒载体
Abstract:Objective To construct stable transfected cell lines of non-small cell lung cancer A549 with overexpression and knockdown LncRNA RP11-521C20.3. Methods According to lncRNA RP11-521C20.3 gene sequence, primers were designed and amplified. The target gene was then connected to a vector that had been cleaved with Sbf I and EcoRI enzymes to construct the recombinant plasmid pcSLenti-pA-RP11-521C20.3-CMV-SFH-EGFP-P2A-Puro-WPRE. This plasmid was transfected into 293T cells to package the Lentivirus containing the lncRNA RP11-521C20.3 plasmid. An shRNA (RP11-521C20.3) was constructed and connected to the pSLenti-U6-shRNA-CMV-EGFP-F2A-Puro-WPRE vector after being modified with AgeI and EcoRI enzymes. This vector was then transfected into 293T cells after verification. Recombinant plasmids pcSLenti-pA-RP11-521C20.3-CMV-SFH-EGFP-P2A-Puro-WPRE and pSLenti-U6-shRNA (RP11-521C20.3)-CMV-EGFP-F2A-Puro-WPRE were then constructed using the lentivirus-mediated method and introduced into A549 cells. Finally, RT-qPCR technology was used to detect the expression of lncRNA RP11-521C20.3 at the gene level. Results The expression level of lncRNA RP11-521C20.3 mRNA in the OV-lncRNA RP11-521C20.3 group (overexpression group) was higher than that in the NC-lncRNA RP11-521C20.3 group (overexpression control group) (P < 0.001), with a fold change of (20.43±0.69). The expression level of lncRNA RP11-521C20.3 mRNA in the sh-lncRNA RP11-521C20.3 group (knockdown group) was lower than that in the sh-NC group (knockdown control group) (P < 0.001), with a fold change of (0.21±0.08). Conclusion In this study, a stable cell line with overexpression and knockdown of lncRNA RP11-521C20.3 was successfully constructed, which laid an important foundation for the subsequent study of the role of lncRNA RP11-521C20.3 in the pathogenesis of chronic obstructive pulmonary disease (COPD). -
Key words:
- lncRNA RP11-521C20.3 /
- Non-small cell lung cancer /
- A549 /
- Lentiviral vector
-
生殖道沙眼衣原体(chlamydia trachomatis,CT)感染是我国重点监测的性传播疾病,也是全球最常见的性传播疾病,全世界每年新增病例超过1.3亿例[1]。云南省的生殖道沙眼衣原体发病率也呈逐年上升趋势,2012年至2017年生殖道沙眼衣原体感染发病率年均增长率25.35%,且感染者也主要集中在15~44岁年龄组[2-3]。生殖道沙眼衣原体感染可引发眼结膜、咽部、直肠等多器官感染,若治疗不及时,还可导致女性盆腔炎、异位妊娠、不孕不育等并发症[4]。同时,由于感染引发的生殖器部位炎症或溃疡,也使HIV的易感性和传播风险大大增加[5-6]。研究表明[7-10],沙眼衣原体感染可无任何局部或全身症状,导致感染者未能接受到有效治疗,增加了二次感染其他病原体包括HIV的可能性,同时HIV感染也会增加CT持续感染及全身散播的危险,其中HIV阳性女性中感染沙眼衣原体的流行率为9.7%~16.2%。婚检人群包含了一般适婚人群和主要的性活跃人群,对该人群进行性传播疾病的监测有助于评估普通人群的感染状况。为此,本研究对云南省2019年部分州(市)婚检人群开展了生殖道沙眼衣原体感染调查,为制定疾病控制措施和分析防控效果提供依据。
1. 资料与方法
1.1 调查地点
云南省按照其地理位置可分为滇南、滇西、滇东以及滇中地区,本次研究根据地理位置,结合云南省各州市近3 a暗娼人群HIV平均感染率在全省的排序情况将全省各州市分为高中低3个流行水平,分别选取高流行地区(红河州和临沧市)、中流行地区(德宏州和西双版纳州)和低流行地区(保山市和普洱市)共6个州市作为调查现场。
1.2 调查对象
采用固定场所连续抽样方式,招募前往所选取的6个调查州市当地医疗保健机构进行婚前检查,并愿意参加调查并签署知情同意书者作为调查对象。本次调查共纳入1671人,其中红河州343人(20.5%),临沧市315人(18.9%),德宏州301人(18.0%),西双版纳州69人(4.1%),保山市316人(18.9%),普洱市327人(19.6%)。其中,男性841人(50.3%),女性830人(49.7%),年龄为18~57岁,平均(27.74±6.317)岁;以汉族为主,占61.6%(1030/1671),文化程度主要以初中为主,占46.2%(772/1671);以云南省为主,占96.0%(1604/1671),职业主要为农民,占56.9%(951/1671),月平均收入主要在2000~3000元之间,占39.6%(662/1671)。
1.3 调查内容与方法
采用通过专家咨询、KMO和巴特利特检验(KMO = 0.691,P < 0.001)等方法确保效度和信度达到要求的问卷收集调查对象的一般人口学信息、性行为情况、是否感染性病和就医情况等信息。问卷调查结束后,采集调查对象尿液样本,对于已有性行为的女性,同时采集宫颈分泌物标本,用于生殖道沙眼衣原体核酸检测。
1.4 实验室检测
生殖道沙眼衣原体核酸检测使用罗氏核酸检测试剂盒,检测原理为PCR荧光探针法。使用Cobas x480自动核酸提取仪从罗氏配套的尿液和宫颈拭子保存管中直接提取核酸,Cobas z480荧光定量PCR仪扩增核酸并判定结果。采用试剂盒配套的阳性对照、阴性对照和内参标准品对扩增检测进行全面过程质控,所有操作均按照说明书严格执行。
1.5 统计学处理
使用EpiData3.1软件建立数据库,SPSS19.0软件进行数据统计学分析。计数资料以例数或百分比[n(%)]表示。一般人口学特征和性行为特征、性病感染和就医等情况比较采用单因素Logistic回归进行分析,以是否感染CT作为因变量(是 = 1,否 = 0),以相关因素作为自变量。将单因素分析中P < 0.1的变量进行多因素Logistic回归分析,计算各因素校正后的比值比(AOR)及95%的可信区间(95%CI),检验水准为α = 0.05,P < 0.05为差异有统计学意义。
2. 结果
2.1 研究对象中CT感染情况
在调查的1671例研究对象中,共检测出CT核酸阳性111例,其中男性42例,女性通过宫颈检测出29例,尿液54例,指南[11]指出,宫颈和尿液检测中其中任一项为阳性,则判定为阳性,因此女性共检测出69例。总的人群感染率为6.64%(111/1671,95%CI:5.40%~7.80%),男性感染率4.99%(42/841,95%CI:3.50%~6.50%),女性感染率8.31%(69/830,95%CI:6.40%~10.20%)。普洱市和德宏州的感染率较高,分别为8.87%(29/327,95%CI:5.80%~12.00%),8.64%(26/301,95%CI:5.40%~11.80%)。18~20岁的感染率为20.83%(20/96,95%CI:12.60%~29.10%)。文化程度为初中及以下的婚检人群CT的感染率为7.83%(85/1085,95%CI:6.20%~9.40%),见表1。
表 1 婚检人群CT感染影响因素分析Table 1. Analysis of influencing factors of CT infection in premarital examination population变量 合计 感染人数(n) 感染率(%) 单因素分析 多因素分析 P OR(95%CI) P AOR(95%CI) 州市 0.054 0.043* 保山市 316 12 3.80 — 1.000 — 1.000 红河州 343 15 4.37 0.971 0.986(0.468~2.078) 0.556 1.301(0.542~3.119) 西双版纳州 69 4 5.80 0.333 1.685(0.586~4.846) 0.202 2.200(0.655~7.382) 德宏州 301 26 8.64 0.012* 2.317(1.119~4.477) 0.014* 2.649(1.219~5.755) 临沧市 315 25 7.94 0.178 1.612(0.804~3.232) 0.160 1.877(0.780~4.518) 普洱市 327 29 8.87 0.048* 1.954(1.005~3.801) 0.013* 2.708(1.231~5.954) 年龄(岁) < 0.001* < 0.001* ≥41 89 5 5.62 — 1.000 — 1.000 31~40 324 19 5.86 0.930 1.047(0.380~2.886) 0.738 1.192(0.426~3.341) 21~30 1162 67 5.77 0.952 1.029(0.404~2.622) 0.558 1.332(0.510~3.480) 18~20 96 20 20.83 0.005* 4.364(1.562~12.194) 0.007* 4.346(1.468~12.707) 性别 男 841 42 4.99 — 1.000 — 1.000 女 830 69 8.31 0.007* 1.725(1.161~2.564) 0.084 1.446(0.952~2.196) 民族 汉族 1030 61 5.92 — 1.000 其他 641 50 7.80 0.178 1.299(0.888~1.900) 文化程度 高中及以上 586 26 4.44 — 1.000 — 1.000 初中及以下 1085 85 7.83 0.009* 1.831(1.166~2.875) 0.028* 1.854(1.071~3.211) 户籍 本省 1604 106 6.61 — 1.000 非本省 67 5 7.46 0.783 1.140(0.449~2.895) 职业 农民 951 72 7.57 — 1.000 — 1.000 其他 720 39 5.42 0.081 0.699(0.468~1.045) 0.454 1.243(0.703~2.199) 月平均经济收入(元) 0.408 ≥5000 235 12 5.11 — 1.000 3000~5000 461 26 5.64 0.709 1.104(0.657~1.855) 2000~3000 662 51 7.70 0.433 0.791(0.440~1.422) ≤2000 313 22 7.01 0.358 0.712(0.345~1.469) *P < 0.05。 2.2 CT感染的相关因素分析
使用Logistic回归分析感染CT的相关因素,单因素分析后将P < 0.1的因素纳入多因素分析中。多因素分析结果显示,相比保山市,德宏州(AOR = 2.649,95%CI:1.219~5.755)和普洱市(AOR = 2.708,95%CI:1.231~5.954)CT的感染率相对较高;年龄18~20岁(AOR = 4.346,95%CI:1.468~12.707)和文化程度为初中及以下的(AOR = 1.854,95%CI:1.071~3.211)更可能感染CT,见表1。
3. 讨论
本研究对云南省婚检人群中CT感染情况进行了调查,婚检人群在一定程度上可以代表一般人群,从而对评估一个地区的性传播疾病的水平具有参考价值。根据以往的调查,云南省暗娼人群中CT的感染率为14.43%[12],而男男性行为人群(men who have sex with men,MSM)中CT感染率为18.2%[13]。本调查显示,云南省婚检人群中CT的感染率为6.64%,低于重点人群暗娼和MSM中CT的感染率,但与青年学生中CT的感染率8.52%(68/798)差异无统计学意义(χ2 = 2.150,P = 0.084)[14]。女性CT的感染率高于男性,这与以往病例报告的情况相一致[3]。该结果可能是与本次研究中女性阳性感染者首次性行为平均年龄(20.23±3.077)岁,低于男性阳性患者的首次性行为平均年龄(21.44±3.095)岁有关,由于女性首次发生性行为的年龄较小,可能会导致在发生性行为时缺少相应的保护措施,从而进一步增加了女性感染CT的可能性,与本文的研究结果也呈现出一致性。
调查的结果显示,不同州(市)的CT感染率存在显著的差异。与保山市相比,德宏州和普洱市婚检人群CT感染率相对较高,虽然没有统计学差异,但临沧市的CT感染率也达到了7.94%,反应了这些地区具有较高的性病传播风险。对云南省2010年至2017年淋病的时空聚集分析也显示,云南省的西南地区是一个一类聚集地区,包括了普洱市、德宏州和临沧市的大部分县(市、区)[15],这在一定程度上也反应了这些地区性病传播处于较高的水平。但根据2012~2017年生殖道沙眼衣原体病例报告的情况来看,报告发病率前3位的是昆明市、西双版纳州和德宏州,而普洱市和临沧市的报告发病率只有1.44%和0.83%[3],这可能与不同州(市)医疗机构衣原体检测能力和主动筛查意识及力度存在差异有关,提示在全省还需要进一步加强性病的检测能力,加大筛查力度和动员检测,尽可能地发现传染源,并进行规范化的治疗。
本次研究调查中,相比于文化程度为高中及以上的人群而言,文化程度为初中及以下的人群属于婚检人群CT感染的危险因素。研究结果显示[16-17],由于该人群文化程度较低,对于性病的传播和防治知识了解较少,缺少自我保护意识,更加易发生高危行为。因此需要针对该人群的特点,加强性病防治知识的健康教育,提高自我保护意识,减少高危性行为的发生,从而减少CT等相关性病的感染和传播。
2015~2019年我国生殖道沙眼衣原体发病率年均增长10.45%[18],同时部分重点监测的性病低年龄段发病率也在呈逐年上升的趋势[14]。本次调查结果显示,相比于年龄在41岁及其以上人群而言,18~20岁人群是婚检人群CT感染的危险因素,这与珠海市的研究[19]结果相符,同时也与美国、日本等国家的感染情况相一致[20]。本次研究中年龄≤20岁人群,由于缺乏相关的性病防治知识,导致发生首次性行为年龄均在20岁之前(96/96),且近87.5%(84/96)的青少年不能坚持使用安全套,由此可以看出,大多青少年发生首次性行为的年龄较小,且不能坚持使用安全套,这与岑平等[21]的研究结果相一致,这可能与20岁以下人群属于性活跃人群有关。因此应加强对青少年的相关性病防治知识的健康教育,扩大生殖道沙眼衣原体检测的范围。由于CT感染是导致女性生殖感染的常见病原体以及导致女性不孕不育的主要因素之一[22],还会导致男性出现尿道炎、附睾炎、前列腺炎、性功能减退及不育[11],从而影响到生育。目前早发现、早诊断、早治疗是性病防控的最有效措施[12],应加强对婚检人群,尤其是性活跃人群的性病感染情况进行监测,联合各级相关部门,采取多种手段为该人群提供疾病检测,尽可能减少CT的感染与传播,进一步促进优生优育。
由于本次调查是根据云南省暗娼人群HIV平均感染率,在高、中、低流行区中各任意选取了2个州市的部分县区进行样本的采集,虽然同属于性传播疾病,但两者有各自的传播特点,对样本的代表性可能有潜在的影响,但本研究首次在云南省对婚检人群进行CT感染率的调查,为进一步深入研究CT的流行,制定防治措施提供了基础数据。
-
表 1 LncRNA RP11-521C20.3 PCR扩增引物序列步骤
Table 1. LncRNA RP11-521C20.3 PCR amplification primer sequence
步骤 引物与试剂 扩增引物Forward 5′-CCCTGCTTTGGGGTTGTGA-3′ Reverse 5′-GGAGGCTTTGTGGCTTGCT-3′ PCR扩增体系 5×PrimeSTAR®Buffer 10 µL,dNTP Mixtur 4 µL,正、反向引物各1 µL,模板
DNA < 200 ng,PrimeSTAR® HS DNA Polymerase 0.5 µL,灭菌蒸馏水加至50 µL反应条件 98℃ 10sec,55℃ 5sec,72℃ 1 min/kb,30个循环 扩增产物凝胶电泳 1.5%琼脂糖凝胶电泳 胶回收 TaKaRaMiniBEST Agarose Gel DNA Extraction Kit Ver.3.0 表 2 过表达重组载体构建酶切反应体系
Table 2. Enzyme digestion reaction system for construction of overexpressed recombinant vectors
试剂 体积 质粒 2 μg 10 x反应Buffer 5 µL SbfI 1 µL EcoRI 1 µL ddH2O Up to 50 µL 表 3 过表达重组质粒反应体系
Table 3. Overexpressed recombinant plasmid reaction system
试剂 体积 5x反应Buffer 4 µL 插入片段 2 µL 线性化体 1 µL 无缝克隆酶 2 µL CCH2O Up to 20 µL 表 4 过表达重组质粒鉴定体系
Table 4. Identification system of overexpressed recombinant plasmid
试剂 体积 Premix Tag 25 µL 模板 1 µL 引物1(20 µM) 1 µL 引物2(20 µM) 1 µL 超纯水 Up to 50 µL 表 5 慢病毒包装试剂
Table 5. Lentivirus packaging reagent
试剂 体积 载体质粒 20 μg pHelper 1. 0载体质粒 15 μg pHelper2. 0载体质粒 10 μg 转染试剂 Up to 1mL 表 6 shRNA序列
Table 6. shRNA sequence
名称 序列(5′-3′) Y16185-F CcggCCAGATCTGTTGACCAACTTTCAAGAGAAGTTGGTCAACAGATCTGGTTTTTTg Y16185-R aattcaaaaaaCCAGATCTGTTGACCAACTTCTCTTGAAAGTTGGTCAACAGATCTGG GL427NC2-F CcggCCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGGTTTTTTg GL427NC2-R aattcaaaaaaCCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG 表 7 敲减载体构建酶切反应体系
Table 7. Enzyme digestion reaction system for knockdown vector construction
试剂 体积 质粒 2 μg 10x反应Buffer 5 μL AgeI 1 μL EcoRI 1 μL ddH2O Up to 50 μL 表 8 敲减重组体PCR鉴定体系
Table 8. Knock-down recombinant PCR identification system
试剂 体积 Premix Tag 2 μg 模板 1 μL 引物1(20 μM) 1 μL 引物2(20 μM) 1 μL ddH2O Up to 50 μL -
[1] Perret J,Yip S W S,Idrose N S,et al. Undiagnosed and 'overdiagnosed' COPD using postbronchodilator spirometry in primary healthcare settings: A systematic review and meta-analysis[J]. BMJ Open Respir Res,2023,10(1):e001478. doi: 10.1136/bmjresp-2022-001478 [2] Wilusz J E. Long noncoding RNAs: Re-writing dogmas of RNA processing and stability[J]. Biochimbiophys acta,2016,1859(1):128-138. [3] Ard R,Allshire R C,Marquardt S. Emerging properties and functional consequences of noncoding transcription[J]. Genetics,2017,207(2):357-367. [4] Omote N,Sauler M. Non-coding RNAs as regulators of cellular senescence in idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease[J]. Front Med (Lausanne),2020,7:603047. doi: 10.3389/fmed.2020.603047 [5] Soares do Amaral N,Cruz E Melo N,de Melo Maia B,et al. Noncoding RNA profiles in tobacco- and alcohol-associated diseases[J]. Genes (Basel),2016,8(1):6. doi: 10.3390/genes8010006 [6] Bamodu O A,Wu S M,Feng P H,et al. lnc-IL7R expression reflects physiological pulmonary function and its aberration is a putative indicator of COPD[J]. Biomedicines,2022,10(4):786. doi: 10.3390/biomedicines10040786 [7] Li C,Liu H,Zhang J,et al. LncRNA BMF-AS1 exerts anti-apoptosis function in COPD by regulating BMF expression[J]. Pakistan Journal of Zoology,2020,52(3):893-900. [8] 李玉珍. BMF促细胞凋亡研究进展[J]. 生物化学与生物物理进展,2017,44(9):751-756. [9] Guan R,Yao H,Li Z,et al. Sodium tanshinone IIA sulfonate attenuates cigarette smoke extract-induced mitochondrial dysfunction,oxidative stress,and apoptosis in alveolar epithelial cells by enhancing SIRT1 pathway[J]. Toxicol Sci,2021,183(2):352-362. doi: 10.1093/toxsci/kfab087 [10] Roscioli E,Hamon R,Lester S E,et al. Airway epithelial cells exposed to wildfire smoke extract exhibit dysregulated autophagy and barrier dysfunction consistent with COPD[J]. Respir Res,2018,19(1):234. doi: 10.1186/s12931-018-0945-2 [11] Lee K Y,Park S Y,Park S,et al. Progranulin protects lung epithelial cells from cigarette smoking-induced apoptosis[J]. Respirology,2017,22(6):1140-1148. doi: 10.1111/resp.13023 [12] Hodge S,Hodge G,Holmes M,et al. Increased airway epithelial and T-cell apoptosis in COPD remains despite smoking cessation[J]. Eurrespir J,2005,25(3):447-454. [13] Patel B D,Coxson H O,Pillai S G,et al. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease[J]. Am J Resp Crit Care,2008,178(5):500-505. doi: 10.1164/rccm.200801-059OC [14] Devadoss D,Long C,Langley R J,et al. Long noncoding transcriptome in chronic obstructive pulmonary disease[J]. Am J Resp Cell Mol,2019,61(6):678-688. doi: 10.1165/rcmb.2019-0184TR [15] Qiao X,Hou G,He Y L,et al. The novel regulatory role of the lncRNA-miRNA-mRNA axis in chronic inflammatory airway diseases[J]. Front Mol Biosci,2022,9:927549. doi: 10.3389/fmolb.2022.927549 [16] Tang W,Shen Z,Guo J,et al. Screening of long non-coding RNA and TUG1 inhibits proliferation with TGF-β induction in patients with COPD[J]. Int J Chron Obstruct Pulmon Dis,2016,11:2951-2964. doi: 10.2147/COPD.S109570 [17] Bi H,Zhou J,Wu D,et al. Microarray analysis of long non-coding RNAs in COPD lung tissue[J]. Inflamm Res,2015,64(2):119-126. doi: 10.1007/s00011-014-0790-9 [18] Ming X,Duan W,Yi W. Long non-coding RNA NEAT1 predicts elevated chronic obstructive pulmonary disease (COPD) susceptibility and acute exacerbation risk,and correlates with higher disease severity,inflammation,and lower miR-193a in COPD patients[J]. Int J Clin Exp Pathol,2019,12(8):2837-2848. [19] Gu C,Li Y,Liu J,et al. LncRNA-mediated SIRT1/FoxO3a and SIRT1/p53 signaling pathways regulate type II alveolar epithelial cell senescence in patients with chronic obstructive pulmonary disease[J]. Mol Med Rep,2017,15(5):3129-3134. doi: 10.3892/mmr.2017.6367 [20] Yang L,Wu D,Chen J,et al. Corrigendum to: A functional CNVR_3425.1 damping lincRNA FENDRR increases lifetime risk of lung cancer and COPD in Chinese[J]. Carcinogenesis,2021,42(12):1506-1507. doi: 10.1093/carcin/bgab105 [21] Liu P,Gao H,Wang Y,et al. LncRNA H19 contributes to smoke-related chronic obstructive pulmonary disease by targeting miR-181/PDCD4 Axis[J]. COPD,2023,20(1):119-125. doi: 10.1080/15412555.2023.2165906 [22] Liu P, Zhang H, Zeng H, et al. LncRNA CASC2 is involved in the development of chronic obstructive pulmonary disease via targeting miR-18a-5p/IGF1 axis[J]. Ther Adv Respir Dis, 2021, 15: 17534666211028072. [23] 孟凡荣,陈琛,万海粟,等. 慢病毒载体及其研究进展[J]. 中国肺癌杂志,2014,17(12):870-876. [24] Fischer S,Cassivi S D,Xavier A M,et al. Cell death in human lung transplantation: Apoptosis induction in human lungs during ischemia and after transplantation[J]. Ann Surg,2000,231(3):424-431. doi: 10.1097/00000658-200003000-00016 [25] Morris D G,Huang X,Kaminski N,et al. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema[J]. Nature,2003,422(6928):169-173. doi: 10.1038/nature01413 期刊类型引用(1)
1. 邓宝清,叶云凤,宁宁,晏瑞琳,温桂春,黄李成,邓勇峥,袁青,蔡于茂,陈祥生. 不孕不育人群生殖道沙眼衣原体感染影响因素分析. 皮肤性病诊疗学杂志. 2024(02): 82-87 .  百度学术
百度学术其他类型引用(0)
-






 下载:
下载:
 下载:
下载: