Selection of Intensive Adjuvant Therapy for Triple-positive Breast Cancer
-
摘要: 三阳性乳腺癌(triple-positive breast cancer,TPBC)是以激素受体(hormonereceptor,HR)阳性(HR+)和人类表皮生长因子受体(humanepidermalgrowthfactorreceptor 2,HER2)阳性(HER2+)共表达的一类特殊类型的乳腺癌。因其同时受HR信号通路和HER2信号通路共同调控影响,TPBC的治疗策略选择一直是临床关注的热点。TPBC患者强化辅助治疗选择不一,序贯、联合,内分泌治疗和靶向治疗如何精准的谋局落子,尚缺少系统性理论的支持。综述查阅了PUBMED、美国临床试验数据库(ClinicalTrials.gov)、知网等,梳理出近年来TPBC治疗方面的11项随机对照试验结果,为TPBC的辅助强化治疗策略的选择提供临床参考依据。Abstract: Triple-positive breast cancer (TPBC) is a special type of breast cancer characterized by the co-expression of hormone receptors (HR+) and human epidermal growth factor receptor 2 (HER2+). Due to the regulation of the HR signaling pathway and the HER2 signaling pathway, the treatment strategy for TPBC has been a hot topic of clinical attention. There is currently a lack of systematic theoretical support for the selection of intensive adjuvant therapy for TPBC patients, including sequential or combination therapy, endocrine therapy, and targeted therapy. This review article examines 11 randomized controlled trials on TPBC treatment in recent years, obtained from database such as PUBMED, ClinicalTrials.gov, and CNKI, in order to provide clinical reference for the selection of neoadjuvant therapy strategies for TPBC.
-
表 1 TPBC临床研究
Table 1. Clinical Research about TPBC
研究信息 入组人群 入组人数(n) 研究设计 结局 Katherine研究[22]
Ⅲ期试验在曲妥珠单抗进行新辅助化疗后未达到pCR的HER2+患者 1486(其中TPBC有
1074)T-DM1组
曲妥珠单抗组3a DFS率为88.3%VS77.0%,*P < 0.001 ExteNET研究[26]
多中心、随机、双盲
Ⅲ期试验Ⅱ~Ⅲ期、淋巴结阳性、新辅助治疗后未达pCR的HER2+患者 2840(其中TPBC有
1631)曲妥珠单抗辅助治疗后继续使用1 a奈拉替尼
曲妥珠单抗辅助治疗后继续使用1年安慰剂
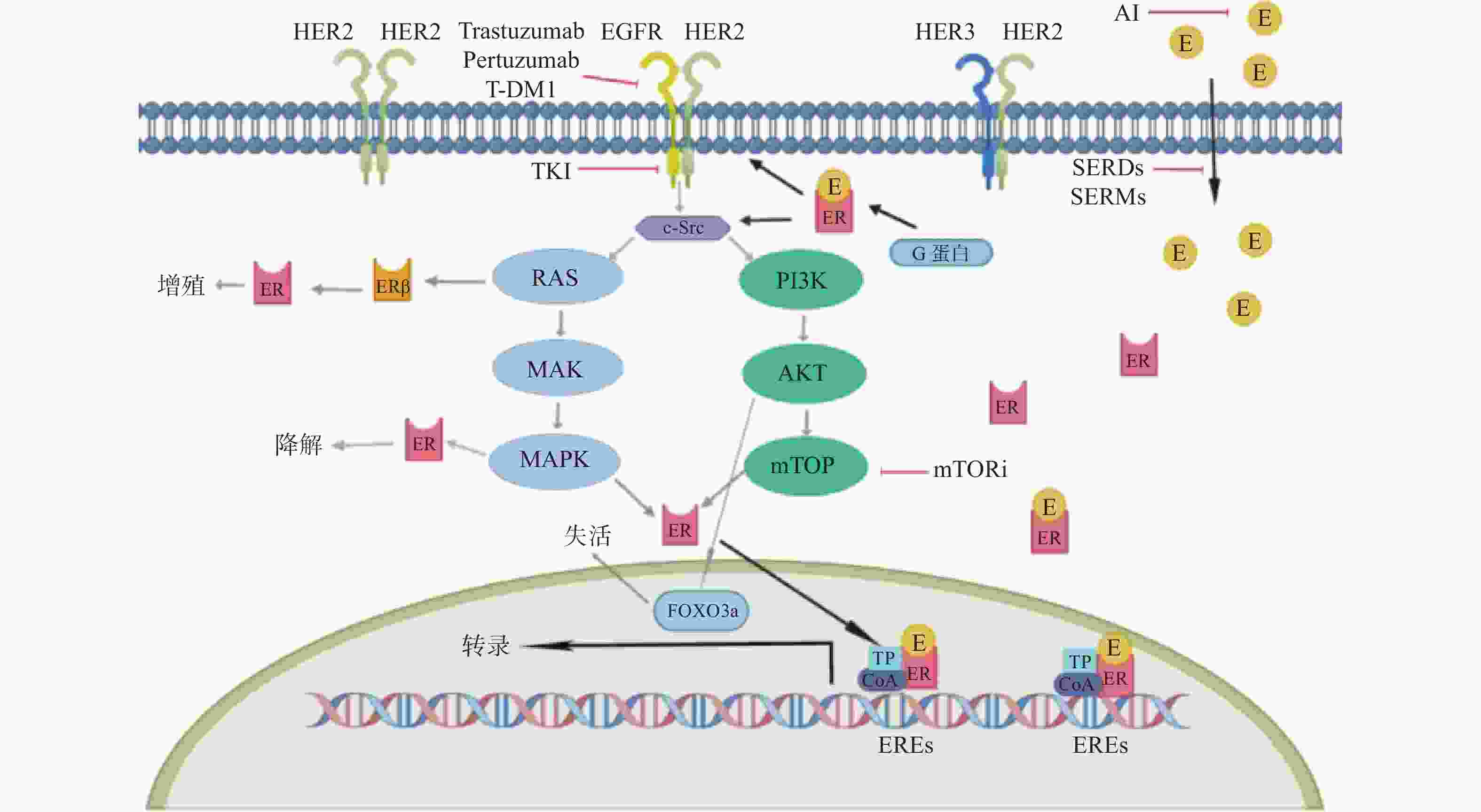
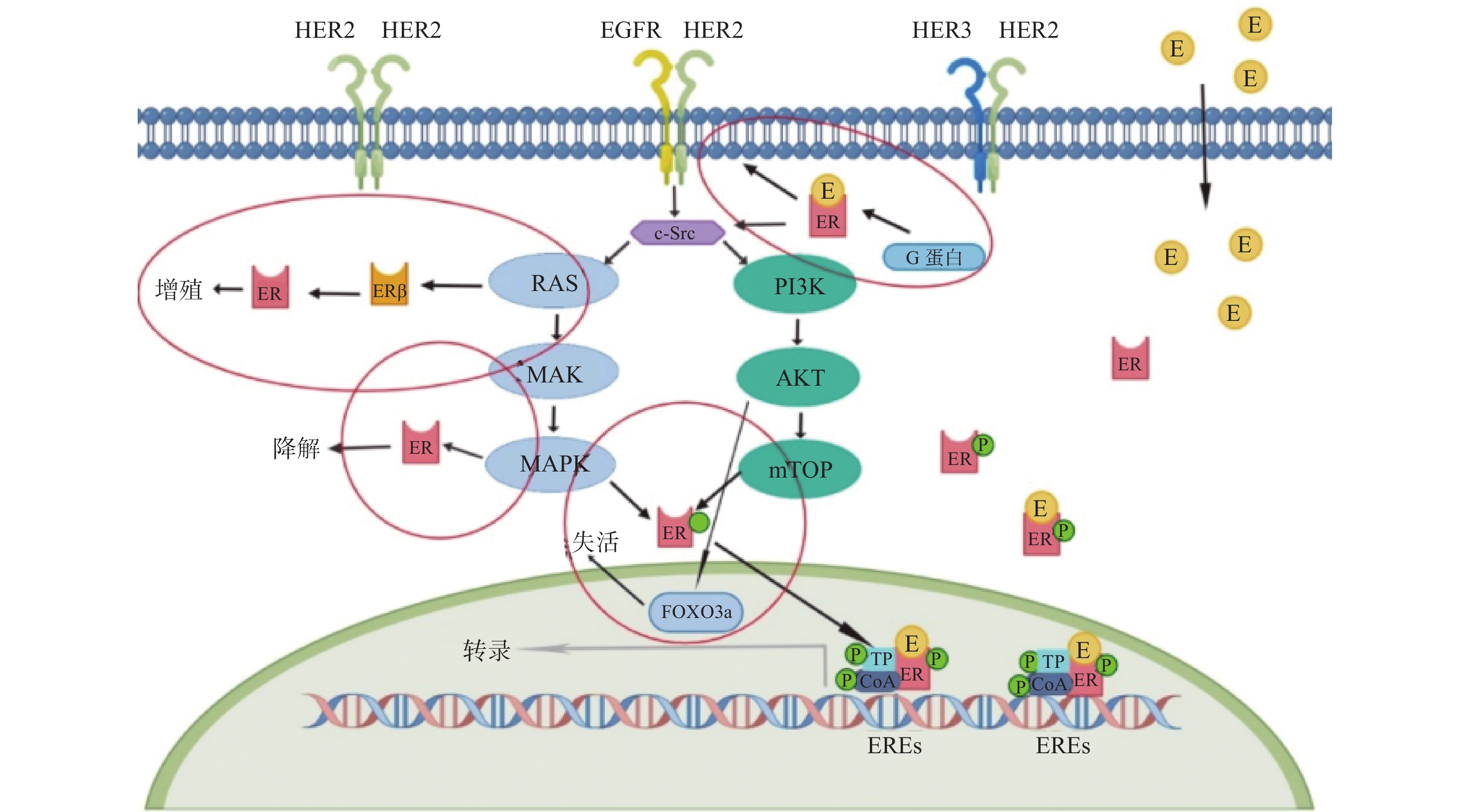
2 a PFS率为93.9%VS 91.6%,*P = 0.0091
5a PFS率为90.2% VS 87.7%,*P = 0.0083
HR阳性亚组的侵袭性无病生存的HR为0.60(95%CI 0.43~0.83)SYSUCC-002研究[29]
NCT01950182
开放、非劣性、随机
Ⅲ期试验
一线转移性TPBC患者 392 曲妥珠单抗+内分泌治疗组(ET组)
曲妥珠单抗+化疗组(CT组)PFS:ET组为19.2个月,CT组为14.8个月,
*P < 0.0001PERTAIN研究[30]
NCT01491737
随机、双臂、开放、多中心Ⅱ期研究转移性TPBC患者 258 曲妥珠单抗+帕妥珠单抗联合AI
曲妥珠单抗联合AI
PFS:双靶组18.89个月VS单靶组15.80个月,*P =
0.0070
ALTERNATIVE研究[31]
大规模随机对照研究以前接受内分泌治疗、曲妥珠单抗治疗和化疗的转移性TPBC患者 355 曲妥珠单抗+拉帕替尼联合AI(L+T组)
拉帕替尼联合AI(L组)
曲妥珠单抗联合AI(T组)PFS:
L+T组11.0个月 VS L组8.3个月,*P= 0.0063
L组8.3个月VS T组5.7个月, P= 0.3159monarcHER研究[36]
NCT02675231
Ⅱ期试验既往晚期阶段接受过至少二线抗HER2靶向治疗的TPBC局部晚期不可手术或转移性患者 237 阿贝西利+曲妥珠单抗+氟维司群(A组)
阿贝西利+曲妥珠单抗(B组)
曲妥珠单抗+化疗(C组)PFS: A组8.3个月VS C组5.7个月,P= 0.051;B组5.7个月VS C组5.7个月, P= 0.77
OS:A组31.1个月VS C组20.7个月,P= 0.086;B组29.2个月 VS C组20.7个月,P= 0.365TAnDEM研究[40]
NCT00022672
Ⅲ期
曾用他莫昔芬治疗的转移性TPBC 207 阿那曲唑+曲妥珠单抗
阿那曲唑PFS:4.8个月VS 2.4个月,*P= 0.0016 eLEcTRA研究[39]
NCT00171847
Ⅲ期转移性TPBC 57 来曲唑
来曲唑+曲妥珠单抗TTP:3.3个月VS 14.1个月,P= 0.23 SOLTI-1303 PATRICIA
研究[32]
Ⅱ期、开放标签、多中心
研究已使用2-4个抗HER2方案(至少包括曲妥珠单抗)治疗后的HER2+局部晚期或转移性乳腺癌患者 71(其中TPBC有37) 曲妥珠单抗+哌柏西利
曲妥珠单抗+哌柏西利+来曲唑PFS6率:
42.8%(12/28)VS 46.4%(13/28),*P= 0.003NCT02657343[23]
Ib/Ⅱ期临床试验已接受过曲妥珠单抗、帕妥珠单抗和T-DM1作为(新)辅助或转移治疗的HER2+患者 Ⅰ期12(其中TPBC有8) 瑞博西尼+T-DM1
瑞博西尼+曲妥珠单抗
瑞博西尼+曲妥珠单抗+氟维司群仅有Ⅰ期结果:仅1例
( 8.3 % )患者病情稳定 > 24周;mPFS = 1.33个月LORDSHIPS研究[33]
NCT03772353
Ⅱ期临床试验
复发或转移的HR+/HER2+晚期乳腺癌 Ⅰ期15 CDK4/6抑制剂达尔西利 + 酪氨酸激酶抑制剂吡咯替尼 + 芳香酶抑制剂来曲唑三药联合方案 总体ORR 66.7 %
经过HER2靶向治疗一线(1L)和二线(2L)ORR:85.7 % VS 50.0 %
总体mPFS 11.3个月,1 L组PFS尚未达到,2 L组PFS为10.9个月*P < 0.05。 -
[1] Siegel R L,Miller K D,Fuchs H E,et al. Cancer statistics,2022[J]. CA:A Cancer Journal for Clinicians,2022,72(1):7-33. doi: 10.3322/caac.21708 [2] Osborne C K,Schiff R. Mechanisms of endocrine resistance in breast cancer[J]. Annual Review of Medicine,2011,62(1):233-247. doi: 10.1146/annurev-med-070909-182917 [3] Konecny G,Pauletti G,Pegram M,et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone eceptor-positive primary breast cancer[J]. JNCI Journal of the National Cancer Institute,2003,95(2):142-153. doi: 10.1093/jnci/95.2.142 [4] Brandão M,Caparica R,Malorni L,et al. What is the real impact of estrogen receptor status on the prognosis and treatment of HER2-positive early breast cancer?[J]. Clinical Cancer Research,2020,26(12):2783-2788. doi: 10.1158/1078-0432.CCR-19-2612 [5] Zhao S,Liu X Y,Jin X,et al. Molecular portraits and trastuzumab responsiveness of estrogen receptor-positive,progesterone receptor-positive,and HER2-positive breast cancer[J]. Theranostics,2019,9(17):4935-4945. doi: 10.7150/thno.35730 [6] Refae S,Pistilli B,Delaloge S. Extended anti-HER2 therapy in early breast cancer: Longer beats shorter?[J]. Current Opinion in Oncology,2016,28(6):469-475. doi: 10.1097/CCO.0000000000000325 [7] Gianni L,Eiermann W,Semiglazov V,et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): Follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort[J]. The Lancet Oncology,2014,15(6):640-647. doi: 10.1016/S1470-2045(14)70080-4 [8] Baselga J,Bradbury I,Eidtmann H,et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised,open-label,multicentre,phase 3 trial[J]. The Lancet,2012,379(9816):633-640. doi: 10.1016/S0140-6736(11)61847-3 [9] Schneeweiss A,Chia S,Hickish T,et al. Long-term efficacy analysis of the randomised,phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer[J]. European Journal of Cancer,2018,89:27-35. doi: 10.1016/j.ejca.2017.10.021 [10] Dhillon S. Neratinib in early-stage breast cancer: A profile of its use in the EU[J]. Clinical Drug Investigation,2019,39(2):221-229. doi: 10.1007/s40261-018-0741-2 [11] Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials[J]. Lancet,2005,365(9472):1687-1717. doi: 10.1016/S0140-6736(05)66544-0 [12] 中国临床肿瘤学会指南工作委员会. 中国临床肿瘤学会(CSCO)乳腺癌诊疗指南2022[M]. 北京: 人民卫生出版社, 2022: 29, 48-49, 65, 86. [13] Piccart M,Procter M,Fumagalli D,et al. Adjuvant pertuzumab and trastuzumab in early HER2-Positive breast cancer in the APHINITY trial: 6 years’ follow-up[J]. Journal of Clinical Oncology,2021,39(13):1448-1457. doi: 10.1200/JCO.20.01204 [14] Giuliano M,Trivedi M V,Schiff R. Bidirectional crosstalk between the estrogen receptor and human epidermal growth factor receptor 2 signaling pathways in breast cancer: molecular basis and clinical implications[J]. Breast Care,2013,8(4):256-262. doi: 10.1159/000354253 [15] Cotrim C Z,Fabris V,Doria M L,et al. Estrogen receptor beta growth-inhibitory effects are repressed through activation of MAPK and PI3K signalling in mammary epithelial and breast cancer cells[J]. Oncogene,2013,32(19):2390-2402. doi: 10.1038/onc.2012.261 [16] Wang Y C,Morrison G,Gillihan R,et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers - role of estrogen receptor and HER2 reactivation[J]. Breast Cancer Research,2011,13(6):R121. doi: 10.1186/bcr3067 [17] Giuliano M,Hu H,Wang Y C,et al. Upregulation of ER signaling as an adaptive mechanism of cell survival in HER2-positive breast tumors treated with anti-HER2 therapy[J]. Clinical Cancer Research,2015,21(17):3995-4003. doi: 10.1158/1078-0432.CCR-14-2728 [18] Schettini F,Buono G,Cardalesi C,et al. Hormone receptor/human epidermal growth factor receptor 2-positive breast cancer: Where we are now and where we are going[J]. Cancer Treatment Reviews,2016,46:20-26. doi: 10.1016/j.ctrv.2016.03.012 [19] Fox E M,Andrade J,Shupnik M A. Novel actions of estrogen to promote proliferation: Integration of cytoplasmic and nuclear pathways[J]. Steroids,2009,74(7):622-627. doi: 10.1016/j.steroids.2008.10.014 [20] Kato S. Estrogen receptor-mediated cross-talk with growth factor signaling pathways[J]. Breast Cancer,2001,8(1):3-9. doi: 10.1007/BF02967472 [21] Yarden R I,Wilson M A,Chrysogelos S A. Estrogen suppression of EGFR expression in breast cancer cells: A possible mechanism to modulate growth[J]. Journal of Cellular Biochemistry,2001,81(S36):232-246. doi: 10.1002/jcb.1142 [22] von Minckwitz G,Huang C S,Mano M S,et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer[J]. New England Journal of Medicine,2019,380(7):617-628. doi: 10.1056/NEJMoa1814017 [23] Goel S,Pernas S,Tan-Wasielewski Z,et al. Ribociclib plus trastuzumab in advanced HER2-positive breast cancer: results of a phase 1b/2 trial[J]. Clinical Breast Cancer,2019,19(6):399-404. doi: 10.1016/j.clbc.2019.05.010 [24] Martin M,Holmes F A,Ejlertsen B,et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised,double-blind,placebo-controlled,phase 3 trial[J]. The Lancet Oncology,2017,18(12):1688-1700. doi: 10.1016/S1470-2045(17)30717-9 [25] Chan A,Delaloge S,Holmes F A,et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): A multicentre,randomised,double-blind,placebo-controlled,phase 3 trial[J]. The Lancet Oncology,2016,17(3):367-377. doi: 10.1016/S1470-2045(15)00551-3 [26] Cognetti F,Di Cosimo S,Bruzzi P,et al. 139P HER2+/HR+ breast cancer patients at high risk of relapse derive benefit from extended adjuvant treatment with neratinib: An exploratory analysis from ExteNET study[J]. Annals of Oncology,2021,32:S420. [27] Yu K,Cai Y,Wu S,et al. Estrogen receptor‐low breast cancer: Biology chaos and treatment paradox[J]. Cancer Communications,2021,41(10):968-980. doi: 10.1002/cac2.12191 [28] Johnston S R D,Harbeck N,Hegg R,et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+,HER2−,node-positive,high-risk,early breast cancer (monarchE)[J]. Journal of Clinical Oncology,2020,38(34):3987-3998. doi: 10.1200/JCO.20.02514 [29] Hua X,Bi X W,Zhao J-L,et al. Trastuzumab plus endocrine therapy or chemotherapy as first-line treatment for patients with hormone receptor–positive and HER2-positive metastatic Breast Cancer (SYSUCC-002)[J]. Clinical Cancer Research,2022,28(4):637-645. doi: 10.1158/1078-0432.CCR-21-3435 [30] Rimawi M,Ferrero J-M,de la Haba-Rodriguez J,et al. First-line trastuzumab plus an aromatase inhibitor,with or without pertuzumab,in human epidermal growth factor receptor 2–positive and hormone receptor–positive metastatic or locally advanced breast cancer (PERTAIN): A randomized,open-label phase II trial[J]. Journal of Clinical Oncology,2018,36(28):2826-2835. doi: 10.1200/JCO.2017.76.7863 [31] Johnston S R D,Hegg R,Im S-A,et al. Phase III,randomized study of dual human epidermal growth factor receptor 2 (HER2) blockade with lapatinib plus trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2-Positive,hormone receptor–positive metastatic breast cancer: updated results of ALTERNATIVE[J]. Journal of Clinical Oncology,2021,39(1):79-89. doi: 10.1200/JCO.20.01894 [32] Ciruelos E,Villagrasa P,Pascual T,et al. Palbociclib and trastuzumab in HER2-positive advanced breast cancer: Results from the phase II SOLTI-1303 PATRICIA trial[J]. Clinical Cancer Research,2020,26(22):5820-5829. doi: 10.1158/1078-0432.CCR-20-0844 [33] Zhang J,Meng Y,Wang B,et al. Dalpiciclib combined with pyrotinib and letrozole in women with HER2-positive,hormone receptor-positive metastatic breast cancer (LORDSHIPS): A phase Ib study[J]. Frontiers in Oncology,2022,12:775081. doi: 10.3389/fonc.2022.775081 [34] Spring L M,Clark S L,Li T,et al. Phase 1b clinical trial of ado-trastuzumab emtansine and ribociclib for HER2-positive metastatic breast cancer[J]. npj Breast Cancer,2021,7(1):103. doi: 10.1038/s41523-021-00311-y [35] Haley B,Batra K,Sahoo S,et al. A phase I/Ib trial of PD 0332991 (palbociclib) and T-DM1 in HER2-positive advanced breast cancer after trastuzumab and taxane therapy[J]. Clinical Breast Cancer,2021,21(5):417-424. doi: 10.1016/j.clbc.2021.03.005 [36] André F,Nadal J C,Denys H,et al. LBA18 Final overall survival (OS) for abemaciclib plus trastuzumab +/- fulvestrant versus trastuzumab plus chemotherapy in patients with HR+,HER2+ advanced breast cancer (monarcHER): A randomized,open-label,phase II trial[J]. Annals of Oncology,2022,33:S1386-S1387. [37] Tolaney S M,Wardley A M,Zambelli S,et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive,HER2-positive advanced breast cancer (monarcHER): A randomised,open-label,phase 2 trial[J]. The Lancet Oncology,2020,21(6):763-775. doi: 10.1016/S1470-2045(20)30112-1 [38] Johnston S,Pippen J,Pivot X,et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor–positive metastatic breast cancer[J]. Journal of Clinical Oncology,2009,27(33):5538-5546. doi: 10.1200/JCO.2009.23.3734 [39] Huober J,Fasching P A,Barsoum M,et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive,hormone-receptor-positive metastatic breast cancer-results of the eLEcTRA trial[J]. The Breast,2012,21(1):27-33. doi: 10.1016/j.breast.2011.07.006 [40] Kaufman B,Mackey J R,Clemens M R,et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2–positive,hormone receptor–positive metastatic breast cancer: Results from the randomized phase III TAnDEM study[J]. Journal of Clinical Oncology,2009,27(33):5529-5537. doi: 10.1200/JCO.2008.20.6847 [41] Lv H, Yan M, Jiang Z. Recent advances in the treatment of hormone receptor-positive/human epidermal growth factor 2-positive advanced breast cancer[J]. Therapeutic Advances in Medical Oncology, 2021, 13: 175883592110133. [42] Yan C,Wei H,Minjuan Z,et al. The mTOR inhibitor rapamycin synergizes with a fatty acid synthase inhibitor to induce cytotoxicity in ER/HER2-positive breast cancer cells[J]. D Calvisi PLoS ONE,2014,9(5):e97697. doi: 10.1371/journal.pone.0097697 [43] Hurvitz S A,Andre F,Jiang Z,et al. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): A phase 3,randomised,double-blind,multicentre trial[J]. The Lancet Oncology,2015,16(7):816-829. doi: 10.1016/S1470-2045(15)00051-0 [44] André F,O’Regan R,Ozguroglu M,et al. Everolimus for women with trastuzumab-resistant,HER2-positive,advanced breast cancer (BOLERO-3): A randomised,double-blind,placebo-controlled phase 3 trial[J]. The Lancet Oncology,2014,15(6):580-591. doi: 10.1016/S1470-2045(14)70138-X [45] Mcdermott M S J,Canonici A,Ivers L,et al. Dual inhibition of IGF1R and ER enhances response to trastuzumab in HER2 positive breast cancer cells[J]. International Journal of Oncology,2017,50(6):2221-2228. doi: 10.3892/ijo.2017.3976 -






 下载:
下载: