Screening of Candidate Strains for Coxsackievirus Group A Virus Type 16 Vaccine
-
摘要:
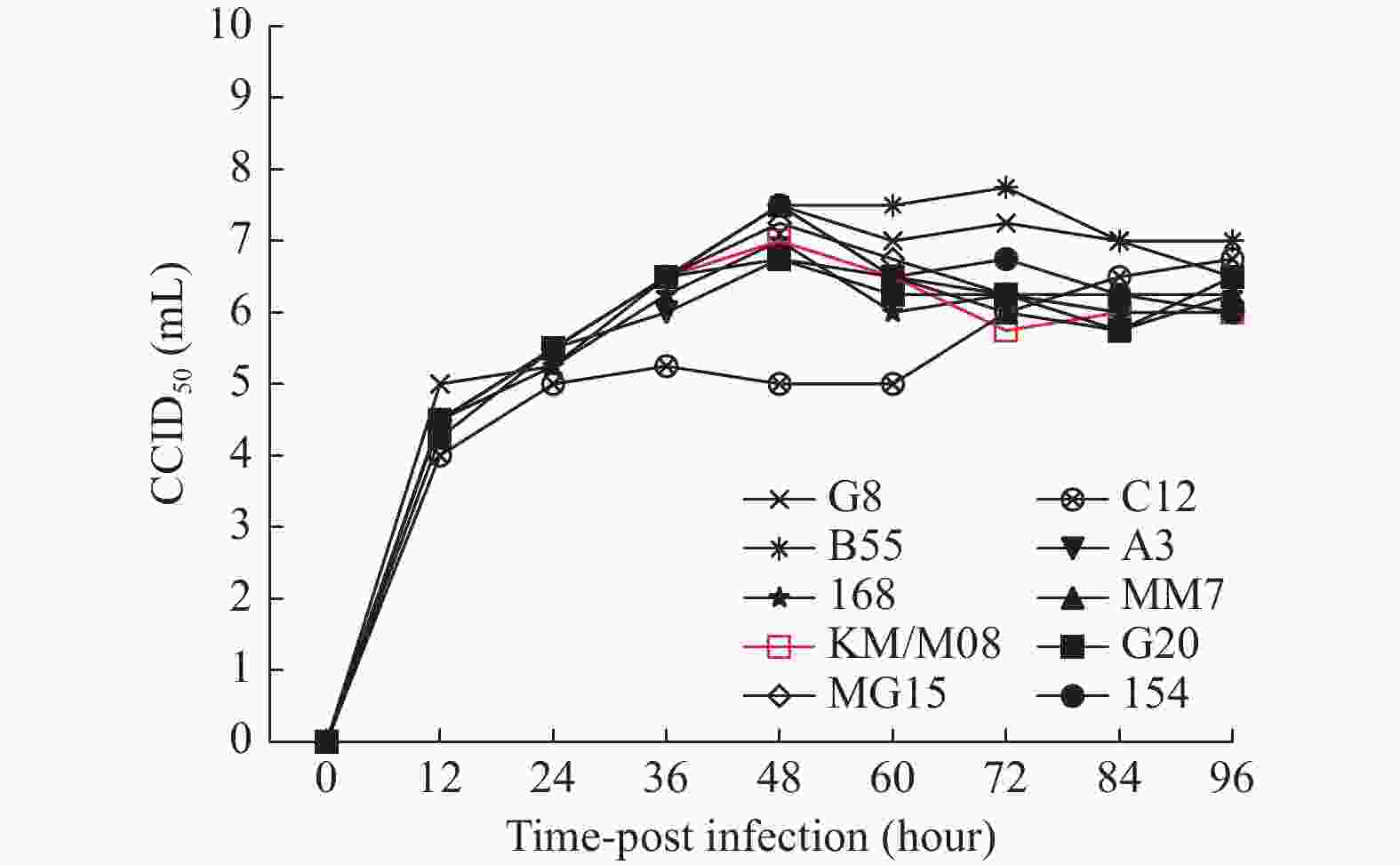
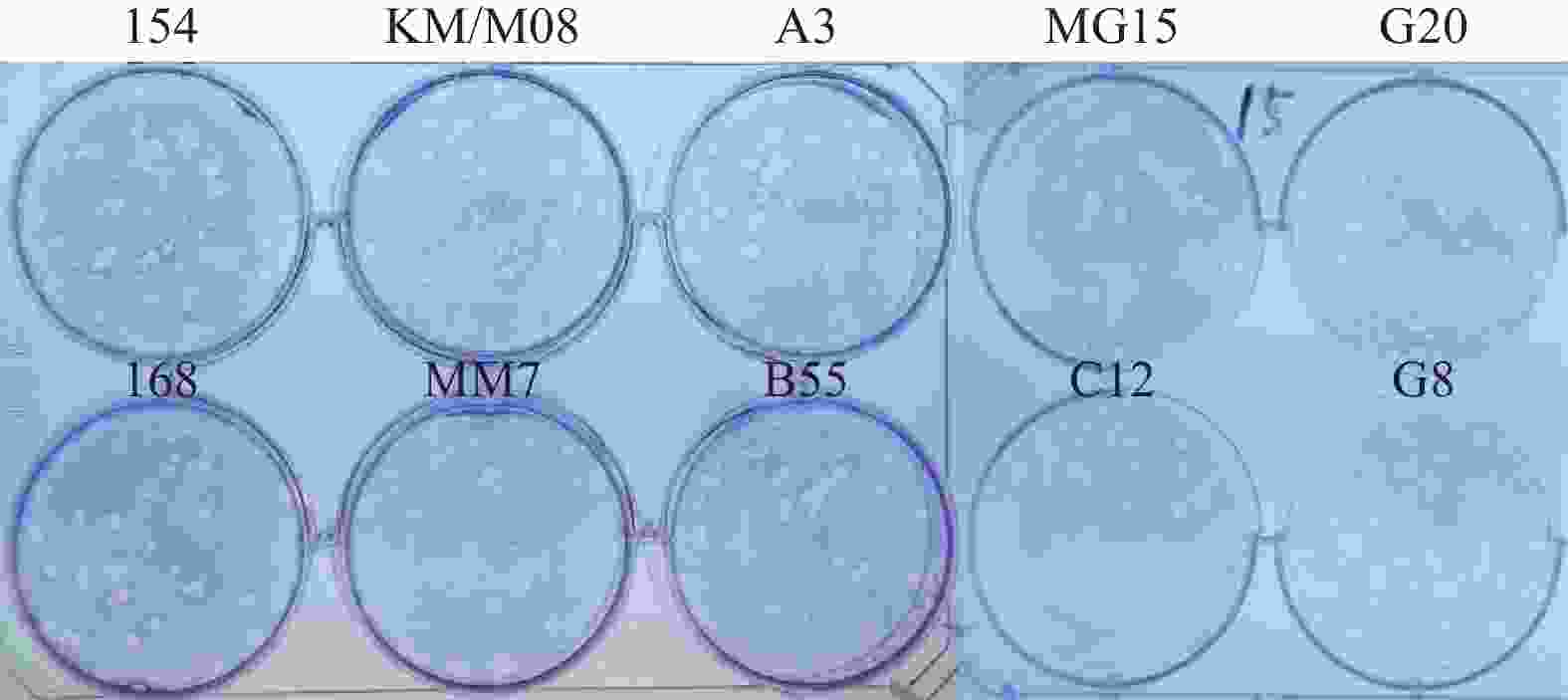
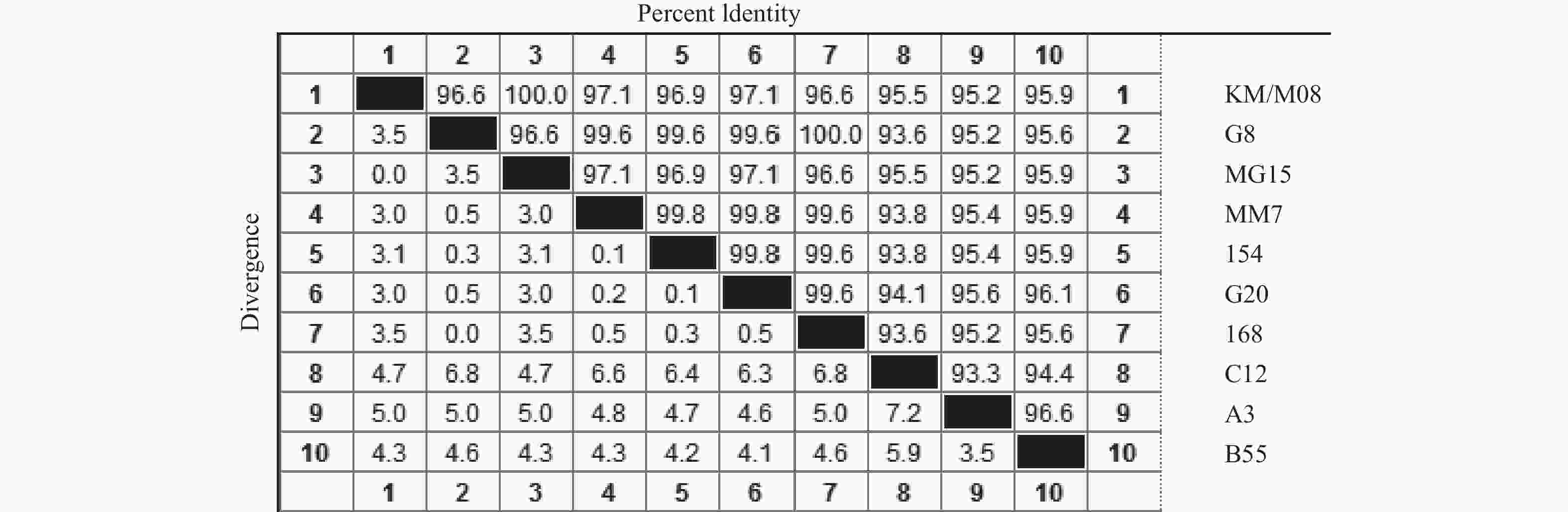
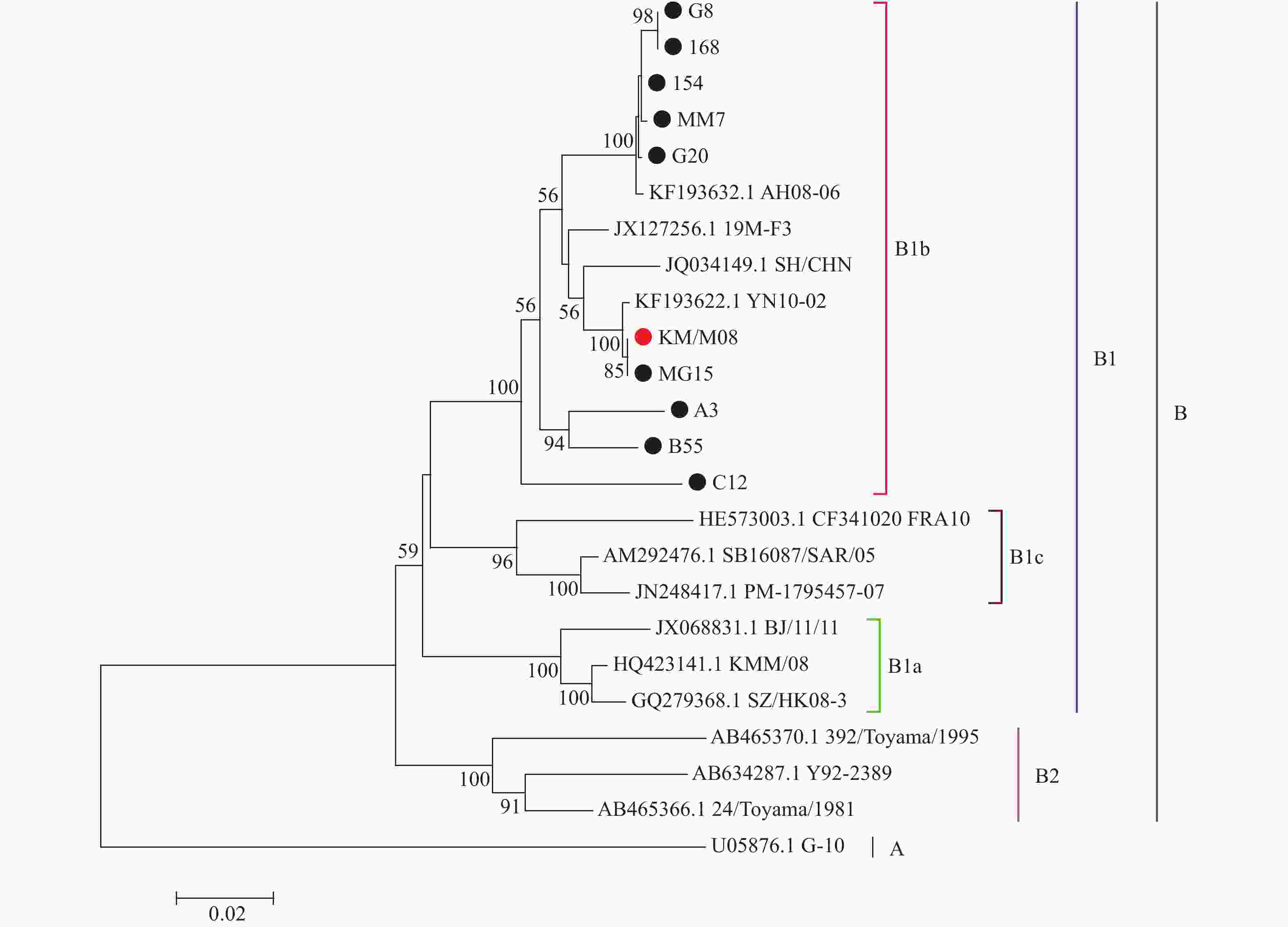
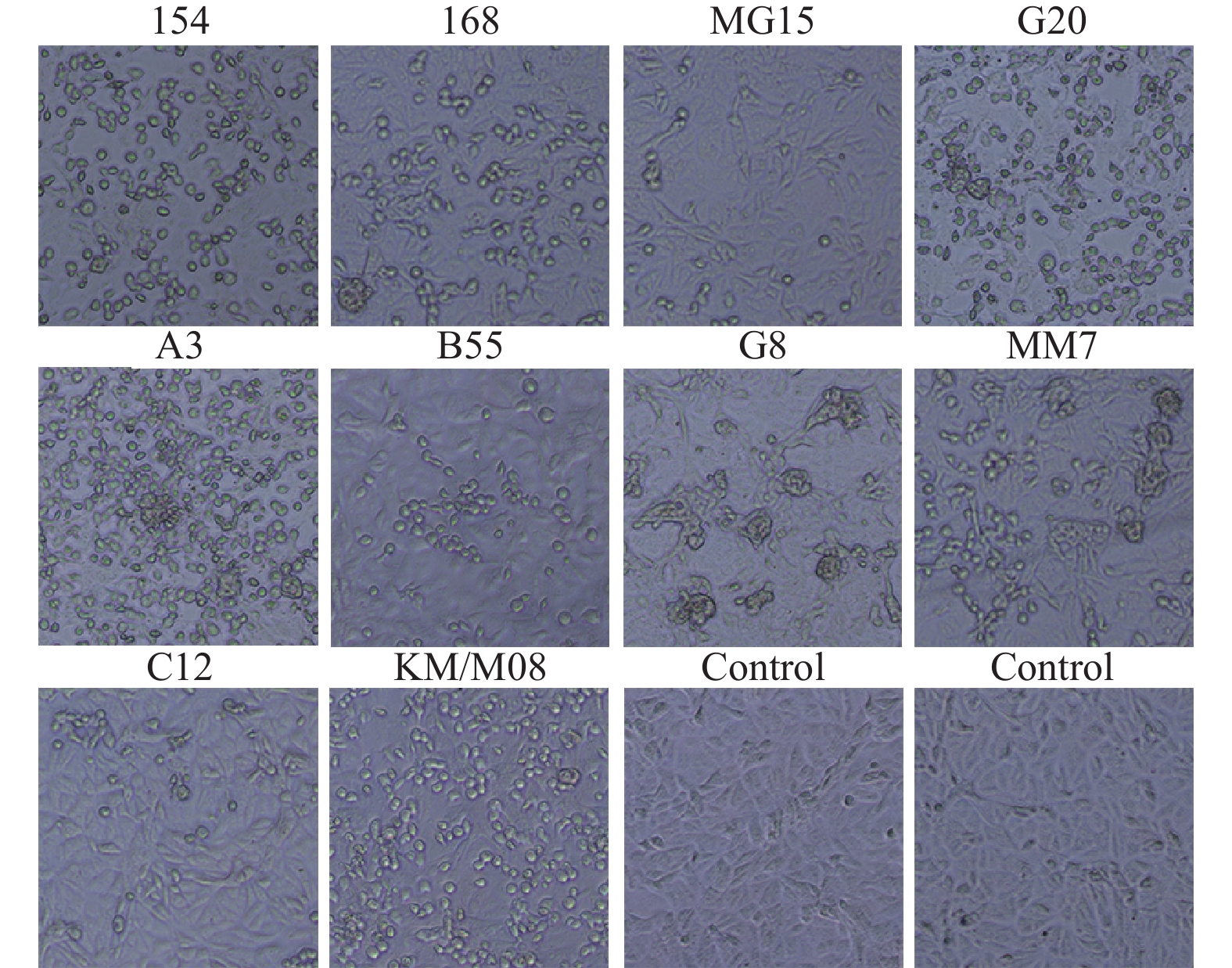
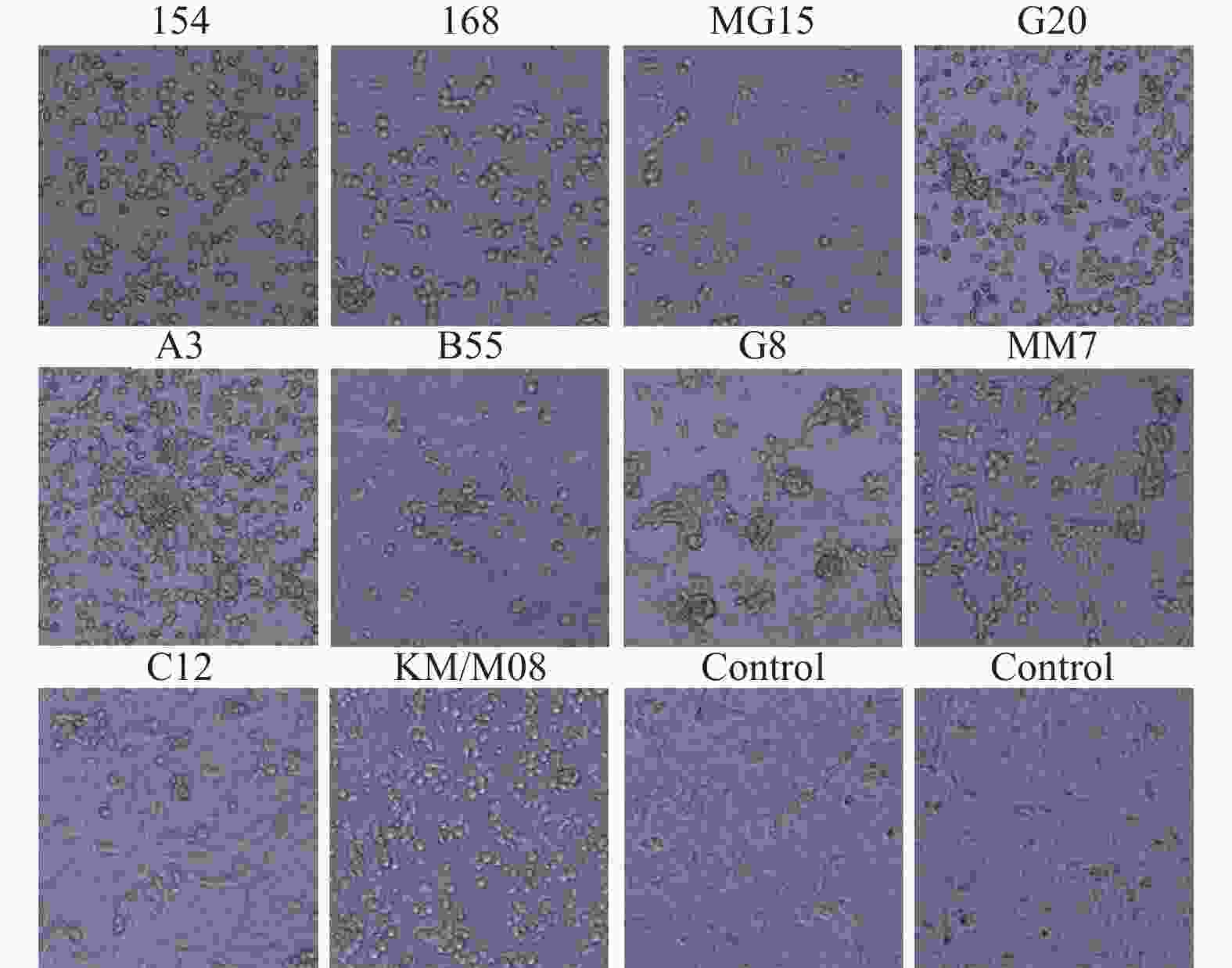
目的 筛选柯萨奇A组病毒16型(CA16)疫苗候选株,为手足口病疫苗研发提供前期基础。 方法 对2019年3月至2020年3月来自昆明市不同地区的35份手足口病患者临床标本进行细胞接种及连续传代,对产生细胞病变的收获液通过CA16标准血清进行鉴别试验,阳性样本通过病毒滴度测定、蚀斑纯化、VP1序列同源性及进化分析,筛选疫苗株。 结果 在收集的35份临床口咽拭子样本中,有23份出现细胞病变,经血清学鉴定10份为CA16病毒,阳性率为43.5%。10株病毒经适应性培养,病毒滴度逐渐升高,但从48 h开始趋于稳定,保持在7.0 lgCCID50。毒株蚀斑显示,所有毒株均可形成0.2~0.8 cm的空斑,呈现规则性差异,KM/M08蚀斑大小比较均一。所有毒株均为B1b型,核苷酸的同源性在93.3%~100%,其中KM/M08与其他毒株的同源性最高,在95.2%~100%之间。 结论 通过病毒增殖动力学曲线及其基因同源性、进化分析,KM/M08在上述各方面评价中都比较符合预期筛选标准,拟作为疫苗候选株进行后续研究。 Abstract:Objective To screen Coxsackievirus A16 (CA16) vaccine candidate strains and provide preliminary basis for the development of vaccine against hand, foot and mouth disease (HFMD). Methods Cell inoculation and continuous passage were performed on the 35 clinical specimens of patients with hand, foot, and mouth disease from different areas of Kunming. The harvested solution with cytopathic effects was identified using CA16 standard serum. Positive samples were screened for vaccine strains through the virus titer determination, plaque purification, VP1 sequence homology and evolutionary analysis. Results Among the 35 collected clinical oropharyngeal swab samples, 23 showed cellular lesions, and 10 were identified as CA16 virus by serology, with a positive rate of 43.5%. After the adaptive cultivation, the virus titers of 10 strains gradually increased, but stabilized at 7.0 lgCCID50 from 48 hours onwards. The plaque of the strain showed that all strains can form plaques of 0.2~0.8 cm, showing the regular differences, and the size of KM/M08 plaques was relatively uniform. All strains were B1b type, with nucleotide homology ranging from 93.3~100%. Among them, KM/M08 had the highest homology with other strains, ranging from 95.2~100%. Conclusion Through the analysis of virus proliferation kinetics curve, gene homology, and evolution, KM/M08 meets the expected screening criteria in all aspects of evaluation and is proposed as a vaccine candidate strain for further researches. -
Key words:
- Hand-foot-and-mouth disease /
- CA16 /
- Vaccine strains /
- Evolutionary analysis
-
表 1 VP1扩增引物序列
Table 1. Primer sequences for VP1 amplification
引物名称 序列 (5' –3' ) 扩增长度 (bp) CA16-VP1F GGACTTCGGGTTGCAGTCTTCTG 1229 CA16-VP1R AGTCGTTATGCGTGGCAAGGTGT -
[1] Huang J,Liao Q,Ooi M H,et al. Epidemiology of recurrent hand,foot and mouth disease,China,2008-2015[J]. Emerg Infect Dis,2018,24(3):432-442. [2] 张翠. 手足口病患儿的病原体研究进展[J]. 中国城乡企业卫生,2024,39(9):38-40. [3] Zhang Y,Zhu Z,Yang W,et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China[J]. Virol J,2010,7(1):94. doi: 10.1186/1743-422X-7-94 [4] Li J,Wang J,Xu C,et al. Hand,foot,and mouth disease in China's mainland before it was listed as category C disease in May,2008[J]. Lancet Infect Dis,2017,17(10):1017-1018. doi: 10.1016/S1473-3099(17)30471-1Li J,Wang J,Xu C,et al. Hand,foot,and mouth disease in China's mainland before it was listed as category C disease in May,2008[J]. Lancet Infect Dis,2017,17(10):1017-1018. doi: 10.1016/S1473-3099(17)30471-1 [5] Li R,Liu L,Mo Z,et al. An inactivated enterovirus 71 vaccine in healthy children[J]. N Engl J Med,2014,370(9):829-837. doi: 10.1056/NEJMoa1303224 [6] Yin D Q,Wang C B,Wang C B,et al. Epidemiology characteristics of human coxsackievirus A16 and enterovirus 71 circulating in Linyi,China,from 2009 to 2017[J]. Jpn J Infect Dis,2018,71(6):470-473. doi: 10.7883/yoken.JJID.2018.035 [7] Mao Q,Wang Y,Yao X,et al. Coxsackievirus A16: Epidemiology,diagnosis,and vaccine[J]. Hum Vaccin Immunother,2014,10(2):360-367. doi: 10.4161/hv.27087 [8] Aswathyraj S,Arunkumar G,Alidjinou E K,et al. Hand,foot and mouth disease (HFMD): Emerging epidemiology and the need for a vaccine strategy[J]. Med Microbiol Immunol,2016,205(5):397-407. doi: 10.1007/s00430-016-0465-y [9] Fan S,Wang T,Gao X,et al. Phylogenetic analysis of newcastle disease viruses isolated from wild birds in the Poyang Lake region of China[J]. J Vet Med Sci,2015,77(9):1143-1149. doi: 10.1292/jvms.14-0080 [10] Cox B,Levent F. Hand,foot,and mouth disease[J]. JAMA,2018,320(23):2492. doi: 10.1001/jama.2018.17288 [11] Daba T M,Zhao Y,Pan Z. Advancement of mechanisms of coxsackie virus B3-induced myocarditis pathogenesis and the potential therapeutic targets[J]. Curr Drug Targets,2019,20(14):1461-1473. doi: 10.2174/1389450120666190618124722 [12] Lei H,Wang D,Wang H,et al. Constrictive pericarditis caused by viral myocarditis in children: A brief report[J]. J Cardiothorac Surg,2022,17(1):215. [13] Yang L, Liu Y, Li S, et al. A novel inactivated enterovirus 71 vaccine can elicit cross-protective immunity against coxsackievirus A16 in mice[J]. Vaccine,2016,34(48):5938-5945. [14] Mao Q Y, Wang Y, Bian L, et al. EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD)[J]. Expert Rev Vaccines,2016,15(5):599-606. [15] 郭泽铭,蔡秋虹. LRP6通过IFN通路抑制肠道病毒EV71和柯萨奇病毒CA16[J]. 病毒学报,2022,38(4):858-865. [16] Esposito S,Principi N. Hand,foot and mouth disease: Current knowledge on clinical manifestations,epidemiology,aetiology and prevention[J]. Eur J Clin Microbiol Infect Dis,2018,37(3):391-398. doi: 10.1007/s10096-018-3206-x [17] Baggen J, Thibaut H J, Strating J R P M, et al. The life cycle of non-polio enteroviruses and how to target it[J]. Nature Reviews. Microbiology,2018,16(6):368-381. [18] 牛栋,赵彬彬,武婧,等. EV71、CA16、CA10三价VP1蛋白疫苗的有效性和安全性研究[J]. 中国实验动物学报,2021,29(2):210-218. doi: 10.3969/j.issn.1005-4847.2021.02.011 [19] 杜瑞晓,毛群颖,梁争论. CA16疫苗及其相关研究进展[J]. 中国生物制品学杂,2020,33(1):80-83+87. [20] Bian L, Gao F, Mao Q, et al. Hand, foot, and mouth disease associated with coxsackievirus A10: More serious than it seems[J]. Expert Review of Anti-infective Therapy,2019,17(4):233-242. doi: 10.1016/j.ijid.2018.12.020 -





 下载:
下载: