Analysis of Factors Influencing Recurrence in Osteosarcoma Patients and Construction of Nomogram Prediction Model
-
摘要:
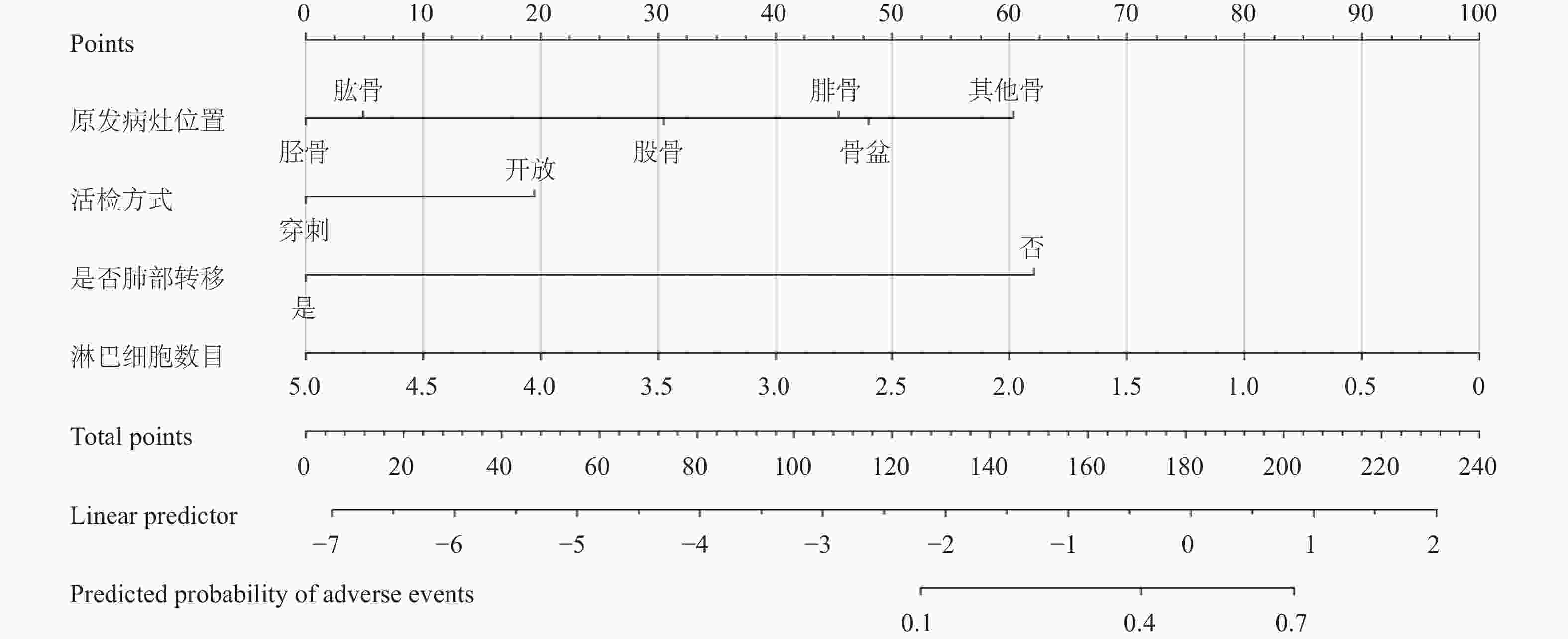
目的 分析影响骨肉瘤患者复发的关键临床影响因素,构建并验证一个基于Nomogram的复发风险预测模型。 方法 回顾性收集2013-2022年在云南省肿瘤医院收治的469例患者临床资料,运用R软件(V4.3.2)进行统计学分析。通过单因素分析和LASSO回归分析初步筛选潜在影响因素,通过多因素Logistic回归分析影响骨肉瘤复发的独立预测因子。基于筛选出的独立影响因素,构建复发风险的列线图(Nomogram)预测模型。采用受试者工作特征曲线(ROC)下面积(AUC)评估模型的区分度。 结果 全队列中,68例患者出现复发,复发率为14.50%。原发病灶位置(胫骨OR = 0.297和其他骨OR = 3.294)、活检方式穿刺(OR = 0.461)、发生肺部转移(OR = 11.873)、高淋巴细胞数目(OR = 0.450)为复发的独立预测因素。胫骨的复发风险低于股骨(P = 0.009),其他骨的复发风险高于股骨(P = 0.008);开放活检患者的复发风险高于穿刺活检患者(P = 0.033);发生肺转移患者的复发风险高于非肺转移患者(P < 0.001);淋巴细胞数越高复发风险越低(P = 0.001)。模型验证的ROC曲线下面积为0.842(95%CI:0.806~0.875),表明模型具有较好的判别能力。 结论 本研究成功构建并验证了一个整合关键临床因素的骨肉瘤复发风险Nomogram预测模型,该模型展现出较好的区分度,能精准、量化地评估个体患者的复发风险。 -
关键词:
- 骨肉瘤 /
- 复发风险 /
- 影响因素 /
- Nomogram预测模型
Abstract:Objectives To identify key clinical factors influencing recurrence in osteosarcoma patients, to construct and validate a Nomogram-based recurrence risk prediction model, thereby providing a quantitative tool for clinical decision-making and recurrence prevention/control. Methods Clinical data of 469 osteosarcoma patients admitted to Yunnan Cancer Hospital between 2013~2022 were retrospectively collected. Statistical analysis was performed using R software (version 4.3.2). Potential influencing factors were initially screened via univariate analysis and LASSO regression analysis. Independent predictors of osteosarcoma recurrence were then identified using multivariate logistic regression analysis. Based on the identified independent factors, a Nomogram prediction model for recurrence risk was constructed. The area under the receiver operating characteristic curve (AUC) was used to evaluate the model's discriminative ability. Results Among the entire cohort, 68 patients experienced recurrence, yielding a recurrence rate of 14.50%. Multivariate analysis identified the following as independent predictors of recurrence: Primary Tumor Location: Tibial lesions (P = 0.009) were associated with a significantly lower recurrence risk compared to femoral lesions (OR = 0.297), while lesions in "Other Bones" (P = 0.008) carried a significantly higher risk (OR = 3.294). Biopsy Method: Needle biopsy(P = 0.033) was associated with a significantly lower recurrence risk compared to open biopsy (OR = 0.461). Lung Metastasis Status: Patients with lung metastasis (P < 0.001) had a significantly higher recurrence risk than those without (OR = 11.873). Lymphocyte Count: A higher lymphocyte count (P = 0.001) was a protective factor, associated with a lower recurrence risk (OR = 0.450). The constructed Nomogram prediction model demonstrated excellent performance: Validation results showed an AUC=0.842(95%CI: 0.806~0.875), indicating outstanding discriminative ability. Conclusions This study successfully constructed and validated a Nomogram prediction model for osteosarcoma recurrence risk integrating key clinical factors. The model demonstrates superior discriminative ability and can accurately and quantitatively assess the recurrence risk for individual patients. This tool thus provides critical reference for guiding clinical treatment decisions. -
Key words:
- Osteosarcoma /
- Recurrence risk /
- Influencing factors /
- Nomogram prediction model
-
表 1 骨肉瘤复发的单因素分析[n(%)/M(P25,P75)]
Table 1. Univariate analysis of factors associated with osteosarcoma recurrence[n(%)/M(P25,P75)]
指标 分类 未复发 复发 χ2/Z P 性别 男 256(86.5) 40(13.5) 0.629 0.428 女 145(83.8) 28(16.2) 民族 汉族 332(85.6) 56(14.4) 0.008 0.929 少数民族 69(85.2) 12(14.8) 吸烟史 无 384(85.3) 66(14.7) 0.252 0.616 有 17(89.5) 2(10.5) 原发病灶位置 股骨 191(84.5) 35(15.5)c 19.973 0.001* 胫骨 117(93.6) 8(6.4)d 肱骨 38(90.5) 4(9.5)d 腓骨 20(80.0) 5(20.0)b 骨盆 7(70.0) 3(30.0)a 其他骨 28(68.3) 13(31.7)a 肿瘤分期 IIA期 55(94.8) 3(5.2)c 13.790 0.001* IIB期 288(86.7) 44(13.3)b Ⅲ期 58(73.4) 21(26.6)a Enneking手术分期 IIA期 52(94.5) 3(5.5)c 43.082 < 0.001* IIB期 303(89.4) 36(10.6)b Ⅲ期 46(61.3) 29(38.7)a 肿瘤最大直径 < 10cm 220(83.7) 43(16.3) 1.655 0.198 ≥10cm 181(87.9) 25(12.1) 病理性骨折 无 361(84.7) 65(15.3) 2.161 0.142 有 40(93.0) 3(7.0) 活检方式 开放 261(83.1) 53(16.9) 4.341 0.037* 穿刺 140(90.3) 15(9.7) 手术方式 保肢手术 231(83.4) 46(16.6) 2.424 0.119 截肢手术 170(88.5) 22(11.5) 乙肝核心抗体 阴性 372(85.9) 61(14.1) 0.769 0.380 阳性 29(80.6) 7(19.4) 乙型肝炎表面抗体 阴性 247(85.2) 43(14.8) 0.066 0.797 阳性 154(86.0) 25(14.0) 是否肺部转移 否 293(94.8) 16(5.2) 63.478 < 0.001* 是 108(67.5) 52(32.5) 年龄(岁) 17(13.5,24) 19(14.25,32.5) −1.642 0.101 BMI(kg/m2) 19.37(17.31,21.96) 19.99(17,23.39) −0.862 0.389 随访时间(月) 31(10,56) 19.5(12,43.25) −1.349 0.177 嗜碱性粒细胞(×10⁹/L) 0.02(0.01,0.03) 0.02(0.01,0.03) −0.112 0.911 嗜酸性粒细胞(×10⁹/L) 0.1(0.06,0.18) 0.09(0.04,0.14) −1.554 0.120 淋巴细胞数目(×10⁹/L) 2(1.57,2.5) 1.79(1.33,2.31) −2.755 0.006* 红细胞平均血红蛋白浓度(g/L) 332(325,340) 334(327.25,340) −1.140 0.254 平均红细胞体积(fL) 86.9(83.68,90.2) 85.4(83.03,89.25) −0.879 0.379 单核细胞计数(×10⁹/L) 0.4(0.32,0.52) 0.44(0.3,0.63) −0.397 0.691 中性粒细胞计数(×10⁹/L) 4.4(3.34,5.79) 4.18(3.05,5.83) −0.747 0.455 降钙素(mmol/L) 0.3(0.26,0.36) 0.29(0.24,0.36) −1.735 0.083 细胞体积大小变异系数(%) 13(12.6,13.6) 12.7(12.35,13.48) −2.008 0.045* 红细胞分布宽度标准差(fL) 41.2(39.08,43.63) 40.55(38.63,42.38) −1.792 0.073 腺苷脱氨酶(U/L) 10(8,12) 9(7,11) −1.749 0.080 碱性磷酸酶(U/L) 184.5(110.25,315.5) 175.5(99,357.75) −0.379 0.705 尿素氮(mmol/L) 4.54(3.74,5.53) 4.32(3.76,5.19) −0.899 0.369 肿瘤标记物(ng/mL) 2.4(2.33,2.48) 2.38(2.28,2.45) −1.543 0.123 胆碱酯酶(U/L) 8229.5 (7140 ,9352.25 )8052 (7149 ,9956.25 )−0.810 0.418 二氧化碳(mmol/L) 23(22,25) 23(21,25) −0.013 0.989 肌酐(μmol/L) 65.5(52,76) 65.5(52.25,79) −0.176 0.860 C-反应蛋白(mg/L) 4.87(1.66,16.72) 4.35(1.15,18.25) −0.541 0.589 血清铁(μmol/L) 12.15(8.1,17.6) 12.95(9.03,19.23) −0.981 0.327 谷氨酰胺转肽酶((U/L)) 18(12,29) 19.5(13,31) −1.384 0.166 球蛋白(g/L) 28(25.95,31) 27.4(25,30.75) −1.410 0.158 钾离子(mmol/L) 4.37(4.14,4.59) 4.34(4.11,4.53) −1.037 0.300 乳酸脱氢酶(U/L) 192(161,258.25) 200(164.5,235.75) −0.146 0.884 总蛋白(g/L) 74(70,78) 73.5(69,77) −1.016 0.310 肿瘤特异性生长因子(U/mL) 54(45,61) 58.5(47,63) −1.660 0.097 APF(μg/L) 1.74(1.19,2.66) 1.92(1.36,2.55) −1.254 0.210 CA125(U/mL) 11.81(8.72,16.41) 13.52(9.68,17.8) −1.500 0.134 CA153(U/mL) 9.99(7.44,14.39) 9.89(7.67,17.33) −0.673 0.501 CA199(U/mL) 7.37(4.35,12.96) 8.55(5.1,13.01) −0.777 0.437 CA242(U/mL) 5(2.38,10.09) 6.14(3.34,9.62) −0.958 0.338 CA724(U/mL) 1.55(0.99,3.28) 1.49(0.89,2.5) −1.283 0.200 CA211(U/mL) 2.1(1.6,2.7) 2.3(1.6,3.3) −1.301 0.193 癌胚抗原(ng/mL) 1.27(0.84,1.94) 1.13(0.79,1.94) −0.329 0.742 铁蛋白铁(ug/L) 180.05(87.73,301.45) 187.1(104.3,323.9) −0.224 0.823 神经元特异性烯醇化酶(ng/mL) 17.03(13.14,23.75) 18.04(13.23,23.39) −0.366 0.715 鳞状细胞癌抗原(ng/mL) 0.85(0.67,1.1) 0.9(0.73,1.2) −0.725 0.468 注:*P < 0.05;a、b、c和d代表复发率存在统计学差异,标记a > 标记b > 标记c > 标记d. 表 2 LASSO回归分析结果表
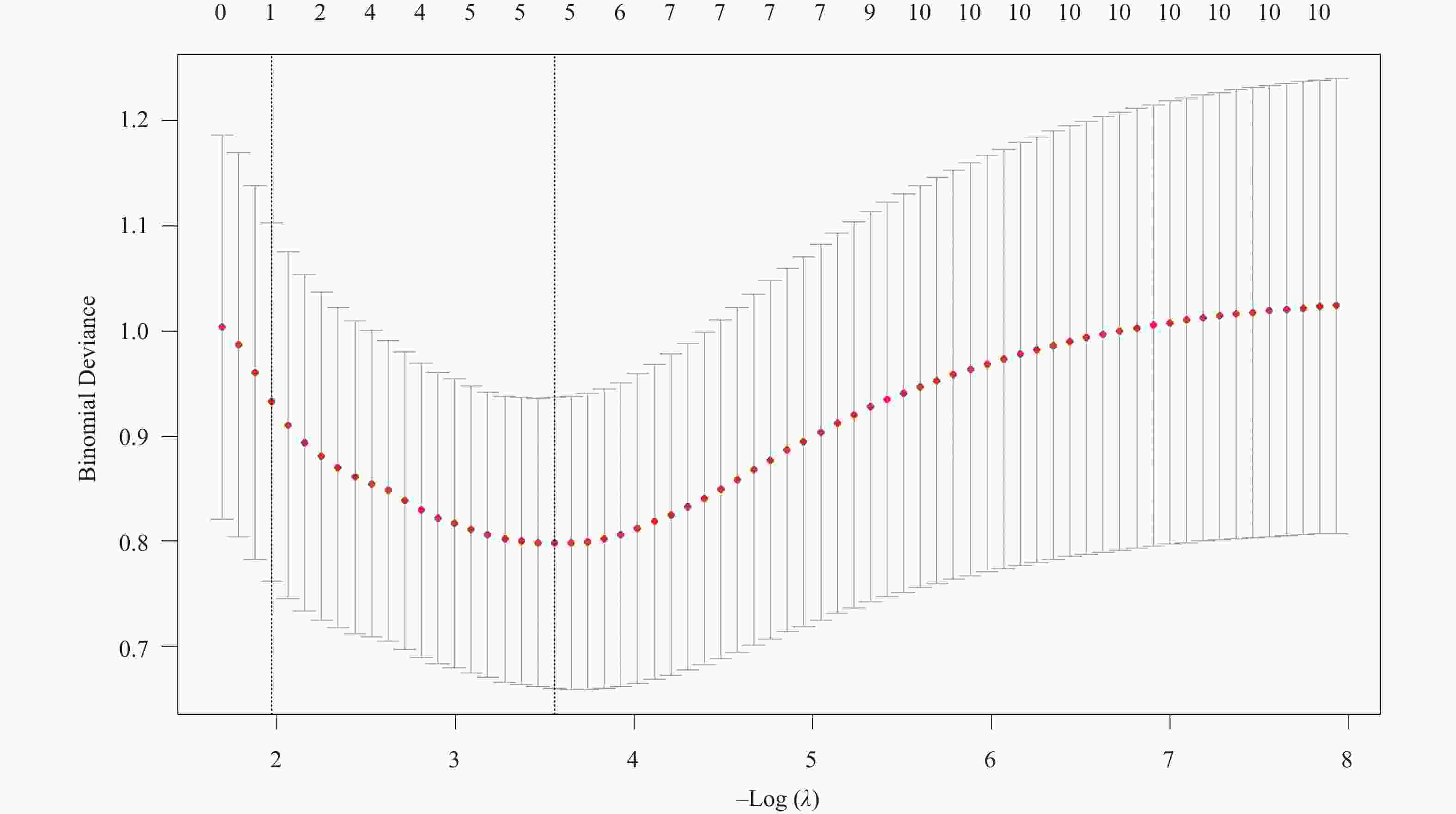
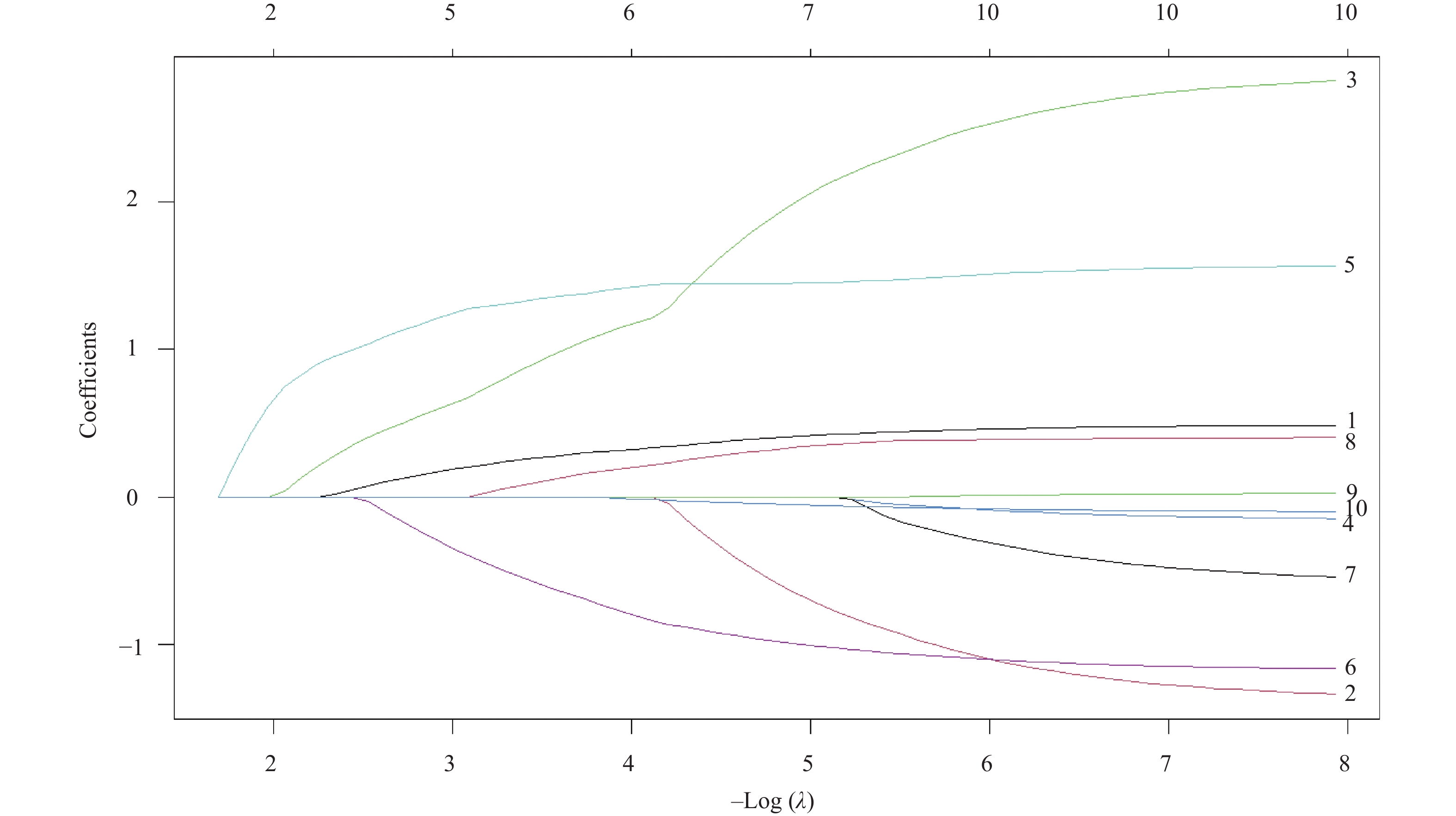
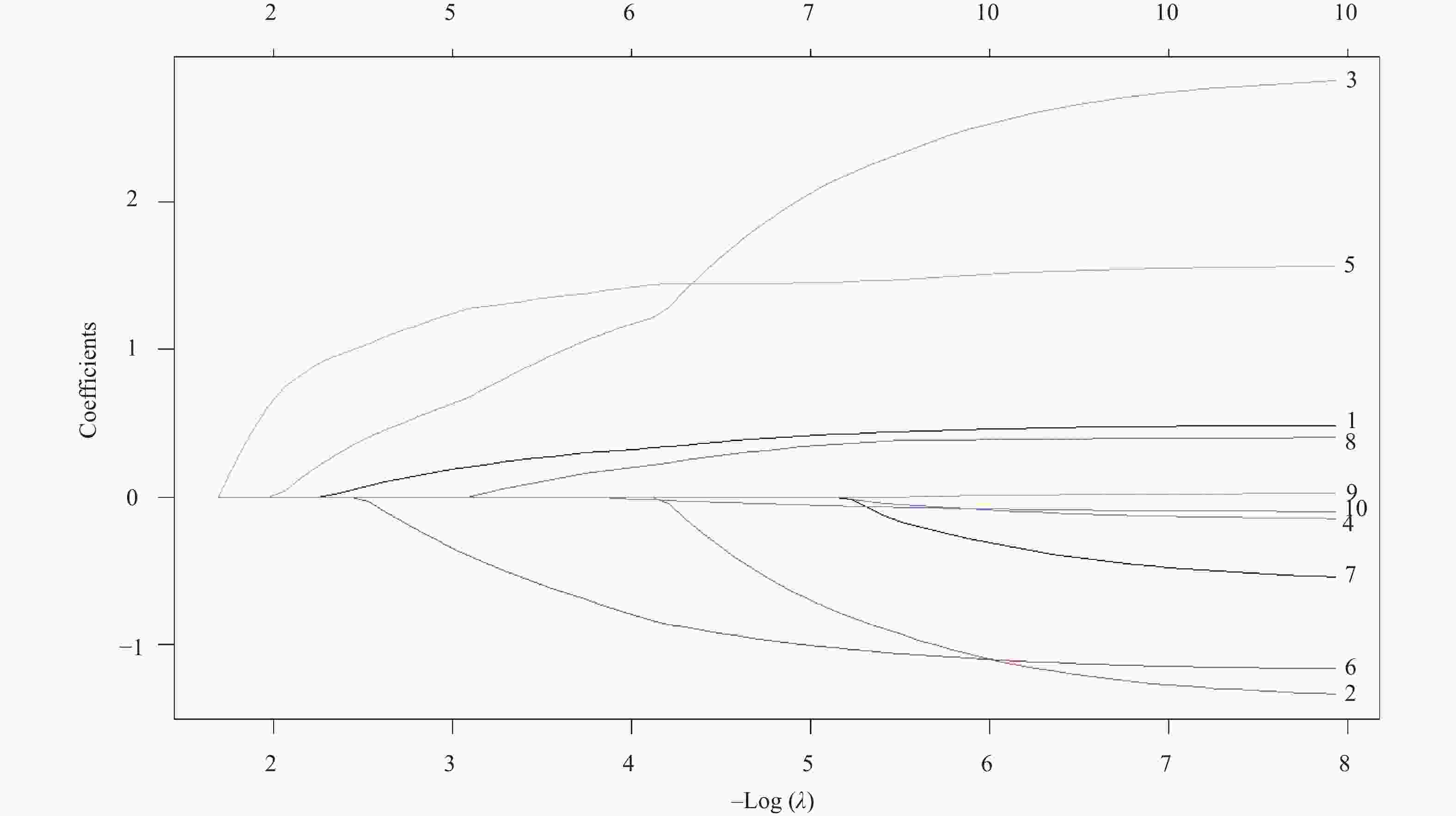
Table 2. Results of Lasso regression analysis
变量名称 λ值 截断系数 − 5.27802 原发病灶位置 0.27514 肿瘤分期 − Enneking手术分期 − 活检方式 0.95991 是否肺部转移 1.34897 淋巴细胞数目(×109/L) − 0.61676 降钙素(mmol/L) − 细胞体积大小变异系数(%) 0.12053 红细胞分布宽度标准差(fL) − 腺苷脱氨酶(U/L) − 肿瘤特异性生长因子(U/mL) − 注:“−”表示零系数,即该变量未进入模型。 表 3 指标的赋值说明表
Table 3. The assignment description of indicators
指标名称 赋值情况说明 原发病灶位置 1 = 股骨;2 = 胫骨;3 = 肱骨;
4 = 腓骨;5 = 骨盆;6 = 其他骨活检方式 1 = 开放;2 = 穿刺 是否肺部转移 0 = 否;1 = 是 淋巴细胞数目(×109/L) 连续变量 细胞体积大小变异系数(%) 连续变量 表 4 骨肉瘤复发二分类多因素Logistic回归分析
Table 4. Multivariate Binary Logistic regression analysis for osteosarcoma recurrence
变量 β 标准误S.E Wald P OR 95%CI 原发病灶位置(参照:股骨) − − 21.336 0.001* 1.000 − 胫骨 −1.214 0.468 6.734 0.009* 0.297 0.119~0.743 肱骨 −1.016 0.609 2.785 0.095 0.362 0.110~1.194 腓骨 0.595 0.622 0.915 0.339 1.813 0.536~6.132 骨盆 0.699 0.887 0.620 0.431 2.011 0.353~11.448 其他骨 1.192 0.452 6.972 0.008* 3.294 1.360~7.982 活检方式(参照:开放) −0.775 0.363 4.562 0.033* 0.461 0.226~0.938 是否肺部转移(参照:否) 2.474 0.347 50.965 < 0.001* 11.873 6.019~23.420 淋巴细胞数目 −0.797 0.249 10.297 0.001* 0.450 0.277~0.733 常量 −1.240 0.511 5.891 0.015 0.289 − *P < 0.05。 表 5 骨肉瘤复发Nomogram预测模型概率与是否复发的RO曲线分析
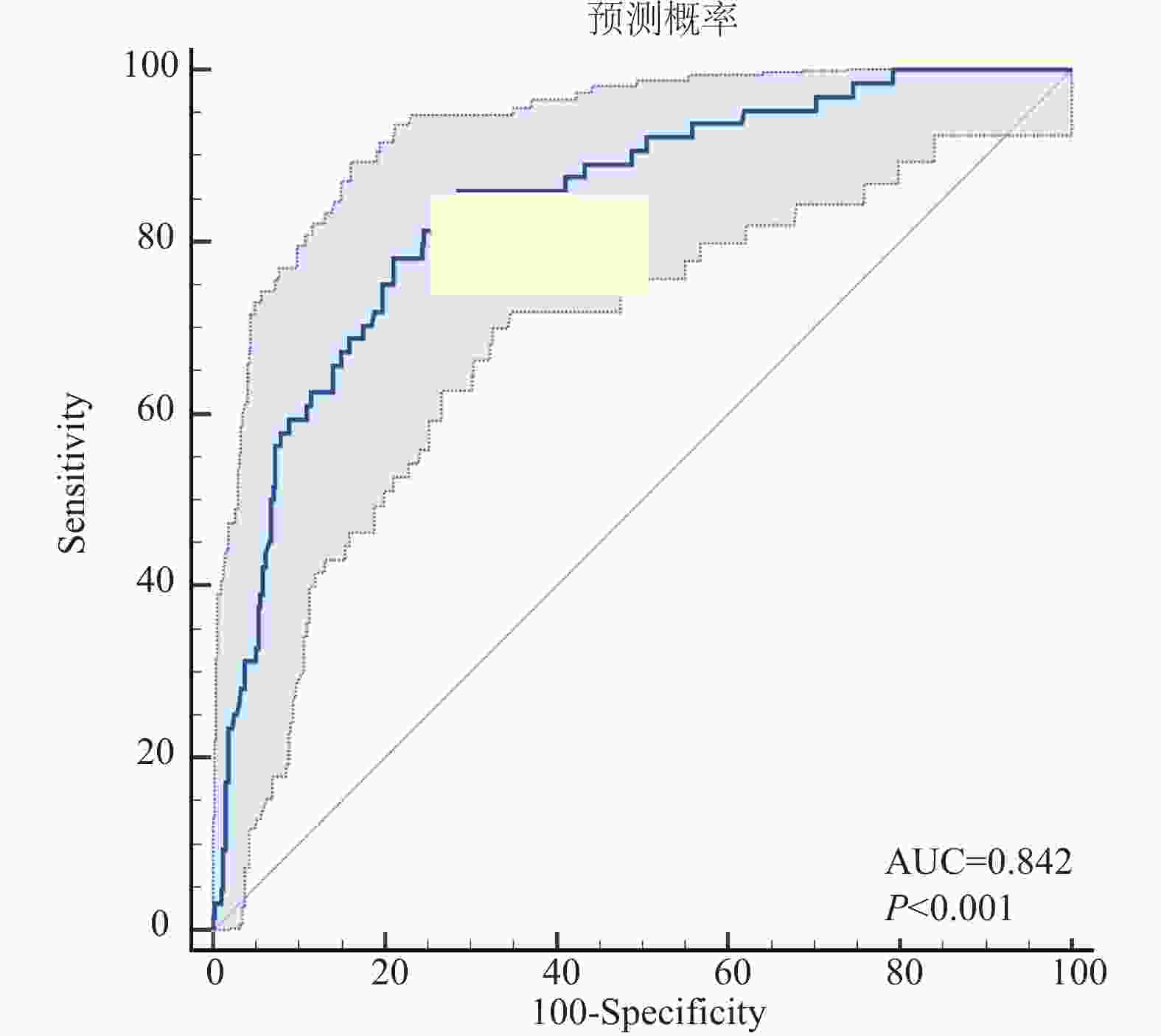
Table 5. The ROC curve of the Nomogram prediction model probability versus actual recurrence status for osteosarcoma recurrence
指标 ACU 95%CI SE P Youden指数 截断值 灵敏度(%) 特异度(%) 模型概率 0.842 0.806~0.875 0.0264 < 0.0001 0.5772 > 0.116 84.37 73.35 -
[1] Yoshida A. Osteosarcoma: Old and new challenges[J]. Surg Pathol Clin, 2021, 14(4): 567-583. doi: 10.1016/j.path.2021.06.003 [2] Danese M D, Groundland J S. Effect of chemotherapy and surgery timing on mortality in upper and lower extremity osteosarcoma[J]. J Natl Cancer Inst, 2025, 117(4): 611-618. doi: 10.1093/jnci/djae229 [3] Beird H C, Bielack S S, Flanagan A M, et al. Osteosarcoma[J]. Nat Rev Dis Primers, 2022, 8: 77. doi: 10.1038/s41572-022-00409-y [4] Lacinski R A, Dziadowicz S A, Melemai V K, et al. Spatial multiplexed immunofluorescence analysis reveals coordinated cellular networks associated with overall survival in metastatic osteosarcoma[J]. Bone Res, 2024, 12(1): 55. doi: 10.1038/s41413-024-00359-z [5] Xie D, Hu C, Zhu Y, et al. Sequential therapy for osteosarcoma and bone regeneration via chemodynamic effect and cuproptosis using a 3D-printed scaffold with TME-responsive hydrogel[J]. Small, 2025, 21(5): e2406639. doi: 10.1002/smll.202406639 [6] Ding W Z, Liu K, Li Z, et al. A meta-analysis of prognostic factors of osteosarcoma[J]. Eur Rev Med Pharmacol Sci, 2020, 24(8): 4103-4112. [7] Balachandran V P, Gonen M, Smith J J, et al. Nomograms in oncology: More than meets the eye[J]. Lancet Oncol, 2015, 16(4): e173-e180. doi: 10.1016/S1470-2045(14)71116-7 [8] 郭卫. 日本骨科协会(JOA)原发恶性骨肿瘤管理临床实践指南重点内容解读[J]. 中国修复重建外科杂志, 2025, 39(7): 814-823. [9] 马国玉, 黄瑾, 杨鑫, 等. 骨肉瘤患者肺转移影响因素分析及预测模型的构建与验证[J]. 肿瘤学杂志., 2025, 31(3): 231-237. [10] 李嘉伊, 任庆兰. 晚期骨肉瘤分子靶向治疗研究进展[J]. 现代医药卫生., 2019, 35(19): 2996-2999. [11] Yao Z, Tan Z, Yang J, et al. Prognostic nomogram for predicting 5-year overall survival in Chinese patients with high-grade osteosarcoma[J]. Sci Rep, 2021, 11(1): 17728. doi: 10.1038/s41598-021-97090-0 [12] 唐顺, 郭卫, 杨荣利, 等. 40岁以上成年骨肉瘤患者的外科治疗及预后因素[J]. 北京大学学报(医学版)., 2015, 47(1): 165-169. [13] 吕卫星, 周胜利, 严米云, 等. 甲氨蝶呤联合雷公藤多苷片治疗骨肉瘤的临床效果及对炎症因子、复发率的影响[J]. 世界复合医学, 2024, 10(10): 78-81. [14] Andreou D, Bielack S S, Carrle D, et al. The influence of tumor- and treatment-related factors on the development of local recurrence in osteosarcoma after adequate surgery. An analysis of 1355 patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols[J]. Ann Oncol, 2011, 22(5): 1228-1235. doi: 10.1093/annonc/mdq589 [15] Koirala P, Roth M E, Gill J, et al. Immune infiltration and PD-L1 expression in the tumor microenvironment are prognostic in osteosarcoma[J]. Sci Rep, 2016, 6: 30093. doi: 10.1038/srep30093 [16] Strauss S J, Frezza A M, Abecassis N, et al. Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up[J]. Ann Oncol, 2021, 32(12): 1520-1536. doi: 10.1016/j.annonc.2021.08.1995 [17] Abd Elmoneim H M, Huwait H F, Nafady-Hego H, et al. Prognostic implications of pd-l1 expression and loss of pten in patients with rhabdomyosarcoma, Ewing’s sarcoma and osteosarcoma[J]. Exp Oncol, 2024, 45(3): 337-350. [18] 闫广宁, 喻玲, 赖续文, 等. 骨肉瘤中PD-1和CTLA-4的表达与患者临床病理特征及预后的相关性[J]. 肿瘤防治研究., 2023, 50(1): 63-68. [19] 褚吉祥, 冯超凡, 何喆, 等. 影像组学-临床特征联合模型预测骨肉瘤术后复发的价值[J]. 现代肿瘤医学, 2025, 6(16): 1-9. [20] 乔相帅, 郑凯, 徐明, 等. 肿瘤生长方式影像学表现对骨肉瘤预后影响的临床研究[J]. 骨科., 2024, 15(4): 332-338. -





 下载:
下载: