TMAO Inhibitors' Effects on Diabetic Sarcopenia in Rats
-
摘要:
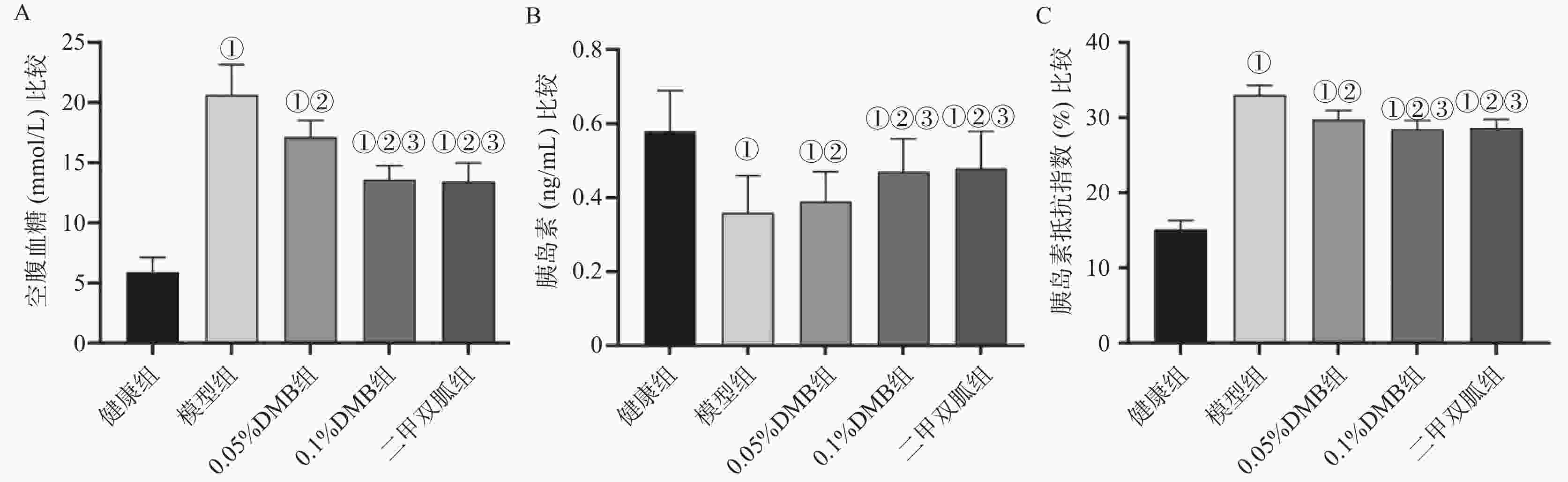
目的 探究氧化三甲胺(trimethylamine N-oxide,TMAO)抑制剂对糖尿病性肌少症大鼠线粒体损伤、骨骼肌功能及微血管衰老的影响。 方法 SPF级别雄性大鼠构建糖尿病性肌少症动物模型,3,3-二甲基-1-丁醇(DMB)干预。检测各组大鼠空腹血糖、胰岛素及胰岛素抵抗指数;抓绳实验、腓肠肌重量、体重检测;腓肠肌病理形态;线粒体膜电位检测及超微结构;微血管衰老相关蛋白基质金属蛋白酶(matrix metalloproteinase-2,MMP-2)、单核细胞趋化蛋白(monocyte chemoattractant protein,MCP-1)、转化生长因子(transforming growth factor β1,TGFβ1)表达。 结果 与正常组大鼠相比,模型组胰岛素、抓绳时间、体重、腓肠肌重量、腓/体比值、线粒体膜电位均降低(P < 0.05),空腹血糖、胰岛素抵抗指数、MMP-2、MCP-1、TGF-β1均升高(P < 0.05);与模型组相比,0.1%DMB组胰岛素、抓绳时间、体重、腓肠肌重量、腓/体比值、线粒体膜电位均升高(P < 0.05),空腹血糖、胰岛素抵抗指数、MMP-2、MCP-1、TGF-β1均降低(P < 0.05)。 结论 TMAO抑制剂可降低空腹血糖及胰岛素抵抗,改善线粒体损伤而提高骨骼肌功能,可能通过抑制MMP-2、MCP-1、TGF-β1表达而减少微血管衰老。 Abstract:Objective To investigate the effects of TMAO inhibitor on mitochondrial damage, skeletal muscle function, and microvascular senescence in rats with diabetic sarcopenia. Methods An animal model of diabetic sarcopenia was established in specific pathogen-free experimental animal (SPF) grade male rats, and intervention was carried out with 3, 3-dimethyl-1-butanol (DMB). The fasting blood glucose, insulin and insulin resistance index of rats in each group were detected. Rope grasping test, gastrocnemius muscle weight, body weight detection; Pathological morphology of the gastrocnemius muscle detection of mitochondrial membrane potential and ultrastructure expression of microvascular aging-related proteins MMP-2, MCP-1 and TGFβ1. Results Compared with the healthy control group, the model group showed significant decreases in insulin level, wire-hanging time, body weight, gastrocnemius muscle weight, gastrocnemius-to-body weight ratio, and mitochondrial membrane potential (P < 0.05), along with significant increases in FBG, HOMA-IR (P < 0.05), and the expression levels of MMP-2, MCP-1, and TGF-β1 (P < 0.05). Compared with the model group, the 0.1% DMB group exhibited significant increases in insulin level, wire-hanging time, body weight, gastrocnemius muscle weight, gastrocnemius-to-body weight ratio, and mitochondrial membrane potential, as well as significant decreases in FBG, HOMA-IR, and the expression levels of MMP-2, MCP-1, and TGF-β1 (P < 0.05). Conclusion TMAO inhibitor can reduce FBG and insulin resistance, alleviate mitochondrial damage, and thereby improve skeletal muscle function in rats with diabetic sarcopenia. This effect is presumably achieved by inhibiting the expression of MMP-2, MCP-1, and TGF-β1 to reduce microvascular senescence. -
Key words:
- Diabetic Sarcopenia /
- Trimethylamine N-Oxide (TMAO) /
- Mitochondria /
- Skeletal Muscle /
- Vascular Aging
-
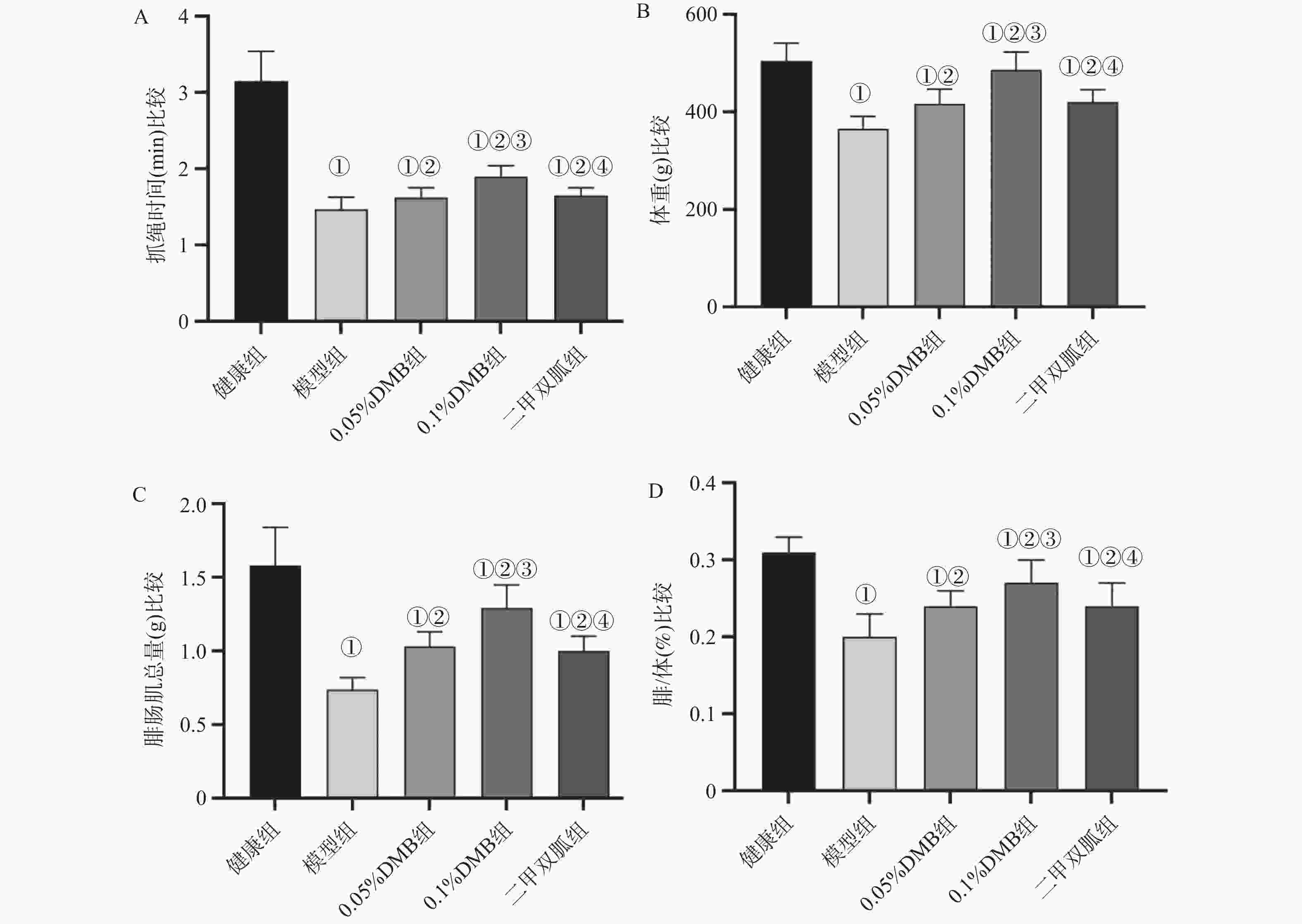
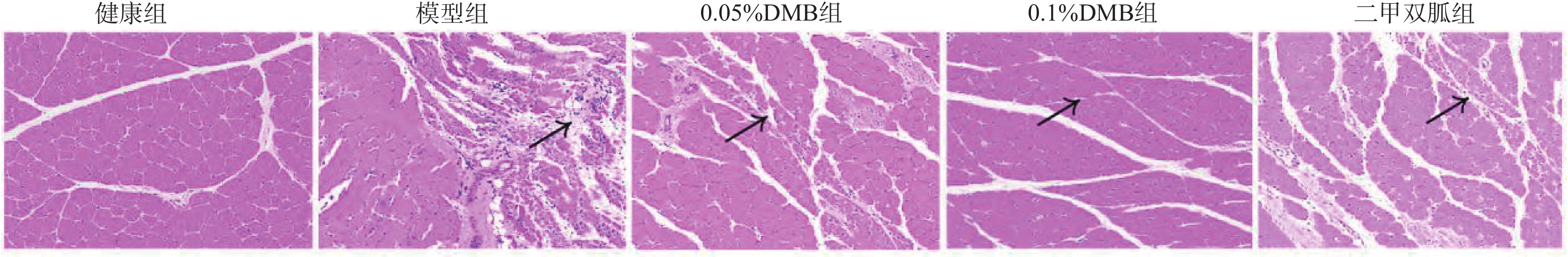
图 3 各组大鼠抓绳时间、体重、腓肠肌总量及腓/体比较[($\bar x \pm s $) ,n = 12]
A:大鼠抓绳时间;B:大鼠体重;C:大鼠腓肠肌总量;D:大鼠腓/体;与正常组相比,*P < 0.05;与模型组相比,#P < 0.05;0.05%DMB组相比,△P < 0.05;与0.1%DMB组相比,$P < 0.05;
Figure 3. shows the comparison of rope grasping time,body weight,total gastrocnemius muscle and fibular/body weight of rats in each group[($\bar x \pm s $) ,n = 12]
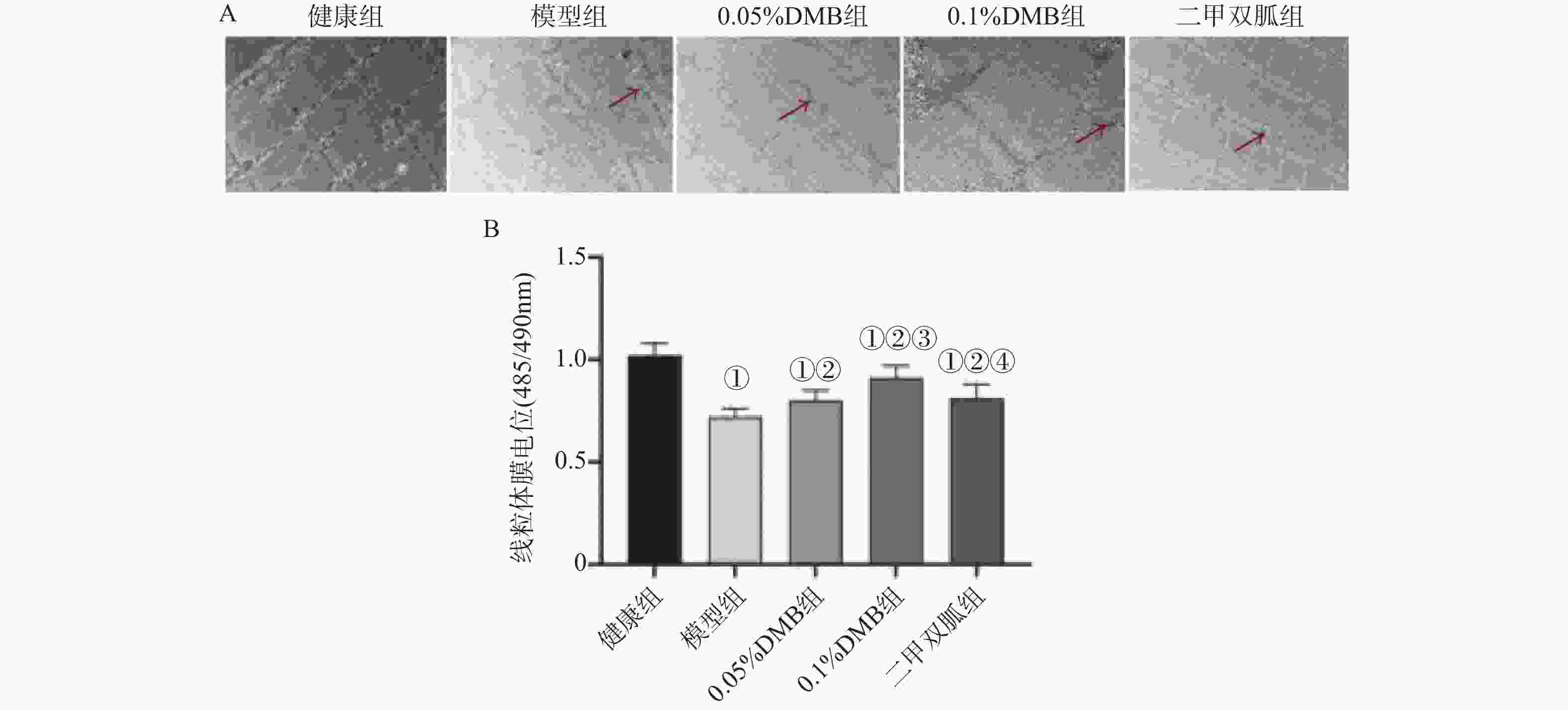
图 4 各组大鼠线粒体超微结构、线粒体膜电位比较[(锇酸染色,
5000 ×),($\bar x \pm s $) ,n = 12]A:线粒体超微结构;B:线粒体膜电位;与正常组相比,*P < 0.05;与模型组相比,#P < 0.05;0.05%DMB组相比,△P < 0.05;与0.1%DMB组相比,$P < 0.05。注:红色箭头为线粒体肿胀。
Figure 4. Comparison of mitochondrial ultrastructure and mitochondrial membrane potential in each group of rats[(osmium acid staining,
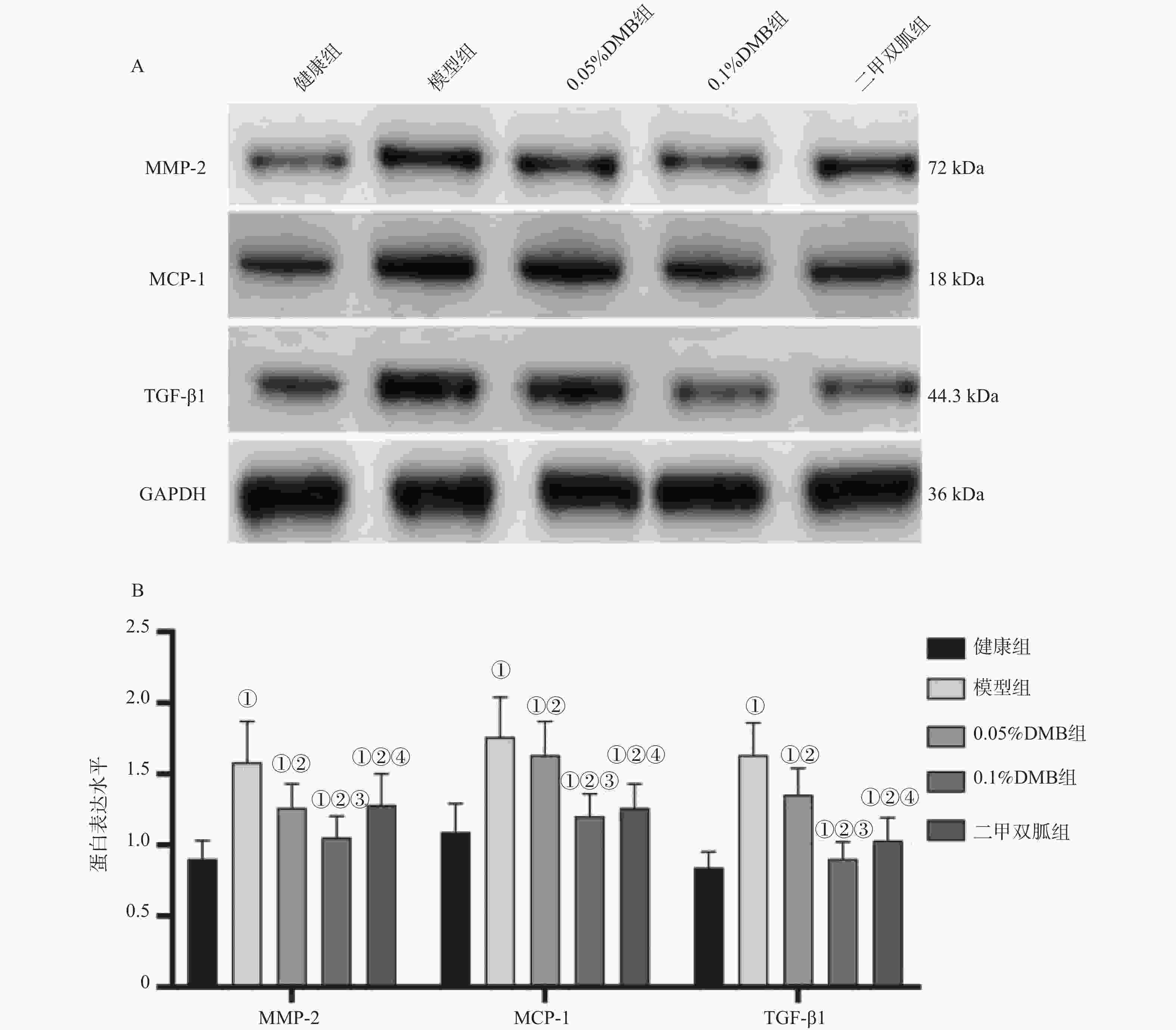
5000 ×),($\bar x \pm s $),n = 12]图 5 各组大鼠MMP-2 、MCP-1及TGF-β1蛋白图及表达比较[($\bar x \pm s $),n = 12]
A:MMP-2 、MCP-1及TGF-β1蛋白图;B:MMP-2 、MCP-1及TGF-β1蛋白表达;与正常组相比,*P < 0.05;与模型组相比,#P < 0.05;0.05%DMB组相比,△P < 0.05;与0.1%DMB组相比,$P < 0.05。
Figure 5. Protein diagrams and expression comparisons of MMP-2,MCP-1 and TGF-β1 in each group of rats [($\bar x \pm s $),n = 12]
-
[1] Sayer A A, Cooper R, Arai H, et al. Sarcopenia[J]. Nat Rev Dis Primers, 2024, 10: 68. doi: 10.1038/s41572-024-00550-w [2] 江涛, 王新航, 张露艺, 等. 中国老年人肌少症患病率的Meta分析[J]. 海南医学, 2022, 33(1): 116-123. [3] 毕雨晴, 殷实. 肌少症发生风险的多维度评估及干预策略[J]. 中国临床保健杂志, 2025, 28(2): 173-180. [4] Sugimoto K, Ikegami H, Takata Y, et al. Glycemic control and insulin improve muscle mass and gait speed in type 2 diabetes: The MUSCLES-DM study[J]. J Am Med Dir Assoc, 2021, 22(4): 834-838. e1. [5] Izzo A, Massimino E, Riccardi G, et al. A narrative review on sarcopenia in type 2 diabetes mellitus: Prevalence and associated factors[J]. Nutrients, 2021, 13(1): 183. doi: 10.3390/nu13010183 [6] Xie Z, Li Y, Li X, et al. A review of mitochondrial dysfunction in diabetic sarcopenia: Mechanisms, diagnosis, and treatment approaches[J]. J Int Med Res, 2025, 53(7): 3000605251355996. [7] 蒋佳红. 2型糖尿病患者中肱踝脉搏波速度在年龄与肌少症的关系中所起的中介作用[D]. 温州医科大学, 2023. [8] Liu D, Liu J, Zhang D, et al. Advances in relationship between cell senescence and atherosclerosis[J]. J Zhejiang Univ (Med Sci), 2022, 51(1): 95-101. [9] Zha A, Li W, Wang J, et al. Trimethylamine oxide supplementation differentially regulates fat deposition in liver, longissimus dorsi muscle and adipose tissue of growing-finishing pigs[J]. Anim Nutr, 2024, 17: 25-35. doi: 10.1016/j.aninu.2023.12.006 [10] Hong Q, Que D, Zhong C, et al. Trimethylamine-N-oxide (TMAO) promotes balloon injury-induced neointimal hyperplasia via upregulating Beclin1 and impairing autophagic flux[J]. Biomed Pharmacother, 2022, 155: 113639. doi: 10.1016/j.biopha.2022.113639 [11] 沈花. 当归补血汤加味对2型糖尿病大鼠脂代谢及肾功能改善作用[J]. 中国处方药, 2022, 20(11): 18-21. [12] 王安彦, 王红梅. 肌少症动物模型的造模方法研究进展[J]. 中华老年多器官疾病杂志, 2024, 23(01): 72-76. [13] Razliqi R N, Ahangarpour A, Ali Mard S, et al. Gentisic acid ameliorates type 2 diabetes induced by nicotinamide-streptozotocin in male mice by attenuating pancreatic oxidative stress and inflammation through modulation of Nrf2 and NF-кB pathways[J]. Life Sci, 2023, 325: 121770. doi: 10.1016/j.lfs.2023.121770 [14] Hefni M E, Witthöft C M, Hellström P, et al. Plasma TMAO concentrations and gut microbiota composition in subjects with and without metabolic syndrome: Results from pilot study[J]. Metabolites, 2025, 15(6): 364. doi: 10.3390/metabo15060364 [15] Luis Román D, Gómez J C, García-Almeida J M, et al. Diabetic Sarcopenia. A proposed muscle screening protocol in people with diabetes: Expert document[J]. Rev Endocr Metab Disord, 2024, 25(4): 651-661. doi: 10.1007/s11154-023-09871-9 [16] Puerarin alleviates atherosclerosis via the inhibition of Prevotella copri and its trimethylamine production [J]. Gut. 2024, 73(12): 1934-1943. [17] Jiang J Y, Zhang Q P, Ren H, et al. Trimethylamine N-oxide aggravates vascular permeability and endothelial cell dysfunction under diabetic condition: in vitro and in vivo study[J]. Int J Ophthalmol, 2024, 17(1): 25-33. doi: 10.18240/ijo.2024.01.04 [18] Constantino-Jonapa L A, Espinoza-Palacios Y, Escalona-Montaño A R, et al. Contribution of trimethylamine N-oxide (TMAO) to chronic inflammatory and degenerative diseases[J]. Biomedicines, 2023, 11(2): 431. doi: 10.3390/biomedicines11020431 [19] Zha A, Li W, Wang J, et al. Trimethylamine oxide supplementation differentially regulates fat deposition in liver, longissimus dorsi muscle and adipose tissue of growing-finishing pigs[J]. Anim Nutr, 2024, 17: 25-35. doi: 10.1016/j.aninu.2023.12.006 [20] Yang W, Zhao Q, Yao M, et al. The transformation of atrial fibroblasts into myofibroblasts is promoted by trimethylamine N-oxide via the Wnt3a/β-catenin signaling pathway[J]. J Thorac Dis, 2022, 14(5): 1526-1536. doi: 10.21037/jtd-22-475 [21] 周梦晴. EPA对2型糖尿病性肌肉衰减症的干预作用及机制研究[D]. 山东: 山东大学, 2024. [22] Peng Y, Wang Y, Hu J, et al. Trimethylamine N-oxide (TMAO) treatment triggers premature ovarian insufficiency (POI) via the activation of mitochondrial pathway apoptosis in granulosa cells[J]. Free Radic Biol Med, 2025, 232: 214-230. doi: 10.1016/j.freeradbiomed.2025.03.007 [23] Eggelbusch M, Shi A, Broeksma B C, et al. The NLRP3 inflammasome contributes to inflammation-induced morphological and metabolic alterations in skeletal muscle[J]. J Cachexia Sarcopenia Muscle, 2022, 13(6): 3048-3061. doi: 10.1002/jcsm.13062 [24] Bordoni L, Petracci I, Feliziani G, et al. Gut microbiota-derived trimethylamine promotes inflammation with a potential impact on epigenetic and mitochondrial homeostasis in caco-2 cells[J]. Antioxidants, 2024, 13(9): 1061. doi: 10.3390/antiox13091061 [25] Tomasova L, Grman M, Ondrias K, et al. The impact of gut microbiota metabolites on cellular bioenergetics and cardiometabolic health[J]. Nutr Metab, 2021, 18(1): 72. doi: 10.1186/s12986-021-00598-5 -





 下载:
下载: