Mechanism on Glu/GABA Regulating Neurotransmitter Balance to Improve Behavioral Abnormalities in Rat Models of Tourette Syndrome
-
摘要:
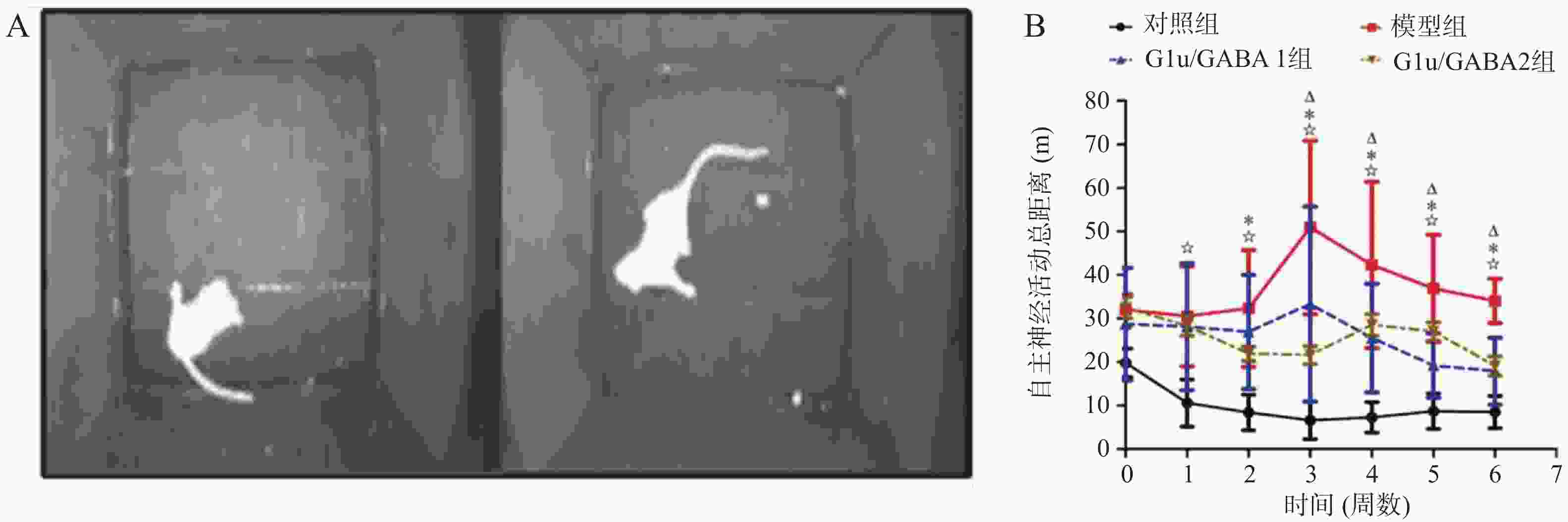
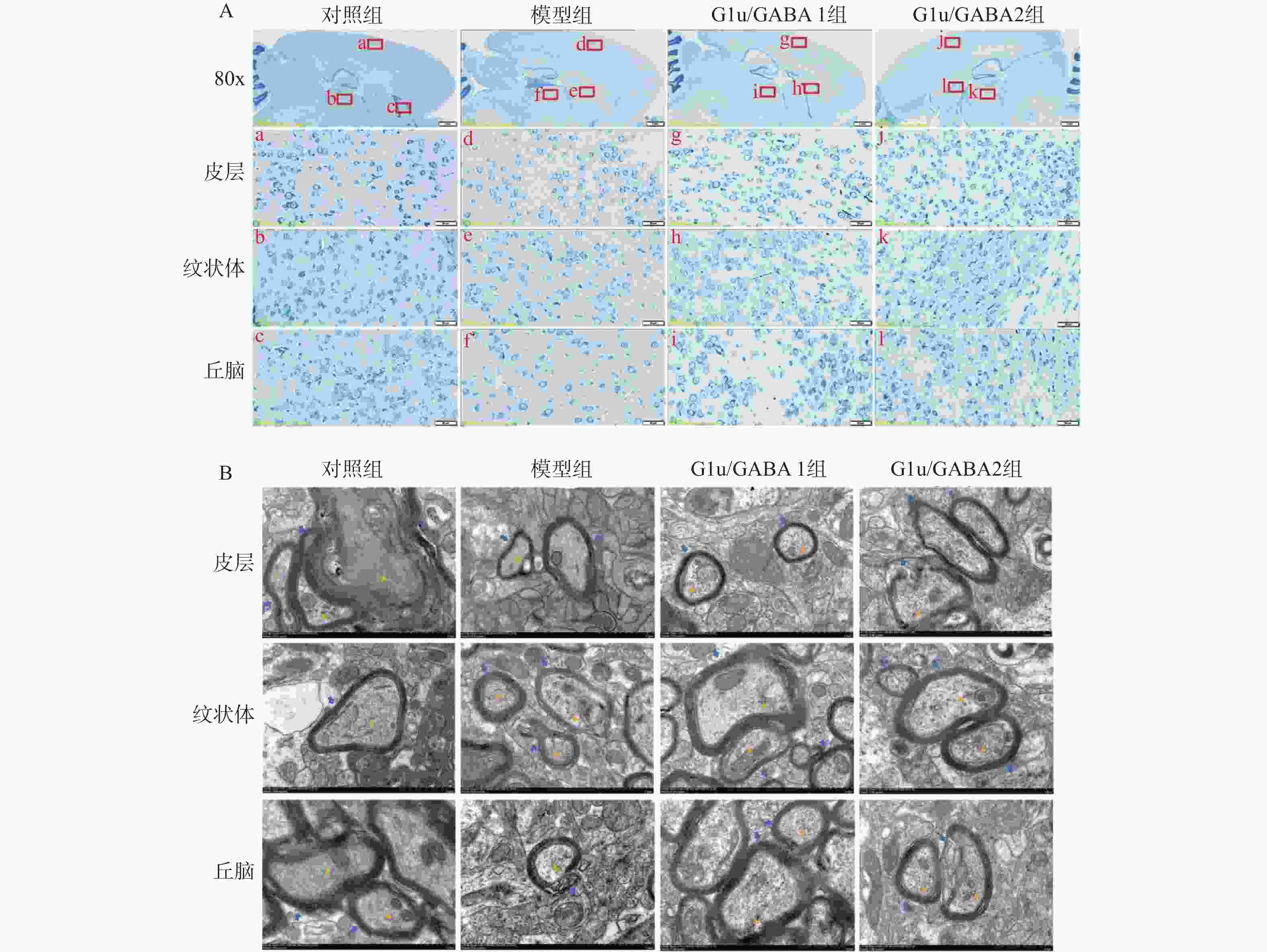
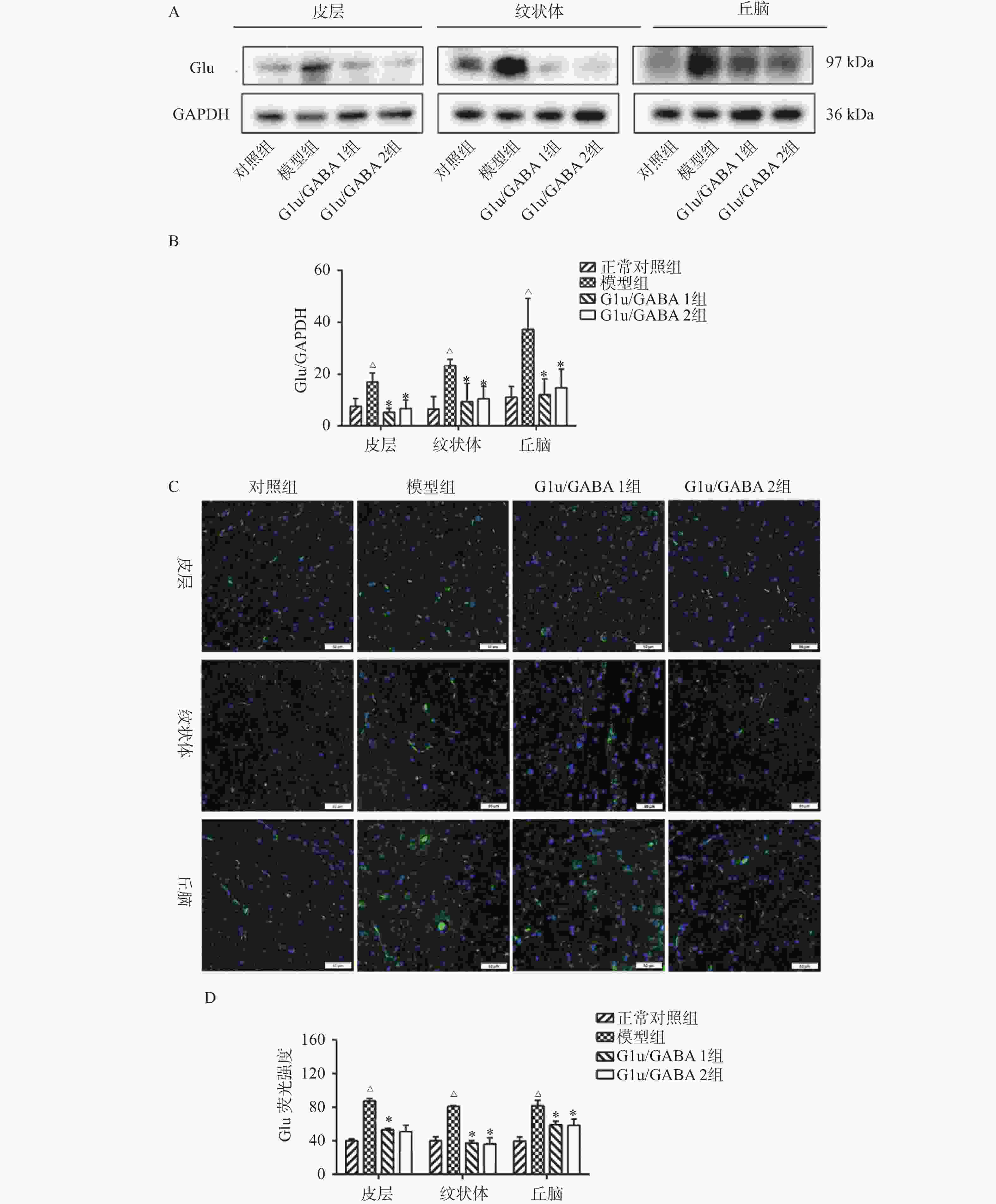
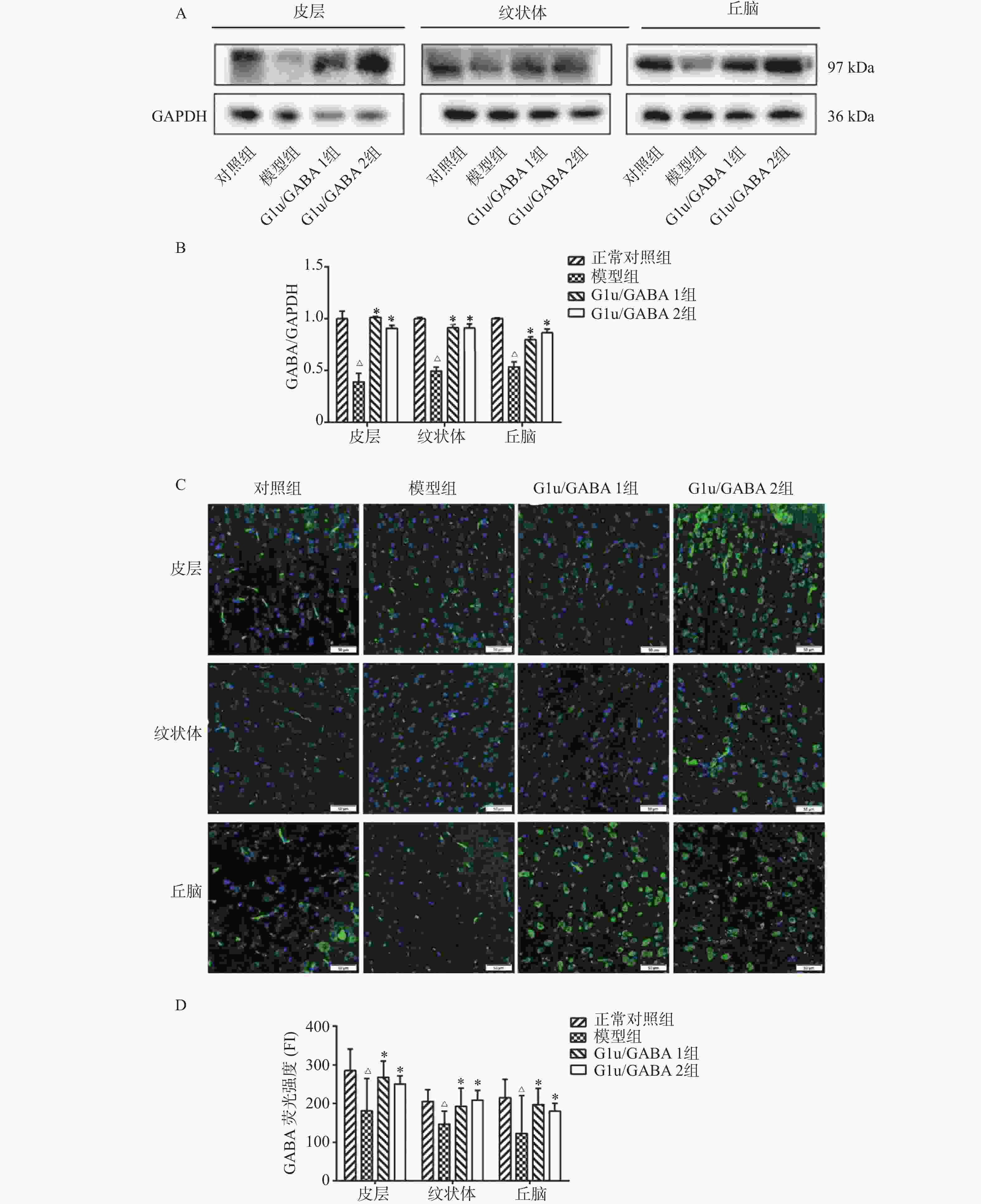
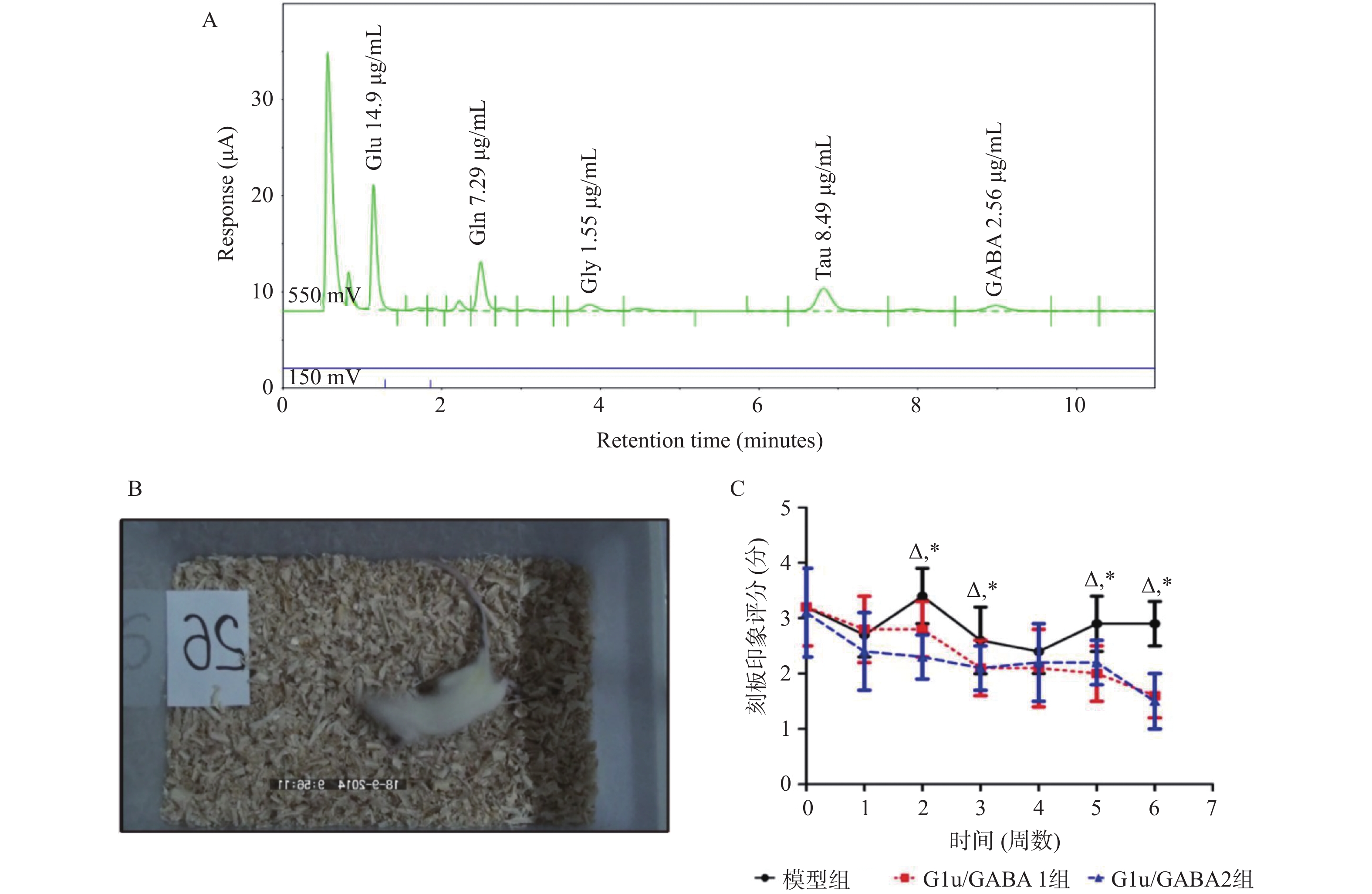
目的 基于Glu/GABA调节神经递质平衡改善抽动秽语综合征(Tourette syndrome,TS)大鼠模型行为异常的机制研究。 方法 选用 48 只 SPF 级 SD 大鼠,随机分为正常对照组、模型组、Glu/GABA 1 组(10 mg/kg)和 Glu/GABA 2 组(20 mg/kg),每组 12 只。除正常对照组外,其余大鼠腹腔注射 250 mg/(kg·d)IDPN 连续 7 d建立 TS 模型,造模成功后各组灌胃干预 6 周。分别于干预前、第 3 周、第 6 周采用盲法进行刻板行为评分,通过自发活动视频系统检测自主活动总距离。干预结束后取材,HE 染色计数皮层、纹状体、丘脑神经元数量,透射电镜观察髓鞘形态;高效液相色谱法检测脑组织 Glu 和 GABA 水平,免疫荧光法测定其荧光强度,以探究 Glu/GABA 对 TS 模型大鼠的影响。 结果 模型组及 Glu/GABA 干预组大鼠刻板行为评分、自主活动总距离均显著高于正常对照组(P < 0.05)。干预 6 周后,Glu/GABA 1 组和 2 组刻板行为评分、自主活动总距离较模型组显著降低(P < 0.001),且两组间无统计学差异(P > 0.05)。模型组皮层、纹状体、丘脑神经元数量减少,髓鞘板层疏松、轴突破坏;Glu/GABA 干预后神经元数量恢复(P < 0.01),髓鞘结构改善。与正常组相比,模型组脑内 Glu 水平及荧光强度显著升高、GABA 显著降低(P < 0.01);Glu/GABA 干预可逆转上述变化(P < 0.01)。 结论 Glu/GABA可有效降低TS模型大鼠刻板行为评分和自主活动距离,修复皮层、纹状体及丘脑的神经元损伤与髓鞘异常,调节脑内Glu和GABA水平至正常平衡状态,发挥对TS的治疗作用。 -
关键词:
- Glu/GABA /
- 神经递质 /
- 抽动秽语综合征大鼠模型
Abstract:Objective To investigate the mechanism by which Glu/GABA regulation of neurotransmitter balance improves behavioral abnormalities in a rat model of Tourette syndrome (TS). Methods Forty-eight SPF-grade SD rats were randomly divided into four groups: normal control group, model group, Glu/GABA 1 group (10 mg/kg) and Glu/GABA 2 group (20 mg/kg), with 12 rats in each group. Except for the normal control group, the remaining rats received intraperitoneal injections of 250 mg/(kg·d) IDPN for 7 consecutive days to establish TS model. After successful model establishment, each group received gavage intervention for 6 weeks. Stereotyped behavior scores were assessed using blinded methods before intervention, at week 3, and week 6. Total spontaneous activity distance was detected using a video activity monitoring system. After intervention, brain tissue was harvested for HE staining to count neurons in the cortex, striatum, and thalamus, and transmission electron microscopy was used to observe myelin morphology. HPLC was used to detect Glu and GABA levels in brain tissues; immunofluorescence was used to determine their fluorescence intensity, so as to explore the effect of Glu/GABA on TS model rats. Results Stereotyped behavior scores and total spontaneous activity distance in the model group and Glu/GABA intervention groups were significantly higher than in the normal control group (P < 0.05). After 6 weeks of intervention, stereotyped behavior scores and total spontaneous activity distance in Glu/GABA groups 1 and 2 were significantly reduced compared to the model group (P < 0.001), with no statistical difference between the two intervention groups (P > 0.05). Neuron counts in the cortex, striatum, and thalamus of the model group decreased, with myelin lamellae loosening and axonal damage. After Glu/GABA intervention, neuron counts recovered (P < 0.01) and myelin structure improved. Compared with normal group, the model group showed significantly elevated brain Glu levels and fluorescence intensity, and significantly decreased GABA(P < 0.01). Glu/GABA intervention reversed these changes (P < 0.01). Conclusion Glu/GABA effectively reduces stereotyped behavior scores and spontaneous activity distance in TS model rats, repairs neuronal damage and myelin abnormalities in the cortex, striatum, and thalamus, and regulates brain Glu and GABA levels to normal balanced states, thereby exerting therapeutic effects on TS. -
Key words:
- Glu/GABA /
- Neurotransmitter /
- Rat model of Tourette syndrome
-
表 1 Glu/GABA 对 TS 模型大鼠神经元损伤和神经细胞髓鞘形态影响 ($\bar x \pm s $)
Table 1. Effects of glu/gaba on neuronal injury and myelin morphology of nerve cells in ts model rats ($\bar x \pm s $)
组别 神经元数量的相对水平(皮层) 神经元数量的相对水平(纹状体) 神经元数量的相对水平(丘脑) 对照组 1.00 ± 0.05 1.00 ± 0.06 1.00 ± 0.04 模型组 0.70 ± 0.08△ 0.55 ± 0.07△ 0.60 ± 0.06△ Glu/GABA 1组 1.10 ± 0.06* 0.80 ± 0.05* 1.10 ± 0.07* Glu/GABA 2组 1.00 ± 0.05* 0.95 ± 0.04* 1.00 ± 0.05* F 28.65 35.28 25.83 P <0.001# <0.001# <0.001# 与对照组相比,△P < 0.001;与模型组相比,*P < 0.001 ;#P < 0.05。 -
[1] Johnson K A, Worbe Y, Foote K D, et al. Tourette syndrome: Clinical features, pathophysiology, and treatment[J]. Lancet Neurol, 2023, 22(2): 147-158. doi: 10.1016/S1474-4422(22)00303-9 [2] Rajaprakash M, Leppert M L. Attention-deficit/hyperactivity disorder[J]. Pediatr Rev, 2022, 43(3): 135-147. doi: 10.1542/pir.2020-000612 [3] Set K K, Warner J N. Tourette syndrome in children: An update[J]. Curr Probl Pediatr Adolesc Health Care, 2021, 51(7): 101032. [4] Hall M D, Gipson K S, Gipson S Y T, et al. Disrupted cortico-striato-thalamo-cortical circuitry and sleep disturbances in obsessive-compulsive spectrum, chronic tic, and attention-deficit/hyperactivity disorders[J]. Harv Rev Psychiatry, 2025, 33(3): 114-126. doi: 10.1097/HRP.0000000000000429 [5] He J L, Mikkelsen M, Huddleston D A, et al. Frequency and intensity of premonitory urges-to-tic in Tourette syndrome is associated with supplementary motor area GABA+ levels[J]. Mov Disord, 2022, 37(3): 563-573. doi: 10.1002/mds.28868 [6] Hao J, Jiang K, Zhang X, et al. “Glu/GABA-Gln” metabolic loop abnormalities in iminodipropionitrile (IDPN)-induced dyskinetic syndrome[J]. Neurol Sci, 2021, 42(11): 4697-4706. doi: 10.1007/s10072-021-05570-y [7] Sun X, Zhang X, Jiang K, et al. Gastrodin attenuates Tourette syndrome by regulating EAATs and NMDA receptors in the striatum of rats[J]. Neuropsychiatr Dis Treat, 2021, 17: 2243-2255. doi: 10.2147/NDT.S305925 [8] Liu X, Wang X, Cao A, et al. Immune function changes of the IDPN-induced Tourette syndrome rat model[J]. Int J Dev Neurosci, 2021, 81(2): 159-166. doi: 10.1002/jdn.10085 [9] Yu W, Shi X, Cui X, et al. Jian-Pi-Zhi-Dong-Decoction regulates the expression of glutamate transporters to attenuate glutamate excitotoxicity and exerts anti-tics effects in Tourette syndrome model rats[J]. Neuropsychiatr Dis Treat, 2018, 14: 3381-3392. doi: 10.2147/NDT.S185169 [10] Yang L, Wang X, Liu X, et al. Striatal syntaxin 1A is associated with development of Tourette syndrome in an iminodipropionitrile-induced animal model[J]. Dis Markers, 2022, 2022: 1148191. [11] Dąbrowska-Bouta B, Strużyńska L, Sidoryk-Węgrzynowicz M, et al. Memantine improves the disturbed glutamine and γ-amino butyric acid homeostasis in the brain of rats subjected to experimental autoimmune encephalomyelitis[J]. Int J Mol Sci, 2023, 24(17): 13149. doi: 10.3390/ijms241713149 [12] Peng B, Yang Q, B Joshi R, et al. Role of alcohol drinking in Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis[J]. Int J Mol Sci, 2020, 21(7): 2316. doi: 10.3390/ijms21072316 [13] Guo C, Ma Y Y. Calcium permeable-AMPA receptors and excitotoxicity in neurological disorders[J]. Front Neural Circuits, 2021, 15: 711564. doi: 10.3389/fncir.2021.711564 [14] Kim K, Yoon H. Gamma-aminobutyric acid signaling in damage response, metabolism, and disease[J]. Int J Mol Sci, 2023, 24(5): 4584. doi: 10.3390/ijms24054584 [15] 王纳. 基于肠-脑轴研究青龙止动颗粒治疗抽动秽语综合征的作用机理[D]. 上海: 上海中医药大学, 2021. -





 下载:
下载: