Predicting Active Components of Chinese Herbal Medicines for Treating Bone Aging Based on Reverse Network Pharmacology and Molecular Docking
-
摘要:
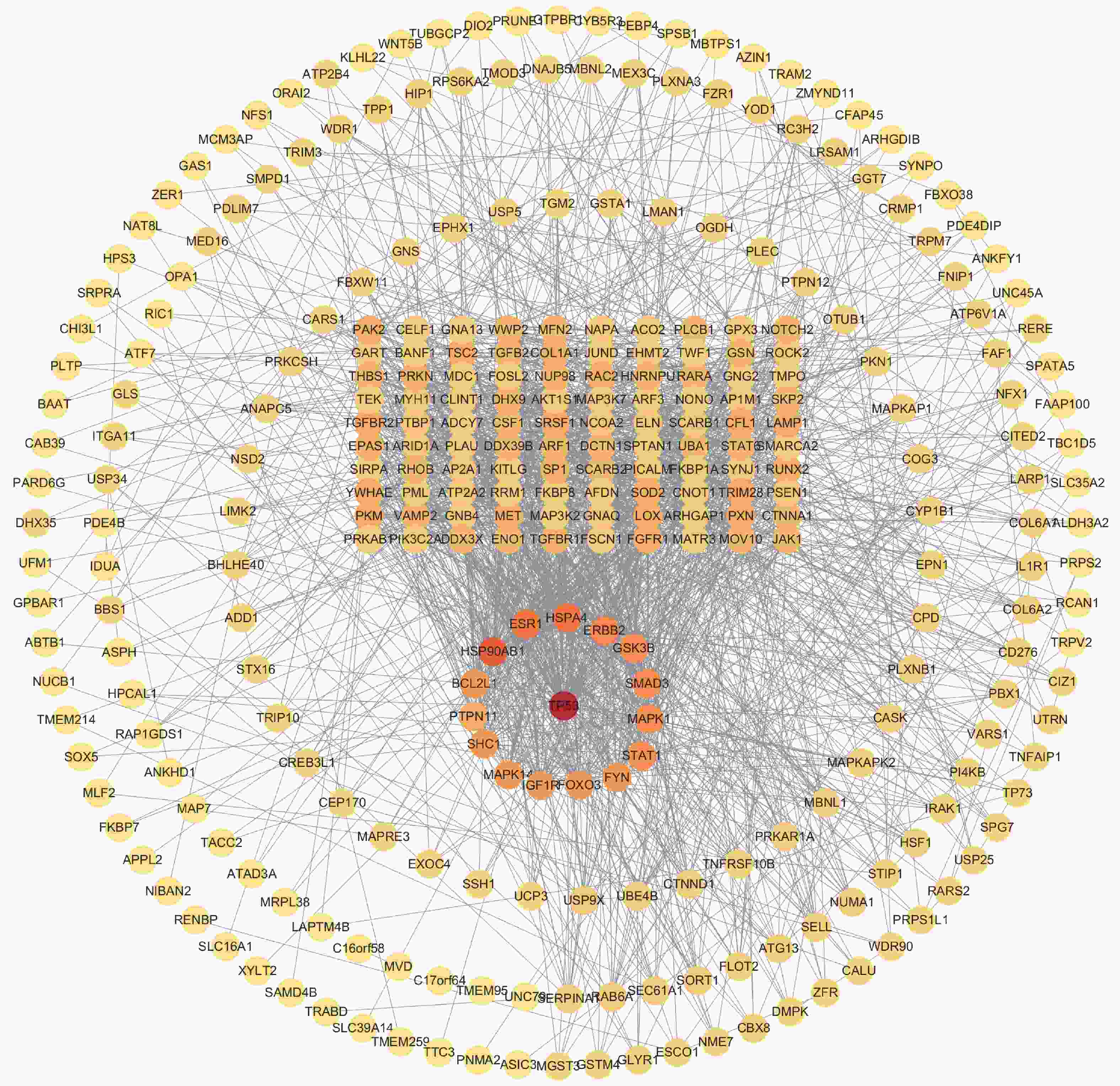
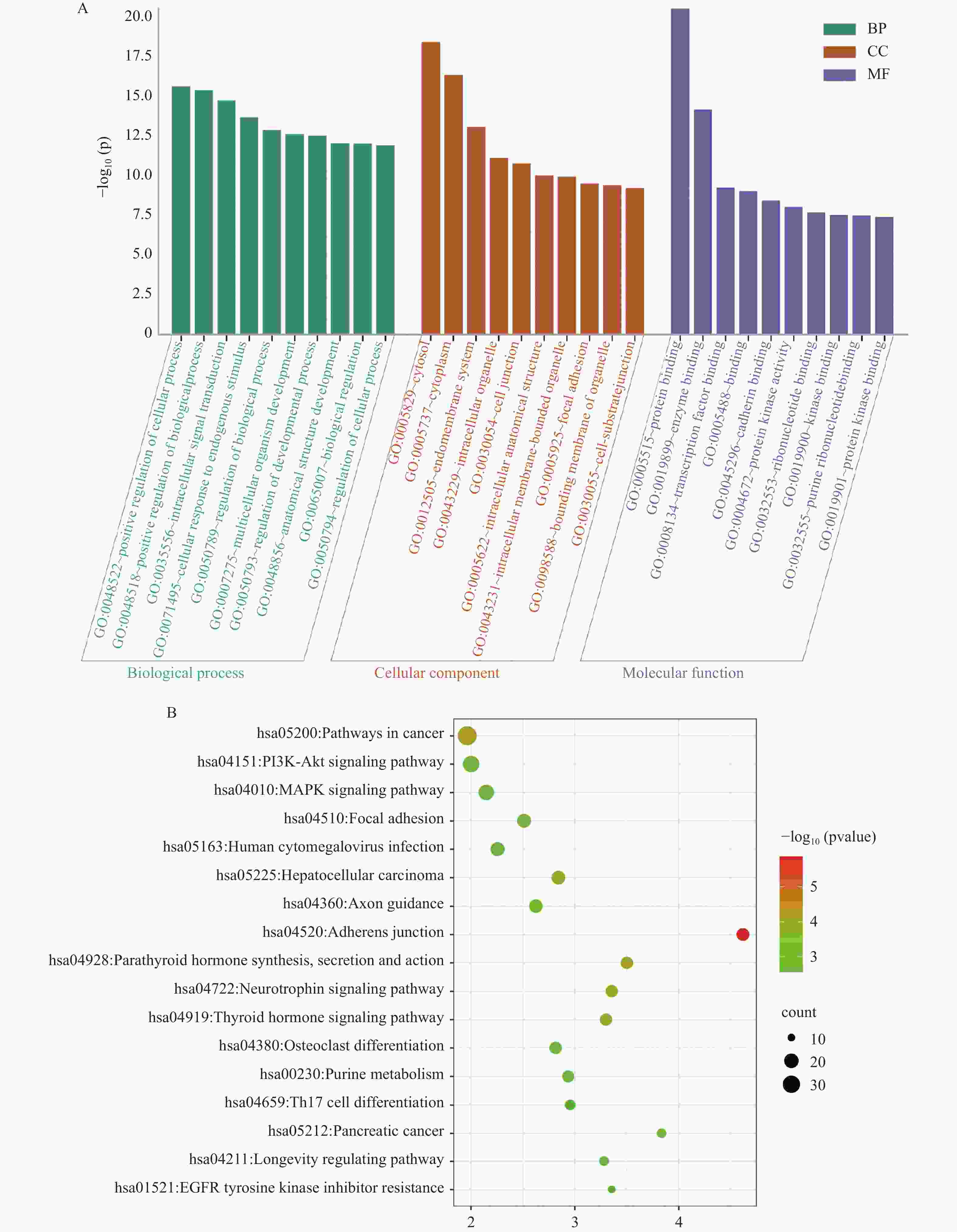
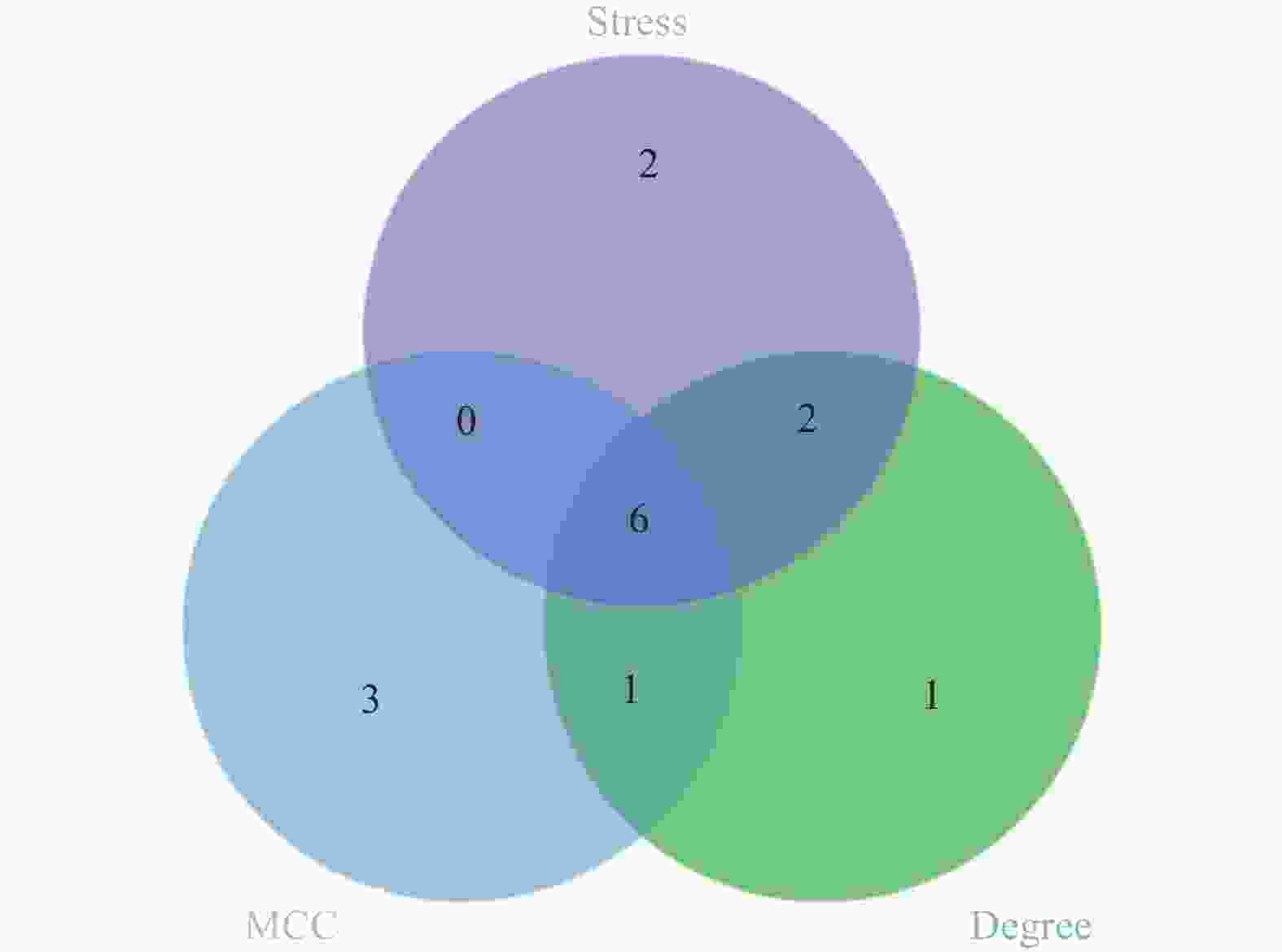
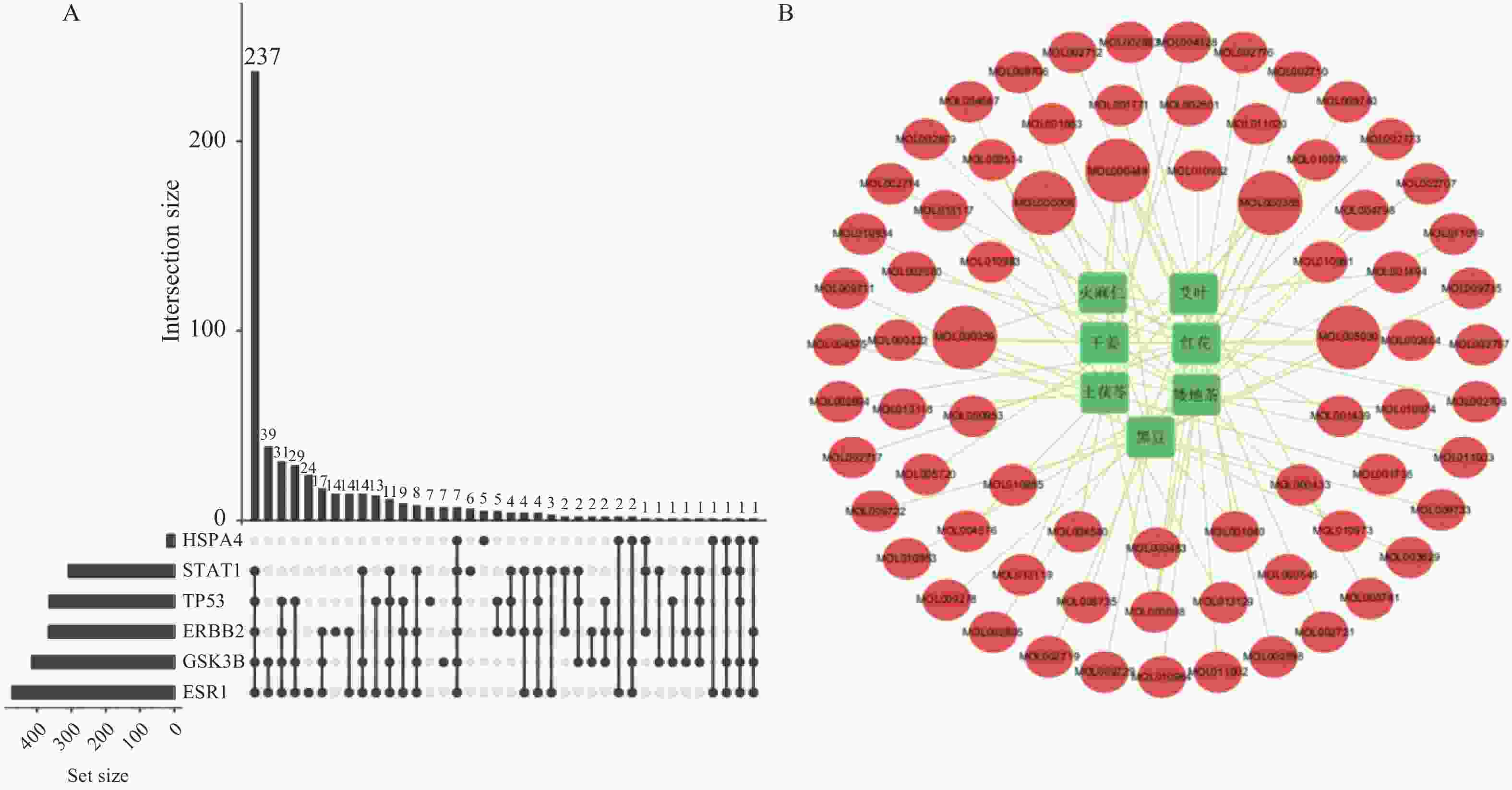
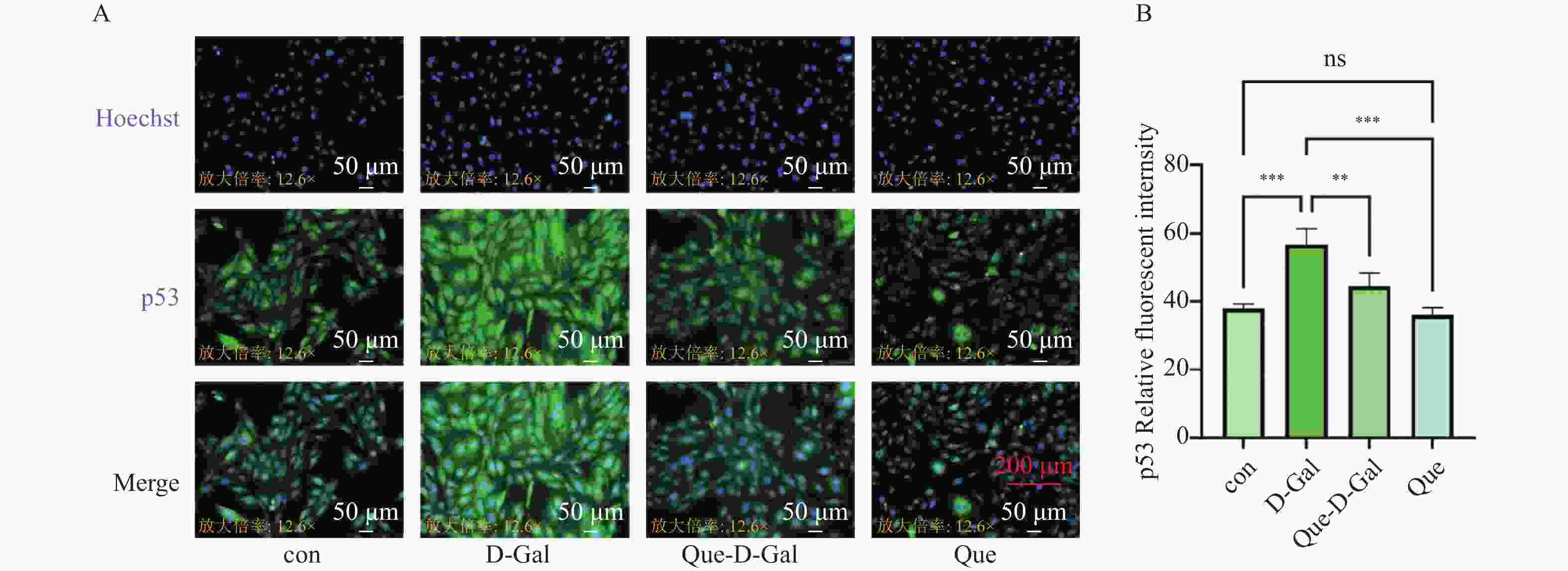
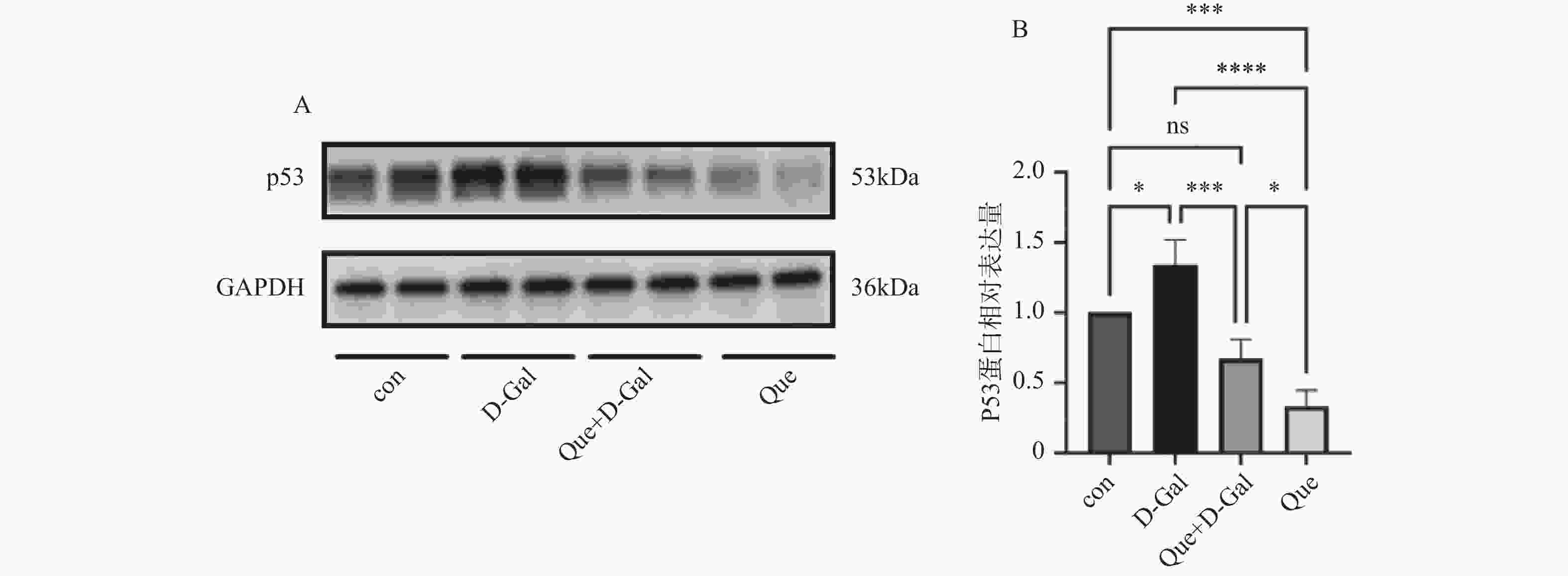
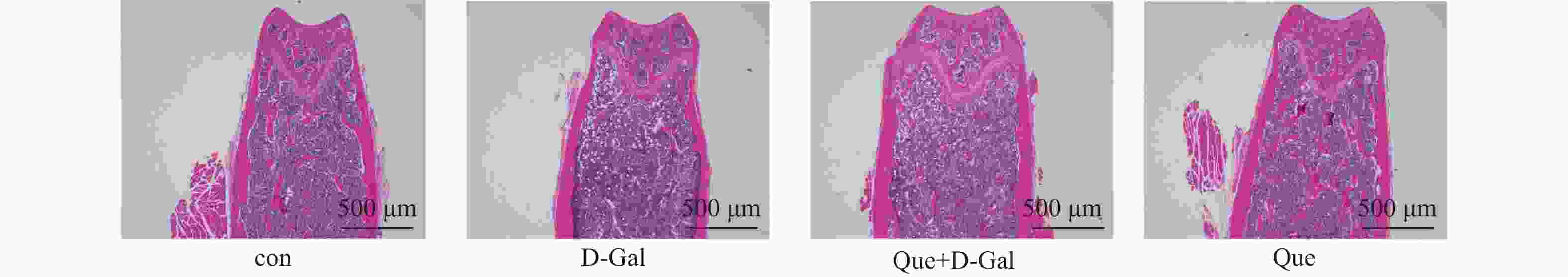
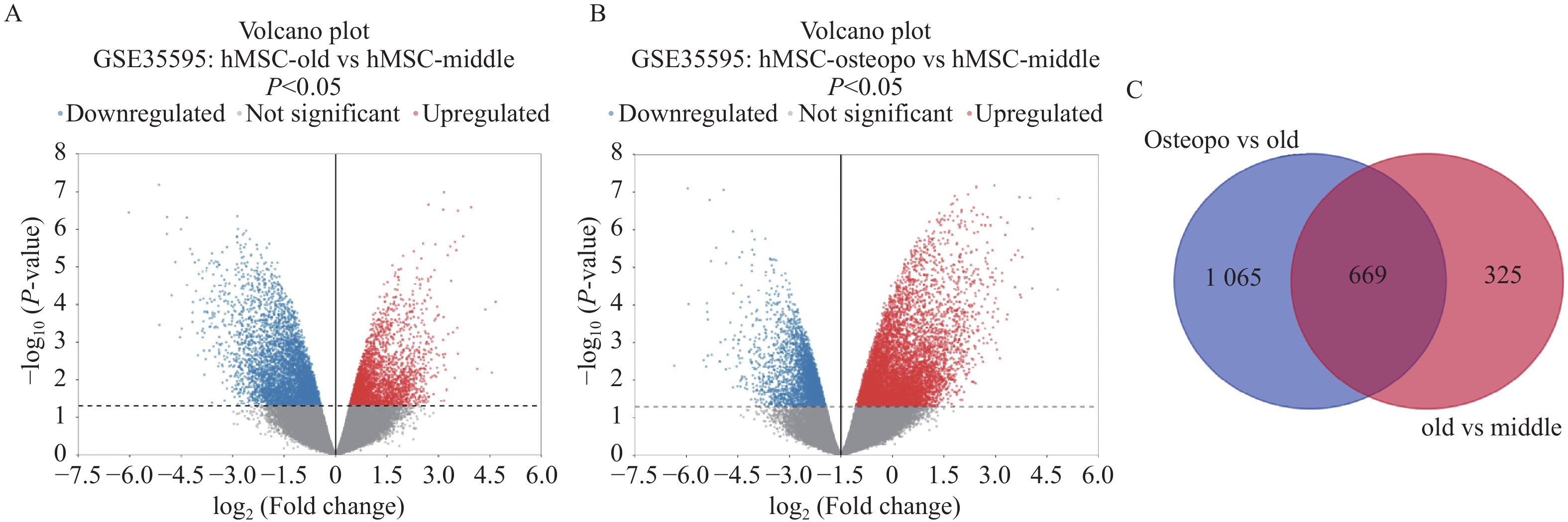
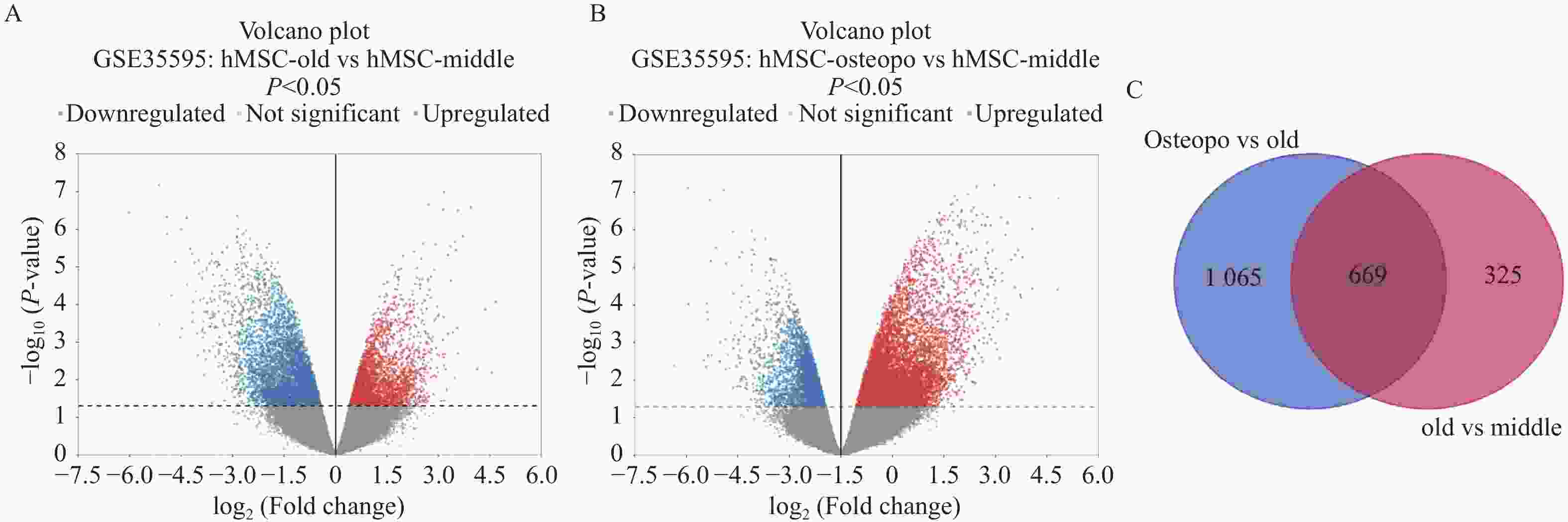
目的 应用逆向网络药理学和分子对接技术探寻治疗骨衰老的潜在中草药活性成分及其作用机制,并进行初步实验验证。 方法 从GEO数据库获取转录组数据,用GEO2R分析差异基因得到骨衰老特征基因群,构建PPI网络识别关键靶点,进行GO和KEGG富集分析。通过Degree、MCC 、Stress三种算法取交集获得疾病核心靶点,利用核心靶点逆向寻找具有治疗骨衰老潜力的中草药,并进行药物活性分析,将药物活性成分与疾病核心靶点进行分子对接,进一步筛选代表药物,构建衰老骨细胞模型和动物模型初步验证其疗效。细胞实验分为4组:对照组(Control) 、骨衰老组(D-Gal)、槲皮素+骨衰老组(Que+D-Gal)以及槲皮素组(Que),利用D-半乳糖(10 g/L)构建衰老骨细胞模型,槲皮素(10 μM)处理D-Gal诱导的衰老骨细胞,通过SA-β-Gal染色观察细胞衰老情况,免疫荧光和Western blot检测细胞内p53的蛋白表达。将24只C57BL/6J小鼠随机分为4组,每组6只,组别与细胞分组相同,用D-半乳糖(500 mg/kg)颈背部皮下注射构建骨衰老小鼠模型,槲皮素(50 mg/kg)灌胃,通过股骨切片HE染色观察骨保护效果。 结果 获得669个骨衰老特征基因,主要富集于PI3K-AKT、MAPK、破骨细胞分化、长寿调节等通路,核心靶点为TP53、HSPA4、ESR1、ERBB2、GSK3B、STAT1。逆向收集到能共同作用于骨衰老6个核心靶点的中草药为矮地茶、艾叶、干姜、黑豆、红花、火麻仁、土茯苓,主要活性成分为谷甾醇、豆甾醇、槲皮素、木犀草素等,将疾病6个核心靶点与药物活性成分进行分子对接,发现豆甾醇、槲皮素、木犀草素与6个核心靶点均为较强结合(结合能≤-7 kcal/mol),综合考虑结合能与口服利用度,将槲皮素(Quercetin)为本研究的代表药物。细胞实验SA-β-Gal染色发现,与D-Gal组比较,Que+D-Gal组衰老细胞阳性面积明显减少(P < 0.01);免疫荧光和Western blot验证发现,与D-Gal组比较,Que+D-Gal组的p53荧光信号强度明显降低(P < 0.01),p53蛋白表达显著降低(P < 0.001)。小鼠股骨HE染色发现,与D-Gal组比较,Que+D-Gal组骨小梁数量增加。 结论 本研究通过逆向网络药理学从大数据库中筛选到矮地茶、艾叶等中药的活性成分,如豆甾醇、槲皮素、木犀草素等可能调控衰老关键靶点TP53,与骨代谢相关通路基因如ESR1、GSK3B等协同作用,从而发挥骨保护作用。 Abstract:Objective To explore potential active components of Chinese herbal medicines (CHMs) for treating bone aging and their mechanisms of action using reverse network pharmacology and molecular docking techniques, with preliminary experimental validation. Methods Transcriptomic data were obtained from the GEO database. GEO2R was used to analyze differentially expressed genes, identifying a characteristic bone aging gene set. A Protein-Protein Interaction (PPI) network was constructed to identify key targets, followed by Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses. Core disease targets were identified by intersecting results from three algorithms (Degree, MCC, and Stress). These core targets were used to reversely identify CHMs with potential anti-bone aging effects, followed by drug activity analysis. Molecular docking was performed between active components and core disease targets to further screen representative compounds. Senescent bone cell models and animal models were established for preliminary efficacy verification. Cell experiments were divided into 4 groups: control group (Control), bone aging group (D-Gal), quercetin + bone aging group (Que+D-Gal), and quercetin group (Que). An aging bone cell model was constructed using D-galactose (10 g/L), and quercetin (10 µM) was used to treat D-Gal-induced senescent cells. Cellular senescence was observed by SA-β-Gal staining, and p53 protein expression was detected by immunofluorescence and Western blot. Twenty-four C57BL/6J mice were randomly divided into 4 groups (6 mice per group) with the same grouping as cells. A bone aging mouse model was established via subcutaneous injection of D-galactose (500 mg/kg) on the dorsal neck, and quercetin (50 mg/kg) was administered by gavage. HE staining of femoral sections was used to observe bone protective effects. Results A total of 669 characteristic bone aging genes were identified, primarily enriched in as PI3K-AKT, MAPK, osteoclast differentiation, and longevity regulation pathways. Core targets were TP53, HSPA4, ESR1, ERBB2, GSK3B, and STAT1. Reverse screening identified CHMs acting on all six core targets: Ardisia japonica , Artemisia argyi , Zingiber officinale, Glycine max , Carthamus tinctorius , Cannabis sativa , and Smilax glabra. The main active components included β-sitosterol, stigmasterol, quercetin, and luteolin. Molecular docking between the six core targets and the active components revealed that stigmasterol, quercetin, and luteolin exhibited strong binding (binding energy ≤ -7 kcal/mol) to all six core targets. Considering both binding energy and oral bioavailability, quercetin was selected as the representative drug for this study. SA-β-Gal staining showed that the positive area of senescent cells in the Que+D-Gal group was significantly reduced compared to the D-Gal group(P < 0.01). Immunofluorescence and Western blot confirmed that that p53 fluorescence signal intensity (P < 0.01) and p53 protein expression (P < 0.001) were significantly decreased in the Que+D-Gal group compared to the D-Gal group. Conclusion Through reverse network pharmacology, this study screened active components from traditional Chinese herbs such as Ardisia japonica and Artemisia argyi from large databases. Components like stigmasterol, quercetin, and luteolin may regulate the key aging target TP53 and synergize with genes in bone metabolism-related pathways (e.g., ESR1, GSK3B), thereby exerting bone protective effects. -
Key words:
- Bone aging /
- Osteoporosis /
- Network pharmacology /
- Molecular docking simulation /
- Quercetin
-
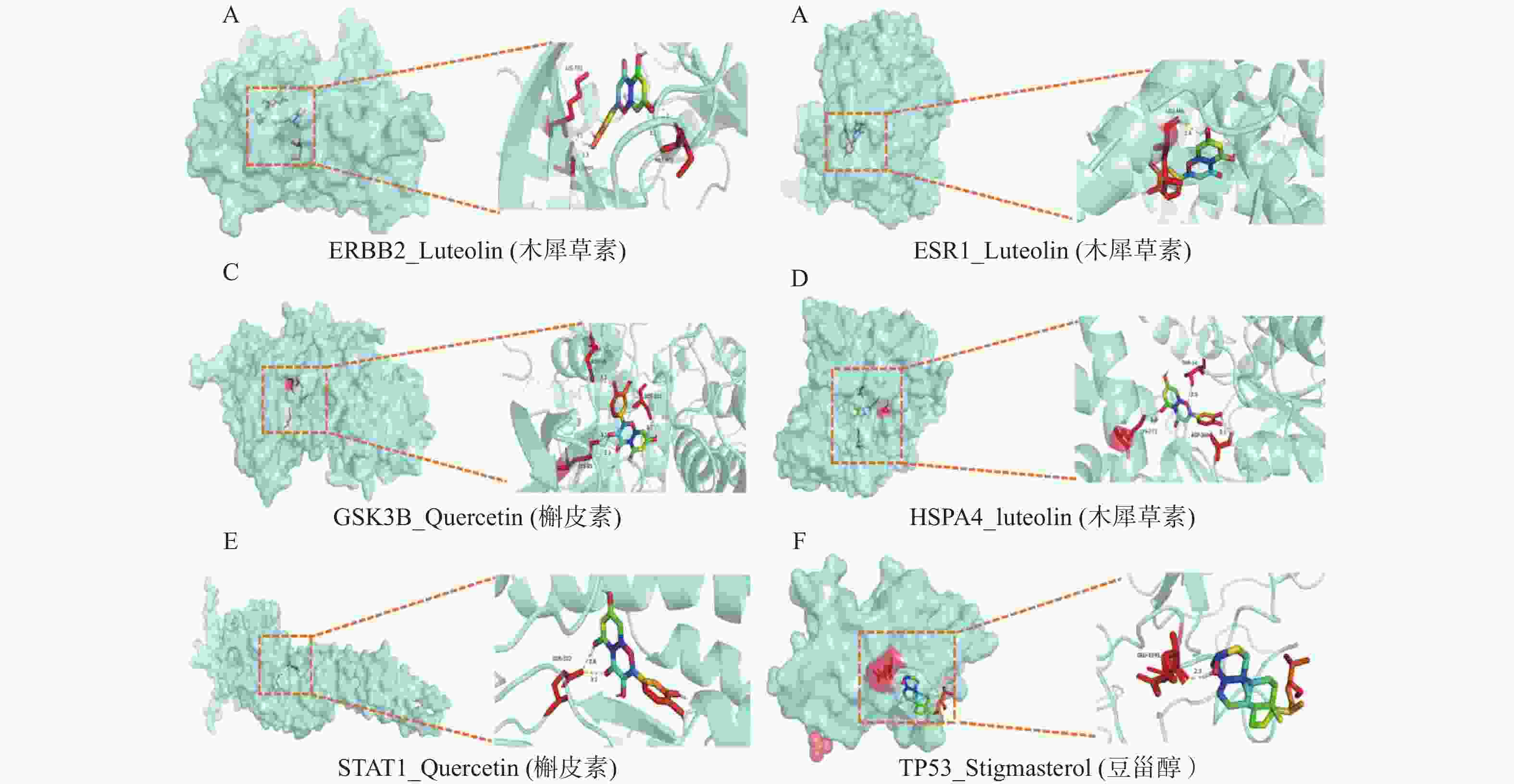
图 6 分子对接可视化图
A:ERBB2_Luteolin(木犀草素);B:ESR1_Luteolin(木犀草素);C:GSK3B_Quercetin(槲皮素);D:HSPA4_luteolin(木犀草素);E:STAT1_Quercetin(槲皮素);F:TP53_Stigmasterol(豆甾醇);GSK3B、HSPA4、STAT1均有两个对接效果最好的活性成分,可视化图中仅呈现出靶点与表2中度值更高的活性成分的结果。
Figure 6. Visualization of molecular docking
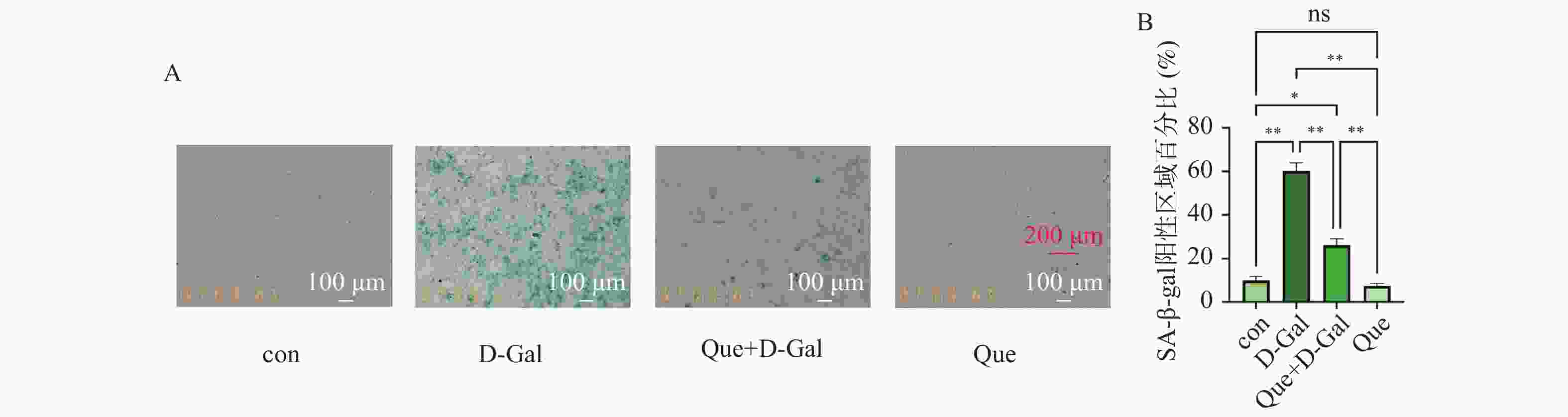
图 7 槲皮素对成骨前体细胞衰老β-半乳糖苷酶染色的影响(×100)
A:SA-β-gal染色检测槲皮素对成骨前体细胞衰老β-半乳糖苷酶染色的影响;Control为空白对照组,D-Gal为D-半乳糖(D-Galactose)诱导的骨细胞衰老模型组,Que为槲皮素(Quercetin)处理组,Que + D-Gal为加入槲皮素处理后的骨细胞衰老模型组;胞浆呈蓝绿色的为衰老细胞;B:各组SA-β-gal染色阳性区域百分比统计分析(×100,标尺:100 μm);*P < 0.05;**P < 0.01;ns: P > 0.05。
Figure 7. Effect of Quercetin on SA-β-gal staining of MC3T3 (×100)
表 1 部分药物活性成分信息
Table 1. Information on active ingredients of herbs
MOL ID 名称 度值 OB(%) DL MOL000449 豆甾醇(Stigmasterol) 5 43.83 0.76 MOL000358 β-谷甾醇(Beta-sitosterol) 5 36.91 0.75 MOL000098 槲皮素(Quercetin) 4 46.43 0.28 MOL000359 谷甾醇(Sitosterol) 3 36.91 0.75 MOL000006 木犀草素(Luteolin) 2 36.16 0.25 MOL000422 山柰酚(Kaempferol) 2 41.88 0.24 MOL005030 二十碳烯酸(Gondoic acid) 2 30.7 0.20 表 2 核心靶点与核心活性成分分子对接结合能信息(kcal/mol)
Table 2. Molecular docking binding energy information of core targets and core active ingredients (kcal/mol)
成分 基因 ERBB2 ESR1 GSK3B HSPA4 STAT1 TP53 豆甾醇(Stigmasterol) −7.9 −7.1 −7.7 −7.7 −7.0 −9.7 β-谷甾醇(Beta-sitosterol) −7.4 −6.9 −7.8 −6.6 −6.0 −8.2 槲皮素(Quercetin) −9.3 −7.2 −9.1 −8.3 −7.7 −8.3 谷甾醇(Sitosterol) −8.4 −6.5 −9.1 −7.2 −6.3 −9.1 木犀草素(Luteolin) −9.5 −8.3 −8.8 −8.3 −7.7 −8.4 -
[1] Horvath T, Leoni T, Reschenhofer P, et al. The impact of ageing, socio-economic differences and the evolution of morbidity on future health expenditure-a dynamic microsimulation[J]. BMC Health Serv Res, 2025, 25(1): 952. [2] 中华医学会骨质疏松和骨矿盐疾病分会. 原发性骨质疏松症诊疗指南(2022)[J]. 中国全科医学, 2023, 26(14): 1671-1691. doi: 10.12464/j.issn.0253-9802.2025-0086 [3] 《老年性骨质疏松症中西医结合诊疗指南》工作组, 史晓林, 刘康. 老年性骨质疏松症中西医结合诊疗指南[J]. 中国骨质疏松杂志, 2024, 30(7): 937-946. doi: 10.3969/j.issn.1006-7108.2024.07.001 [4] Ukon Y, Kaito T, Hirai H, et al. Cellular senescence by loss of Men1 in osteoblasts is critical for age-related osteoporosis[J]. Aging Cell, 2024, 23(10): e14254. [5] Xu Y, Yang Y, Hua Z, et al. BMP2 immune complexes promote new bone formation by facilitating the direct contact between osteoclasts and osteoblasts[J]. Biomaterials, 2021, 275: 120890. doi: 10.1016/j.biomaterials.2021.120890 [6] Wang J, Zhang Y, Wang S, et al. Bone aging and extracellular vesicles[J]. Sci Bull, 2024, 69(24): 3978-3999. doi: 10.1016/j.scib.2024.10.013 [7] Li K, Hu S, Chen H. Cellular senescence and other age-related mechanisms in skeletal diseases[J]. Bone Res, 2025, 13(1): 68. doi: 10.1038/s41413-025-00448-7 [8] Reid I R, Billington E O. Drug therapy for osteoporosis in older adults[J]. Lancet, 2022, 399(10329): 1080-1092. doi: 10.1016/S0140-6736(21)02646-5 [9] 高增杰, 杨扉扉, 李丽莉, 等. 抗骨质疏松症中药的作用机制研究进展[J]. 世界中医药, 2025, 20(5): 848-855. doi: 10.3969/j.issn.1673-7202.2025.05.022 [10] Wang J, Teng F. Efficacy of Chinese herbal medicine in patients with osteoporosis: A systematic review and meta-analysis[J]. Front Med, 2025, 12: 1620264. doi: 10.3389/fmed.2025.1620264 [11] Ojo O A, Ogunlakin A D, Ajayi-Odoko O A, et al. Bioprospection of indigenous herbal formulations for diabetes care: in vitro, network pharmacology, and molecular dynamics studies[J]. BMC Complementary Med Ther, 2025, 25(1): 225. [12] Lotfi M S, Jamali H, B Rassouli F. Network pharmacology and in silico study of quercetin and structurally similar flavonoids as osteogenesis inducers that interact with oestrogen receptors[J]. Arch Physiol Biochem, 2025, 131(4): 658-669. doi: 10.1080/13813455.2025.2483910 [13] Zhao B, Chen L, Wang W, et al. Anti-osteoporosis activity of lycopene through ESR1∶ Network pharmacology, molecular docking, imaging technology, and experimental validation[J]. Chem Biol Drug Des, 2025, 105(6): e70135. [14] 杨燕红, 贺娟, 金山, 等. 基于逆向网络药理学和分子对接预测银屑病潜在治疗中药的组分构成[J]. 昆明医科大学学报, 2024, 45(12): 49-57. doi: 10.12259/j.issn.2095-610X.S20241207 [15] 饶璐, 丁家和, 魏江平, 等. 槐花通过抑制PI3K/AKT通路减轻炎症反应治疗银屑病[J]. 南方医科大学学报, 2025, 45(9): 1989-1996. [16] Alamro H, Thafar M A, Albaradei S, et al. Exploiting machine learning models to identify novel Alzheimer’s disease biomarkers and potential targets[J]. Sci Rep, 2023, 13: 4979. doi: 10.1038/s41598-023-30904-5 [17] Liu X, Pan F, Sha C, et al. Fuzi decoction ameliorates intervertebral disc degeneration through ferroptosis modulation by suppressing NF-κB pathway[J]. Int Immunopharmacol, 2025, 148: 114155. doi: 10.1016/j.intimp.2025.114155 [18] 叶林霜, 鲁婷, 张美玲, 等. 基于网络药理学和分子对接探讨左归丸“异病同治”阿尔茨海默病和骨质疏松的作用机制[J]. 药物评价研究, 2025, 48(12): 3613-3626. [19] 陈祁青, 马东, 赵继荣, 等. 基于网络药理学及动物实验的杜仲腰痛丸治疗腰椎间盘突出症慢性下肢痛的作用研究[J]. 世界科学技术-中医药现代化, 2023, 25(5): 1702-1713. doi: 10.11842/wst.20220326012 [20] 梁超, 杨暑晨, 许言午, 等. 基于AGEs-RAGE轴探讨左归丸有效成分对衰老骨髓间充质干细胞的影响[J]. 时珍国医国药, 2024, 35(5): 1092-1096. doi: 10.3969/j.issn.1008-0805.2024.05.15 [21] 朱晓龙. 基于Nrf2/xCT/GPX4通路探讨槲皮素对激素诱导MC3T3-E1铁死亡的研究[D]. 锦州: 锦州医科大学, 2024. [22] 黄彦峰, 张丽凤, 黄永毅, 等. 百香果果皮总黄酮对D-半乳糖所致衰老小鼠海马的影响[J]. 中国老年学杂志, 2024, 44(4): 945-948. doi: 10.3969/j.issn.1005-9202.2024.04.046 [23] 招文华. 槲皮素下调DANCR抑制NF——κB骨免疫通路改善PMOP的机制研究[D]. 广州: 广州中医药大学, 2023. [24] 林玲, 向晓东, 帅波, 等. 中药通过减少骨流失改善骨质疏松症研究进展[J]. 中国中医药现代远程教育, 2024, 22(18): 91-93. doi: 10.3969/j.issn.1672-2779.2024.18.029 [25] 刘航, 刘中, 莫中成. 中草药调节破骨细胞分化改善老年骨质疏松[J]. 中国骨质疏松杂志, 2024, 30(10): 1477-1482. doi: 10.3969/j.issn.1006-7108.2024.10.014 [26] 张海文, 张贤, 许太川, 等. 衰老在骨质疏松领域研究现状及趋势的文献可视化分析[J]. 中国组织工程研究, 2026, 30(6): 1580-1591. [27] Liu H, Liu L, Rosen C J. PTH and the regulation of mesenchymal cells within the bone marrow niche[J]. Cells, 2024, 13(5): 406. doi: 10.3390/cells13050406 [28] Kiryaman G, Enabulele I, Banville M L, et al. The evolving role of PTH signaling in osteocytes[J]. Endocrinology, 2025, 166(4): bqaf034. doi: 10.1210/endocr/bqaf034 [29] Lademann F, Tsourdi E, Hofbauer L C, et al. Bone cell-specific deletion of thyroid hormone transporter Mct8 distinctly regulates bone volume in young versus adult male mice[J]. Bone, 2022, 159: 116375. doi: 10.1016/j.bone.2022.116375 [30] Hassani S S, Farhadi E, Esmaeili S A, et al. Deciphering the role of ERK and PI3K/Akt as crosstalk pathways between fibroblast-like synoviocytes and osteoclasts; novel therapeutic approach for rheumatoid arthritis[J]. Mol Biol Rep, 2025, 52(1): 662. doi: 10.1007/s11033-025-10769-9 [31] Cao H, Li Q, Gao Y, et al. Metformin promotes osteogenic differentiation of adipose-derived stem cells in diabetic osteoporosis by regulating autophagy[J]. Cell Biol Int, 2025, 49(10): 1354-1366. doi: 10.1002/cbin.70061 [32] Zhang J, Zhu L, Zhou J, et al. BDNF alleviates senescence and enhances osteogenic differentiation in bone marrow mesenchymal stem cells via the TrkB/PI3K/AKT pathway[J]. Tissue Cell, 2025, 96: 102972. doi: 10.1016/j.tice.2025.102972 [33] Liu Y, Liu Q, Zhang Z, et al. The regulatory role of PI3K in ageing-related diseases[J]. Ageing Res Rev, 2023, 88: 101963. doi: 10.1016/j.arr.2023.101963 [34] 李岳尧, 张民, 杨家驹. 肉苁蓉苷A通过JNK/MAPK通路抑制破骨细胞活性[J]. 中国组织工程研究, 2025, 29(6): 1144-1151. doi: 10.12307/2025.300 [35] Kitazawa S, Haraguchi R, Kitazawa R. Roles of osteoclasts in pathological conditions[J]. Pathol Int, 2025, 75(2): 55-68. doi: 10.1111/pin.13500 [36] 牛园园, 张天驰, 李沐哲, 等. 温肾通络止痛方通过AMPK/mTOR信号通路调控自噬对老年性骨质疏松模型小鼠的干预作用[J]. 中国中西医结合杂志, 2024, 44(1): 84-90. doi: 10.7661/j.cjim.20230923.289 [37] Zheng Y, Deng J, Wang G, et al. P53 negatively regulates the osteogenic differentiation in jaw bone marrow MSCs derived from diabetic osteoporosis[J]. Heliyon, 2023, 9(4): e15188. doi: 10.1016/j.heliyon.2023.e15188 [38] 刘沛, 蒋宜伟, 周玉英, 等. p53在骨质疏松症中的作用机制及中医药干预研究进展[J]. 中国骨质疏松杂志, 2023, 29(7): 1021-1026. doi: 10.3969/j.issn.1006-7108.2023.07.017 [39] 李洋. 朱砂在龟龄集改善D-半乳糖致大鼠衰老中的作用研究[D]. 太原: 山西大学, 2024. [40] Zou H, Hu F, Wu X, et al. LINC01089 governs the miR-1287-5p/HSPA4 axis to negatively regulate osteogenic differentiation of mesenchymal stem cells[J]. Bone Joint Res, 2024, 13(12): 779-789. doi: 10.1302/2046-3758.1312.BJR-2023-0272.R2 [41] Cai Y, Sun H, Song X, et al. The Wnt/β-catenin signaling pathway inhibits osteoporosis by regulating the expression of TERT: An in vivo and in vitro study[J]. Aging, 2023, 15(20): 11471-11488. doi: 10.18632/aging.205136 [42] Xu J, Wakai M, Xiong K, et al. The pro-inflammatory cytokine IL6 suppresses mitochondrial function via the gp130-JAK1/STAT1/3-HIF1α/ERRα axis[J]. Cell Rep, 2025, 44(3): 115403. doi: 10.1016/j.celrep.2025.115403 [43] 代俊泽, 刘毅, 廖翠平, 等. 中药组方治疗糖尿病合并骨质疏松症用药规律挖掘及其作用机制的网络药理学与分子对接研究[J]. 中华中医药学刊, 2024, 42(12): 158-163, 后插55-后插57. doi: 10.13193/j.issn.1673-7717.2024.12.033 [44] 丁佳琪, 周文娟. Wnt/Ca2+/CaMK Ⅱ信号通路在成骨调控及糖尿病骨代谢异常中作用机制的研究进展[J]. 中国口腔种植学杂志, 2025, 30(3): 305-311. [45] 黄越, 柳芳洲, 赵奕菲, 等. 常见黄酮类天然产物对牙槽骨骨稳态的骨免疫调控研究[J]. 中国药学杂志, 2025, 60(2): 153-165. doi: 10.11669/cpj.2025.02.007 [46] 殷润良, 葸慧荣, 李喜香, 等. 基于网络药理学和分子对接技术探究滋阴清骨丸治疗脊柱结核的作用机制[J]. 西部中医药, 2025, 38(6): 78-85. doi: 10.12174/j.issn.2096-9600.2025.06.15 [47] 闫露露. 基于网络药理学和临床观察探讨骨疏丸治疗原发性骨质疏松症(肾虚血瘀型)的疗效和机制[D]. 昆明: 云南中医药大学, 2024. [48] Hassin O, Oren M. Drugging p53 in cancer: One protein, many targets[J]. Nat Rev Drug Discov, 2023, 22(2): 127-144. [49] 靳锐, 周福荣, 包芸. 中药调控P53治疗糖尿病肾病的研究进展[J]. 云南中医中药杂志, 2025, 46(9): 69-77. doi: 10.3969/j.issn.1007-2349.2025.09.017 [50] 徐静逍, 刘佳, 姚姝, 等. 槲皮素对BMSCs成骨分化的影响及作用机制[J]. 昆明医科大学学报, 2025, 46(5): 30-37. -





 下载:
下载: