Effects of Lipid Metabolism-related Genes ASP and PPARα in Atherosclerosis
-
摘要:
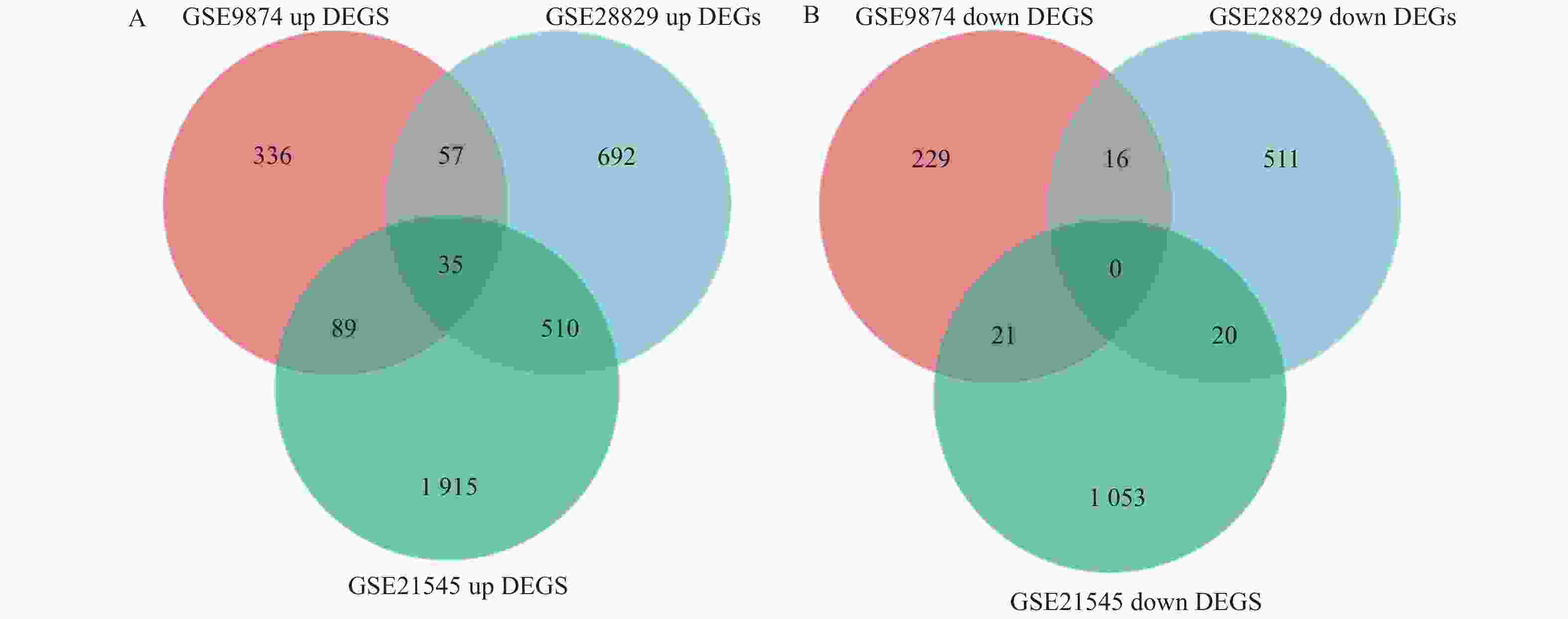
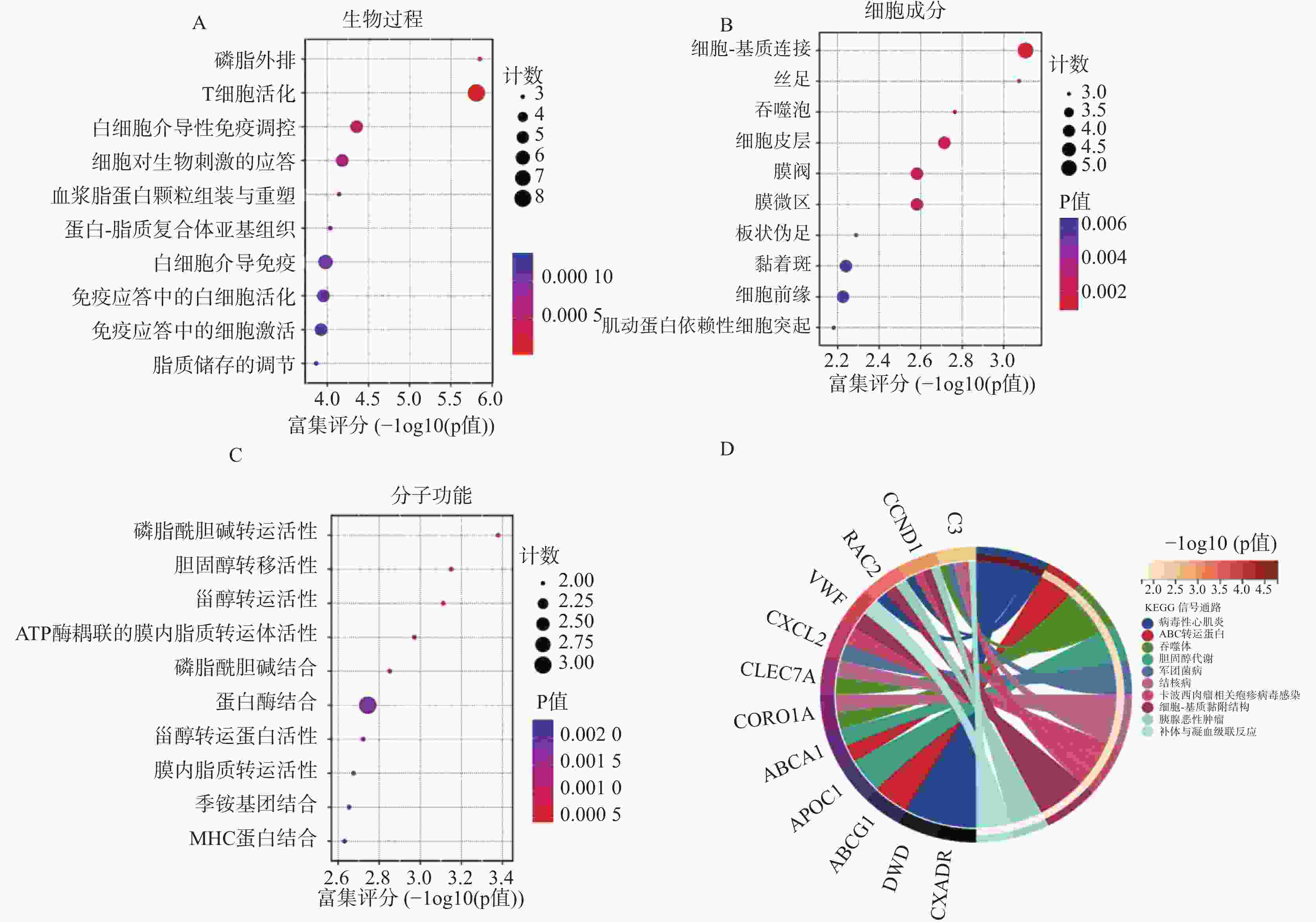
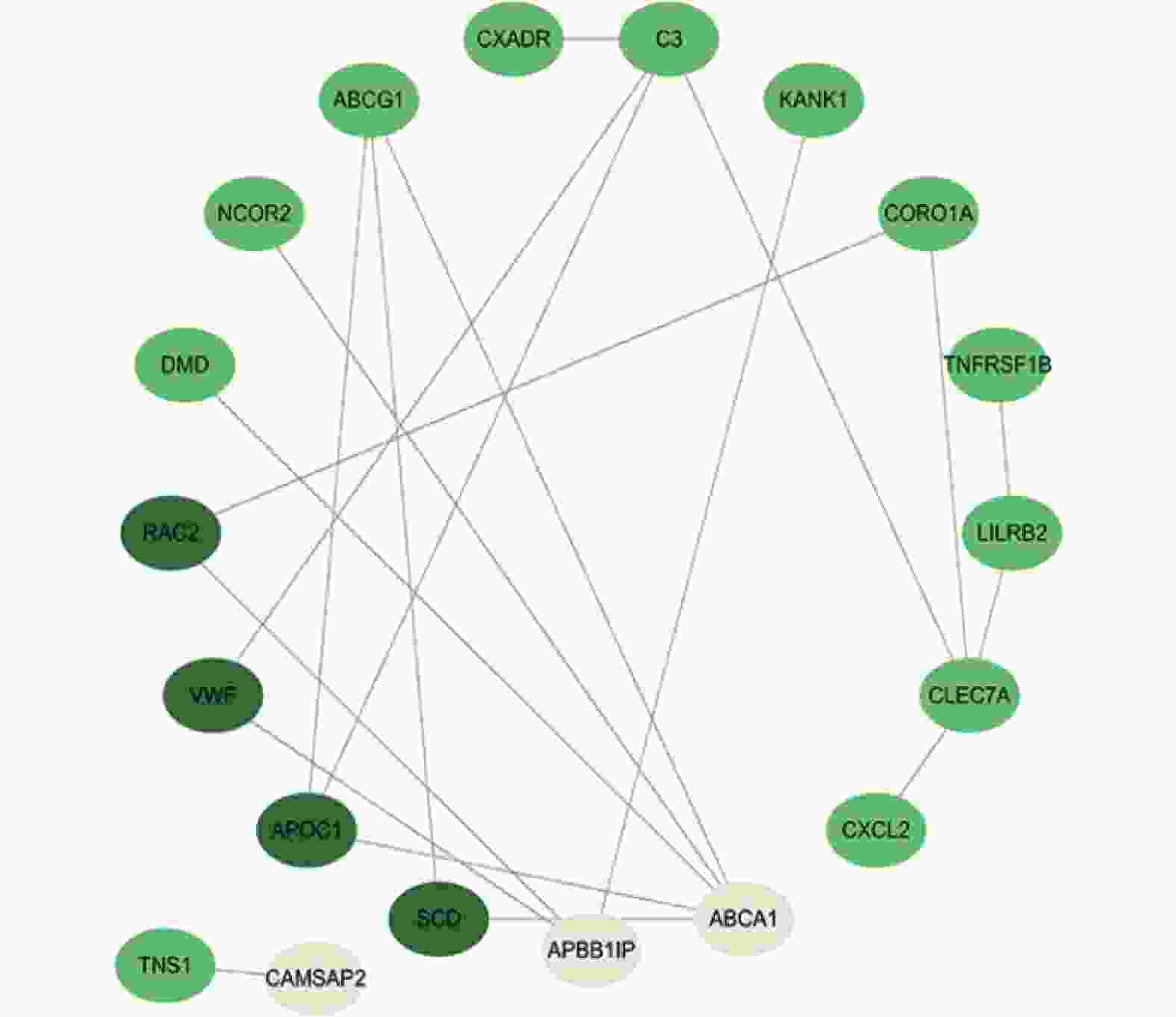
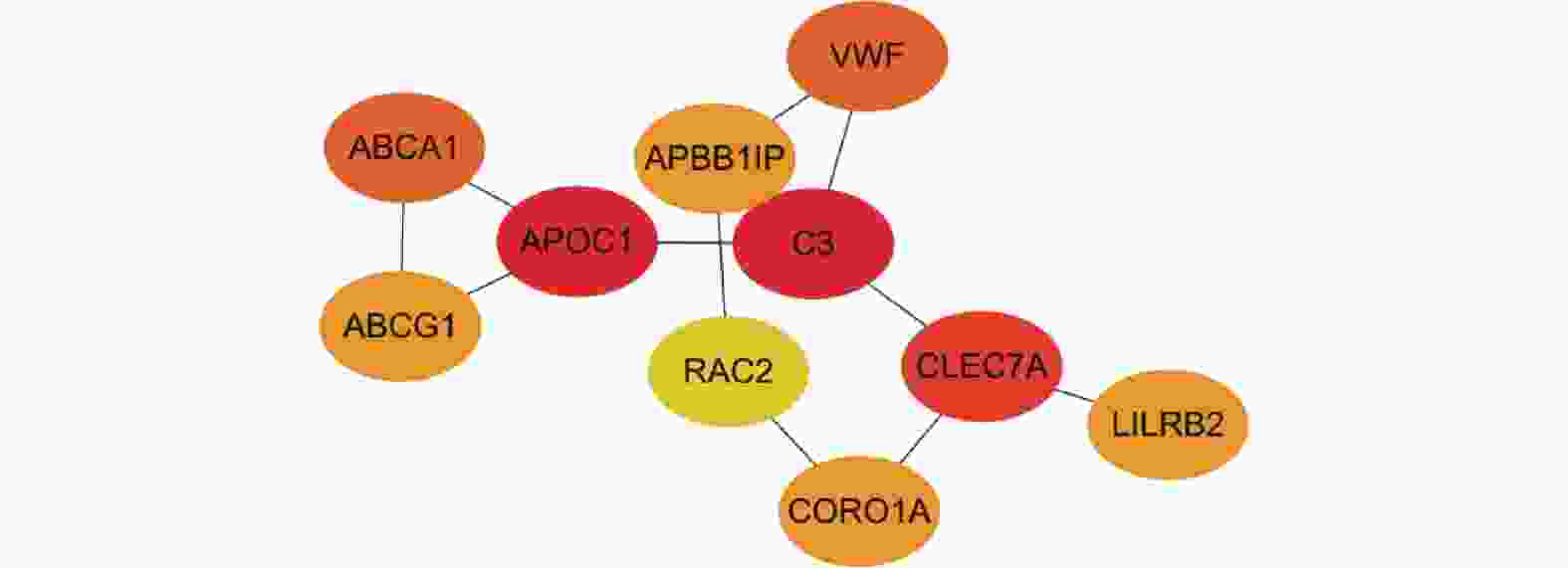
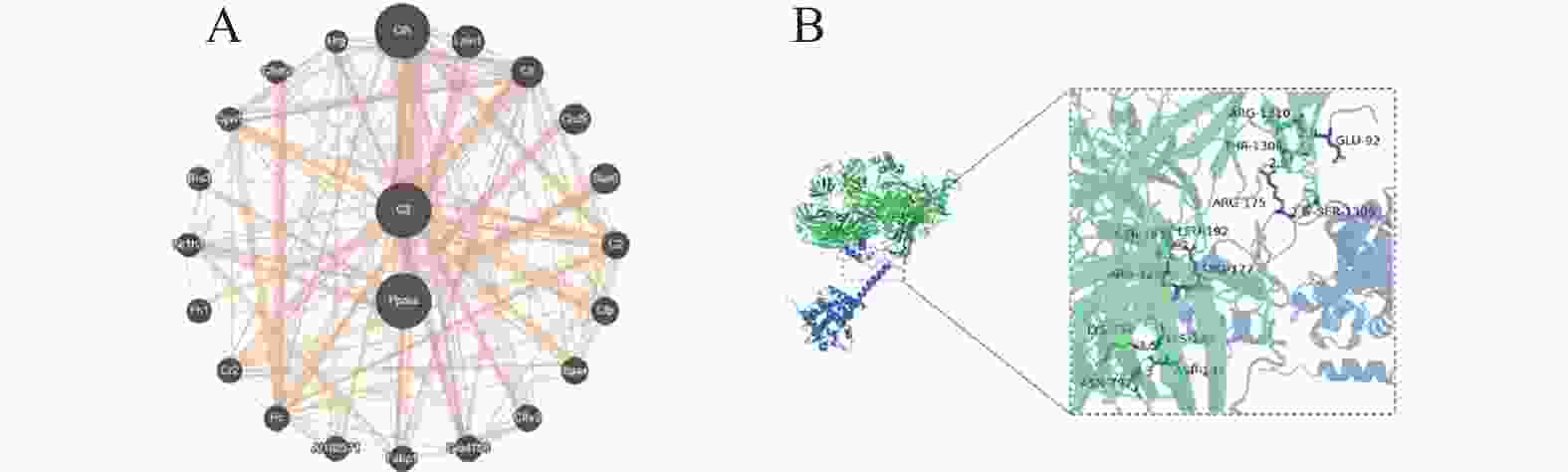
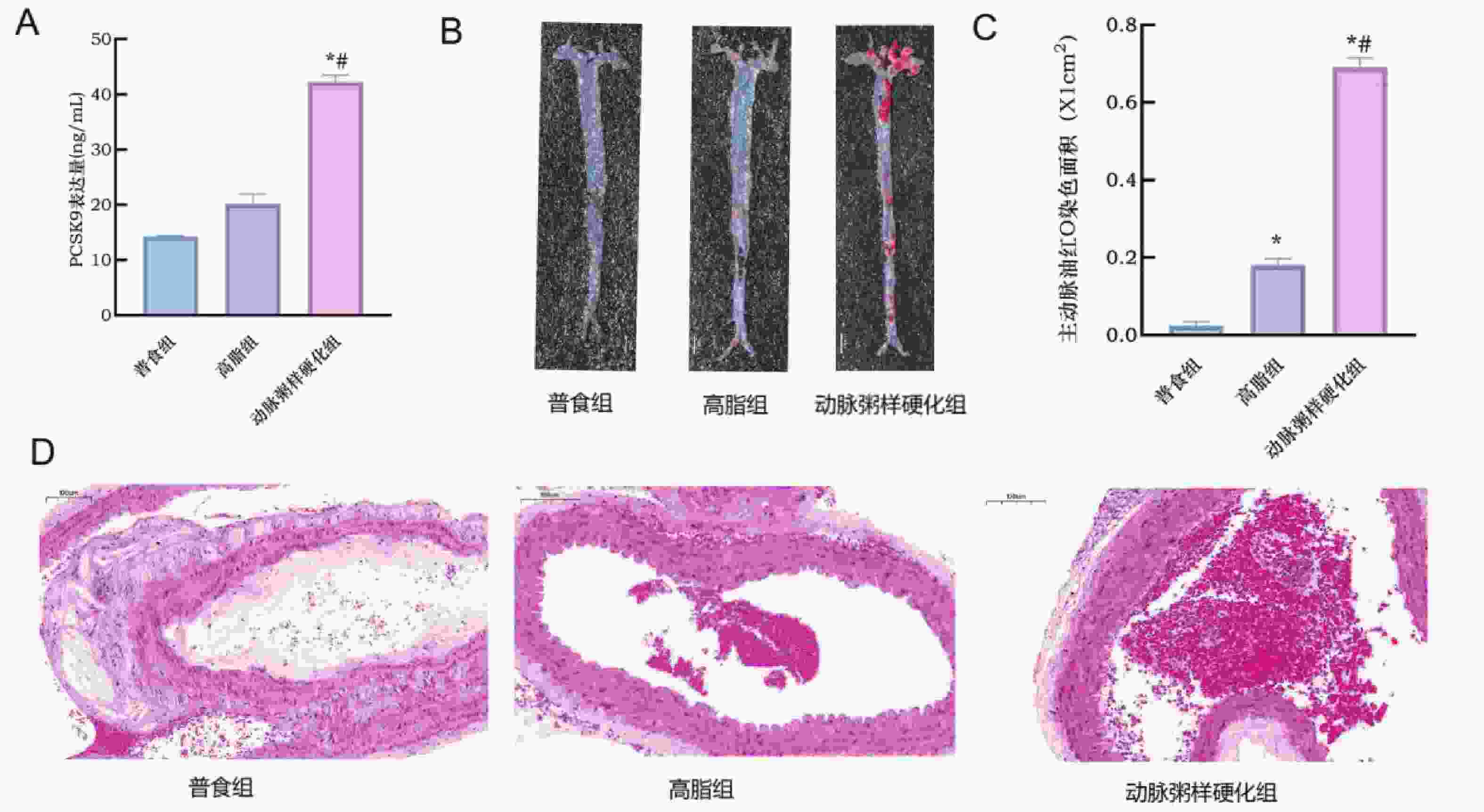
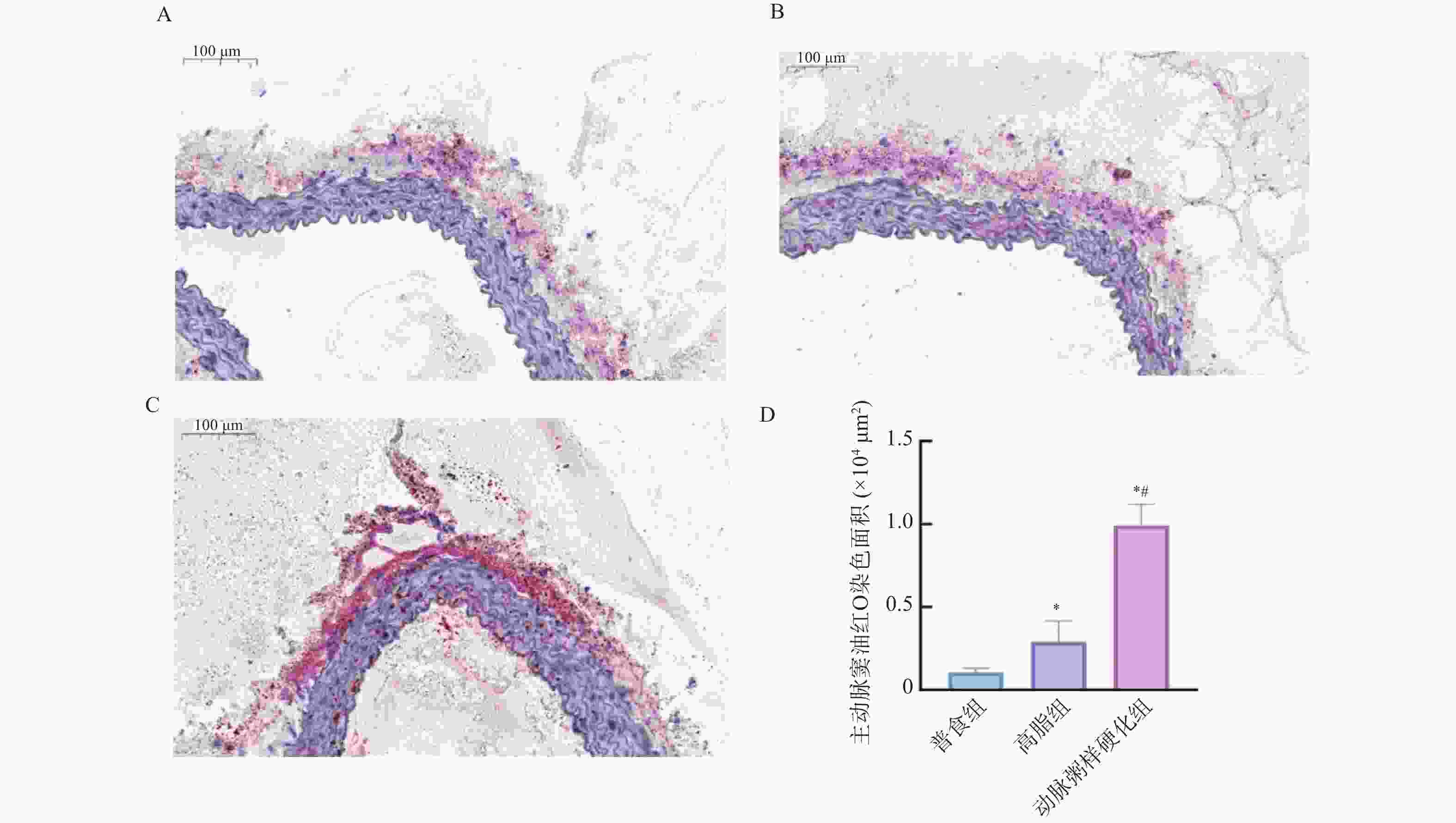
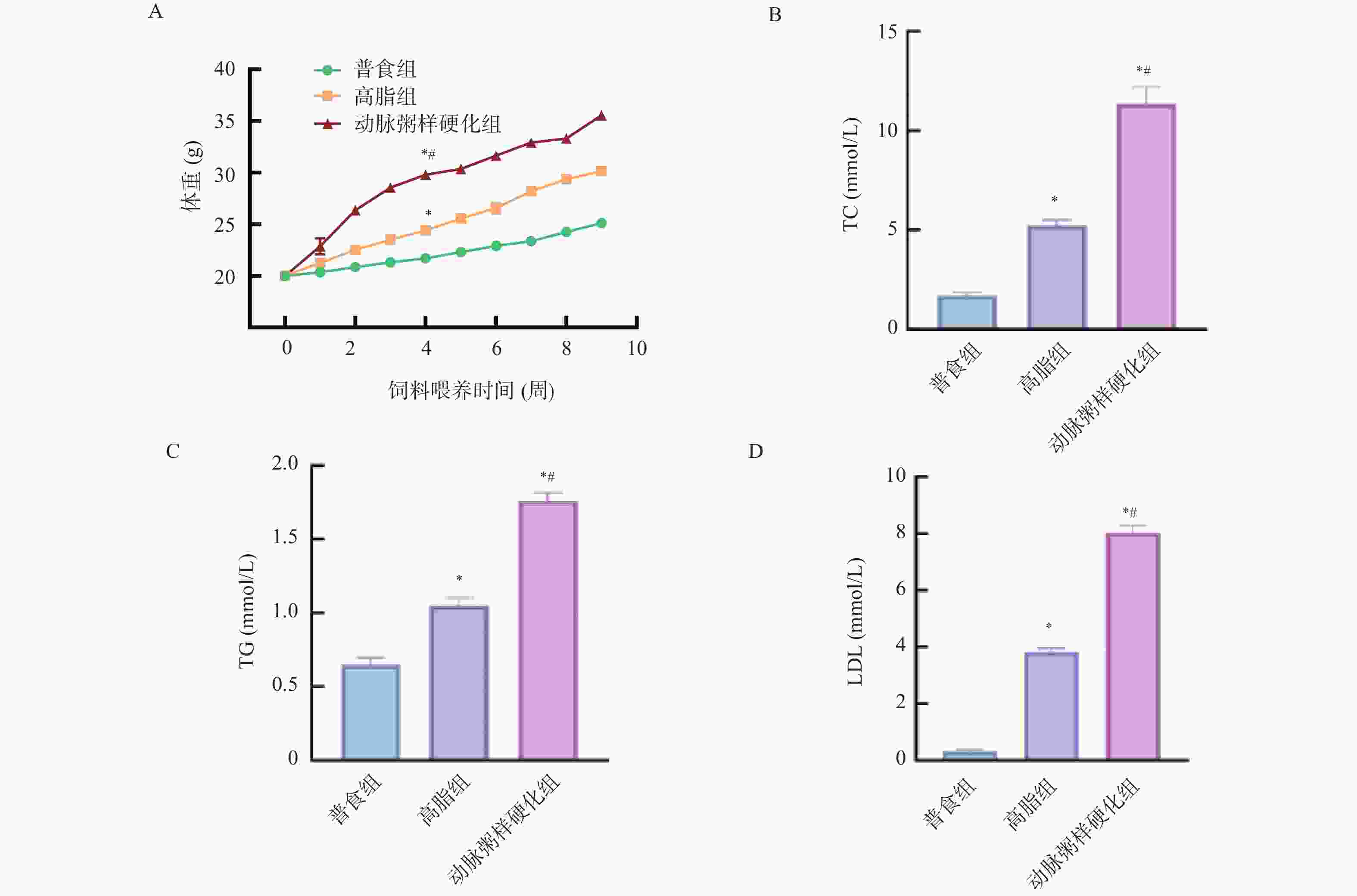
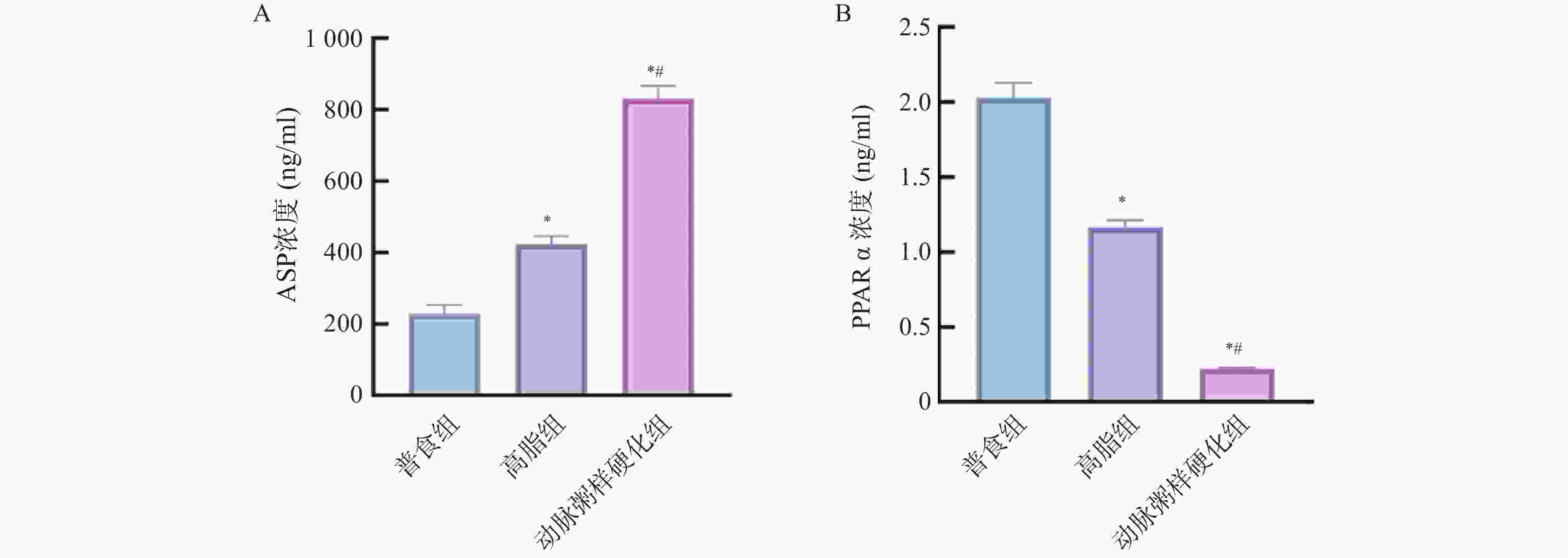
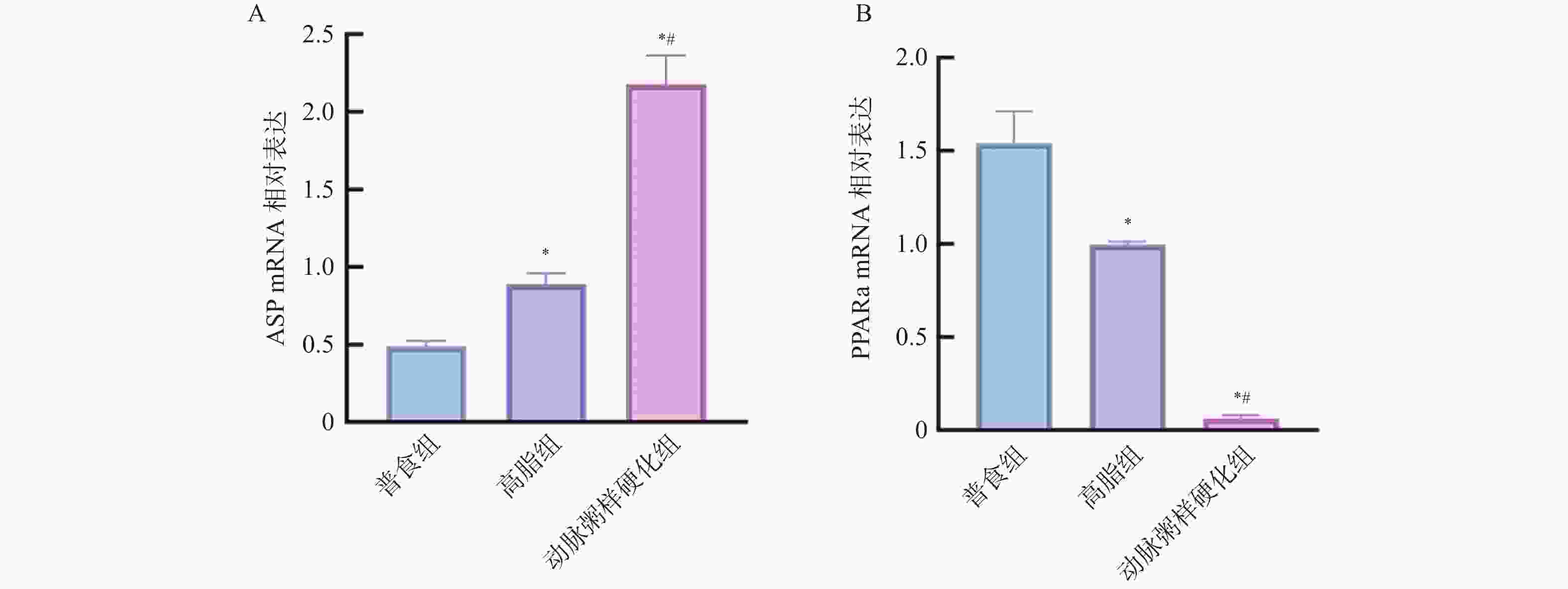
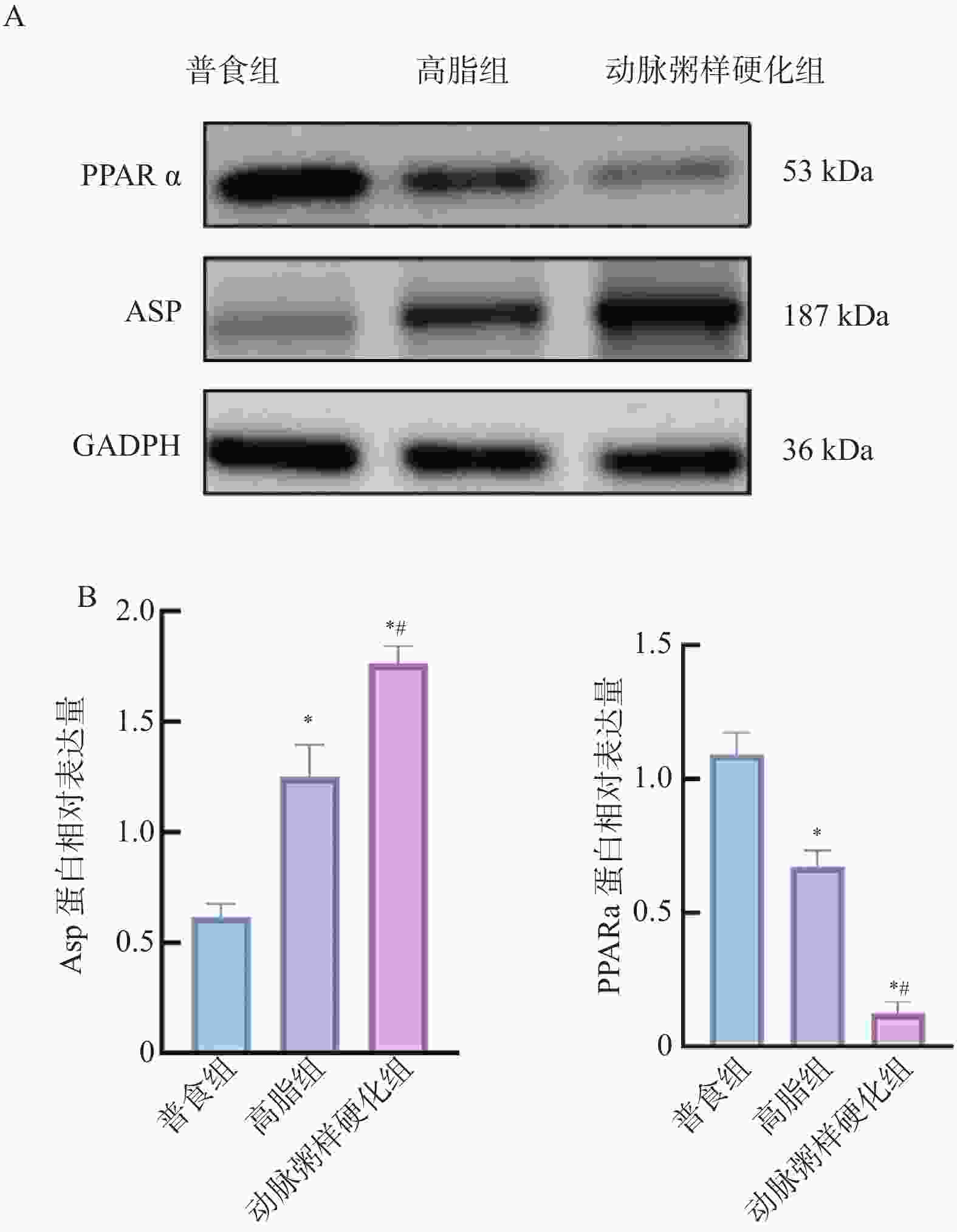
目的 探讨脂代谢相关基因ASP和PPARα在动脉粥样硬化(atherosclerosis,AS)中的作用。 方法 GEO数据库获取AS数据集GSE9874、GSE28829、GSE21545进行交集筛选出共同差异基因(differentially expressed genes,DEGs);对DEGs进行GO、KEGG富集分析;利用String数据库构建蛋白互作网络(protein-protein interaction,PPI),并使用Cytoscape软件筛选关键基因,同时,GeneMANIA数据库寻找关键基因的互作基因并利用HDOCK软件进行分子对接预测,进一步体内实验验证,用C57 小鼠构建动脉粥样硬化模型。采用油红O染色法观察各组小鼠全主动脉、主动脉窦斑块沉积面积;HE染色观察各组主动脉窦组织病理变化;Elisa法检测各组小鼠血脂水平及PCSK9、ASP和PPARα表达水平;RT-qPCR及WB检测主动脉组织中ASP、PPARα的mRNA和蛋白表达水平;CO-IP分析ASP和PPARα蛋白之间的相互作用。 结果 筛选出AS差异表达基因35个,主要富集于胆固醇转移活性、磷脂酰胆碱转运活性、ABC转运蛋白、吞噬体等方面。PPI和cytoscape的结果筛选出ASP为关键基因,且通过GeneMANIA数据库获得ASP的互作基因:PPARα,分子对接预测结果显示两者存在强相互作用并形成稳定结构。体内实验结果表明,与普食组相比,高脂组和动脉粥样硬化组小鼠主动脉、主动脉窦形态表现出不同程度损伤及斑块面积增加;血清中血脂水平显著升高及主动脉组织中ASP mRNA和蛋白水平均显著升高(P < 0.05),PPARα水平则相反;同高脂组相比,动脉粥样硬化组上述指标差异有统计学意义(P < 0.05);CO-IP分析证明ASP和PPARα存在相互作用,且敲低ASP小鼠动脉粥样硬化斑块及血脂水平降低,PPARα表达水平升高(P < 0.05)。 结论 ASP和PPARα参与了AS的发展,且可能与脂代谢密切相关。 -
关键词:
- 动脉粥样硬化 /
- 脂代谢 /
- 促酰化蛋白 /
- 过氧化物酶增殖核受体α
Abstract:Objective To investigate the roles of lipid metabolism-related genes ASP and PPARα in atherosclerosis (AS). Methods Datasets GSE9874, GSE28829, and GSE21545 were retrieved from the GEO database and intersected to identify common differentially expressed genes (DEGs). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed on the DEGs. Protein-protein interaction (PPI) networks were constructed using the String database, and critical genes were screened using Cytoscape software. Additionally, interacting genes of critical genes were identified using the GeneMANIA database, and molecular docking predictions were performed using HDOCK software, with further in vivo experimental validation, Atherosclerosis model was established in C57BL/6J mice. Oil Red O staining was used to observe plaque deposition areas in the whole aorta and aortic sinuses of each group. Hematoxylin-eosin (HE) staining was performed to observe pathological changes in aortic sinus tissues. ELISA was used to detect serum lipid levels and expression levels of PCSK9, ASP, and PPARα. Reverse transcription quantitative PCR (RT-qPCR) and Western blotting (WB) were used to detect mRNA and protein expression levels of ASP and PPARα in aortic tissues. Co-immunoprecipitation (CO-IP) analysis was performed to investigate the interaction between ASP and PPARα proteins. Results A total of 35 differentially expressed genes in AS were identified, primarily enriched in cholesterol transfer activity, phosphatidylcholine transport activity, ABC transporters, and autophagy pathways. PPI and Cytoscape analyses identified ASP as a critical gene. Through the GeneMANIA database, PPARα was identified as an interacting gene of ASP. Molecular docking predictions revealed a strong interaction between the two proteins forming a stable structure. In vivo experiments showed that compared with the normal diet group, the high-fat diet and atherosclerosis groups exhibited varying degrees of damage to aortic morphology with increased plaque area; serum lipid levels were significantly elevated and ASP mRNA and protein levels in aortic tissues were significantly increased (P < 0.05), while PPARα levels showed the opposite pattern. Compared with the high-fat diet group, these indicators in the atherosclerosis group showed statistically significant differences (P < 0.05). CO-IP analysis confirmed the interaction between ASP and PPARα. In ASP-knockdown mice, atherosclerotic plaques and serum lipid levels decreased while PPARα expression levels increased (P < 0.05). Conclusion ASP and PPARα participate in the development of atherosclerosis and may be closely related to lipid metabolism. -
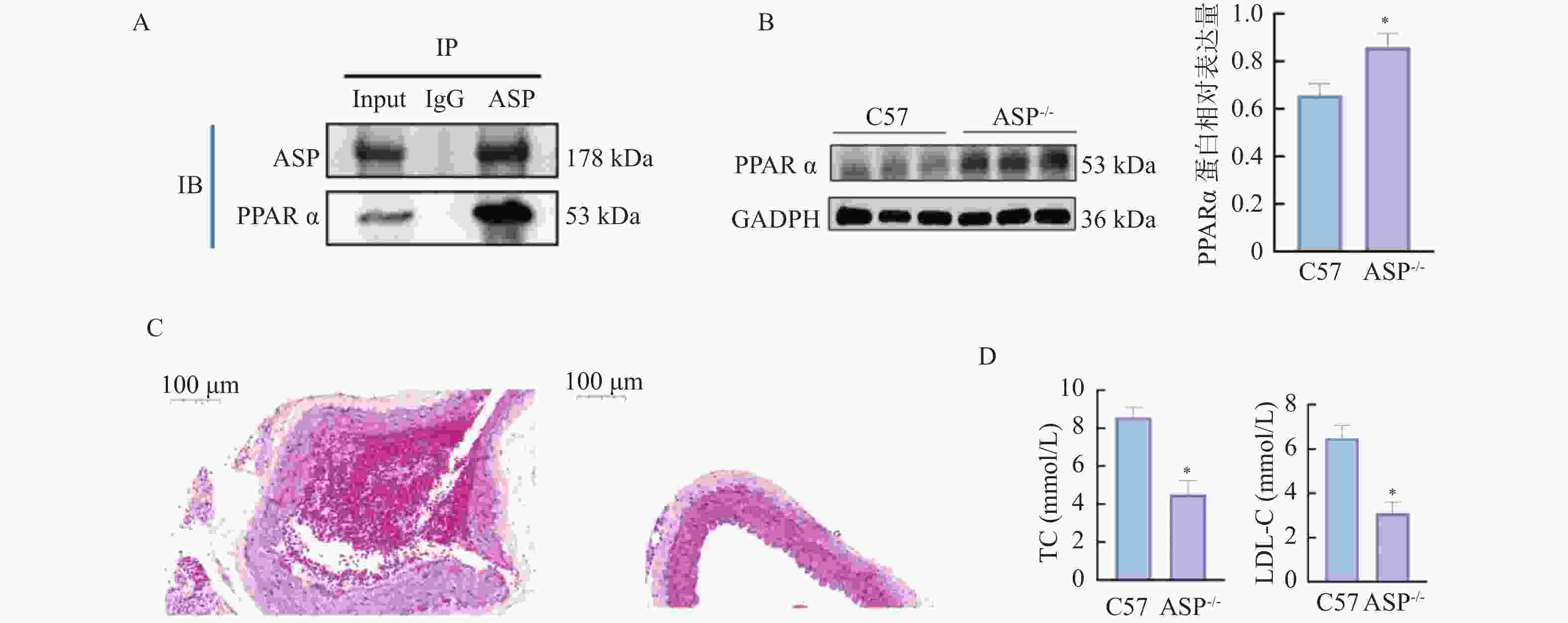
图 13 敲低ASP对小鼠动脉粥样硬化斑块、血脂及关键分子表达的影响($\bar x \pm s $,n = 12)
A:ASP和PPARα经CO-IP后Western blot检测结果;B:各组小鼠主动脉组织中PPARα蛋白相对表达水平;C:各组HE染色图(×40);D:各组小鼠血清中TC、LDL相对表达水平;与C57组比较,*P < 0.05。
Figure 13. Effects of ASP knockdown on atherosclerotic plaques,blood lipid profiles,and key molecule expression in mice($\bar x \pm s $,n = 12)
表 1 AS中基因微阵列数据集特征分析(n)
Table 1. Characterization of gene microarray datasets in AS(n)
数据集 平台信息 样品来源 对照样本数 疾病样本数 GSE9874 GPL570 外周血样本 30 30 GSE28829 GPL570 斑块组织样本 13 16 GSE21545 GPL570 斑块组织样本,外周血样本 97 126 表 2 引物序列
Table 2. Primer sequences
基因 序列(5'-3') 长度(bp) ASP(C3) Forward: CCA CCG CCA AGA ATC GCT ACT TC 23 Reverse: AGC AGC CTT GAC CTC CAC CTC 21 PPARα Forward: GAC AGT GAC AGA CAA CGG CAG TC 23 Reverse: AGG GTG GCA GGA AGG GAA CAG 21 GADPH Forwaed: AGG AGC GAA GAC CCC ACT AAC A 22 Reverse: AGG GGG GCT AAG CAG TTG GT 20 -
[1] Wang X, Xie Z, Zhang J, et al. Interaction between lipid metabolism and macrophage polarization in atherosclerosis[J]. iScience, 2025, 28(4): 112168. doi: 10.1016/j.isci.2025.112168 [2] 宋子凯, 李忠乐, 赵亚明, 等. 脂代谢紊乱破坏线粒体功能导致大鼠动脉粥样硬化形成[J]. 中国实验诊断学, 2024, 28(10): 1218-1224. doi: 10.3969/j.issn.1007-4287.2024.10.017 [3] Fisette A, Lapointe M, Cianflone K. Obesity-inducing diet promotes acylation stimulating protein resistance[J]. Biochem Biophys Res Commun, 2013, 437(3): 403-407. doi: 10.1016/j.bbrc.2013.06.090 [4] Cianflone K, Maslowska M, Sniderman A D. Acylation stimulating protein (ASP), an adipocyte autocrine: New directions[J]. Seminars in Cell & Developmental Biology, 1999, 10(1): 31-41. [5] Montaigne D, Butruille L, Staels B. PPAR control of metabolism and cardiovascular functions[J]. Nat Rev Cardiol, 2021, 18(12): 809-823. doi: 10.1038/s41569-021-00569-6 [6] Luo Y, Luo Y, Chang J, et al. Identification of candidate biomarkers and pathways associated with psoriasis using bioinformatics analysis[J]. Hereditas, 2020, 157(1): 30. doi: 10.1186/s41065-020-00141-1 [7] Wang S, Li X, Bi Y, et al. The impact of inflammation and iron metabolism on gene expression alterations in ischemic stroke: A bioinformatics approach[J]. Sci Rep, 2025, 15: 15233. doi: 10.1038/s41598-025-00369-9 [8] Xu M, Wu X, Liu Z, et al. A novel mouse model of diabetes, atherosclerosis and fatty liver disease using an AAV8-PCSK9-D377Y injection and dietary manipulation in db/db mice[J]. Biochem Biophys Res Commun, 2022, 622: 163-169. doi: 10.1016/j.bbrc.2022.07.031 [9] Chen M J, Xu Y T, Sun L, et al. A novel mouse model of familial combined hyperlipidemia and atherosclerosis[J]. Acta Pharmacol Sin, 2024, 45(6): 1316-1320. doi: 10.1038/s41401-024-01241-8 [10] Ketelhuth D F J. ApoB100-reactive T cells: Does liver tolerance hold the key to modulating adaptive immunity in atherosclerosis?[J]. J Intern Med, 2022, 291(5): 530-532. doi: 10.1111/joim.13436 [11] Ilyas I, Little P J, Liu Z, et al. Mouse models of atherosclerosis in translational research[J]. Trends Pharmacol Sci, 2022, 43(11): 920-939. doi: 10.1016/j.tips.2022.06.009 [12] Sukhorukov V N, Khotina V A, Chegodaev Y S, et al. Lipid metabolism in macrophages: Focus on atherosclerosis[J]. Biomedicines, 2020, 8(8): 262. doi: 10.3390/biomedicines8080262 [13] Emini Veseli B, Perrotta P, De Meyer G R A, et al. Animal models of atherosclerosis[J]. Eur J Pharmacol, 2017, 816: 3-13. doi: 10.1016/j.ejphar.2017.05.010 [14] Ding L, Chang M, Guo Y, et al. Trimethylamine-N-oxide (TMAO)-induced atherosclerosis is associated with bile acid metabolism[J]. Lipids Health Dis, 2018, 17(1): 286. doi: 10.1186/s12944-018-0939-6 [15] Li J, Lin S, Vanhoutte P M, et al. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in apoe-/ - mice[J]. Circulation, 2016, 133(24): 2434-2446. doi: 10.1161/CIRCULATIONAHA.115.019645 [16] 林瑞挺, 张博, 黄灿霞, 等. 冠状动脉粥样硬化性心脏病患者血清补体C3、C4水平与HDL-C的相关性分析[J]. 中山大学学报(医学科学版), 2017, 38(1): 72-77. doi: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2017.0011 [17] Zhu B, Zhai Y, Ji M, et al. Alisma orientalis beverage treats atherosclerosis by regulating gut microbiota in ApoE (-/-) mice[J]. Front Pharmacol, 2020, 11: 570555. doi: 10.3389/fphar.2020.570555 [18] Geng J, Yang C, Wang B, et al. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway[J]. Biomed Pharmacother, 2018, 97: 941-947. doi: 10.1016/j.biopha.2017.11.016 [19] Garcia-Arguinzonis M, Diaz-Riera E, Peña E, et al. Alternative C3 complement system: Lipids and atherosclerosis[J]. Int J Mol Sci, 2021, 22(10): 5122. doi: 10.3390/ijms22105122 [20] Zhou F, Yang L, Sun W, et al. The PPARα/CYP4A14 bile acid pathway is associated with lipid metabolism disorders caused by low birth weight with high-fat diet[J]. Food Nutr Res, 2023, 67. [21] Mooli R G R, Rodriguez J, Takahashi S, et al. Hypoxia via ERK signaling inhibits hepatic PPARα to promote fatty liver[J]. Cell Mol Gastroenterol Hepatol, 2021, 12(2): 585-597. doi: 10.1016/j.jcmgh.2021.03.011 [22] Li F H, Huang X L, Wang H, et al. Protective effect of Yi-Qi-Huo-Xue Decoction against ischemic heart disease by regulating cardiac lipid metabolism[J]. Chin J Nat Med, 2020, 18(10): 779-792. [23] Hennuyer N, Duplan I, Paquet C, et al. The novel selective PPARα modulator (SPPARMα) pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis[J]. Atherosclerosis, 2016, 249: 200-208. doi: 10.1016/j.atherosclerosis.2016.03.003 [24] Jiang H, Guo M, Dong L, et al. Levels of acylation stimulating protein and the complement component 3 precursor are associated with the occurrence and development of coronary heart disease[J]. Exp Ther Med, 2014, 8(6): 1861-1866. doi: 10.3892/etm.2014.2018 [25] Li A C, Binder C J, Gutierrez A, et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma[J]. J Clin Invest, 2004, 114(11): 1564-1576. doi: 10.1172/JCI18730 -






 下载:
下载: