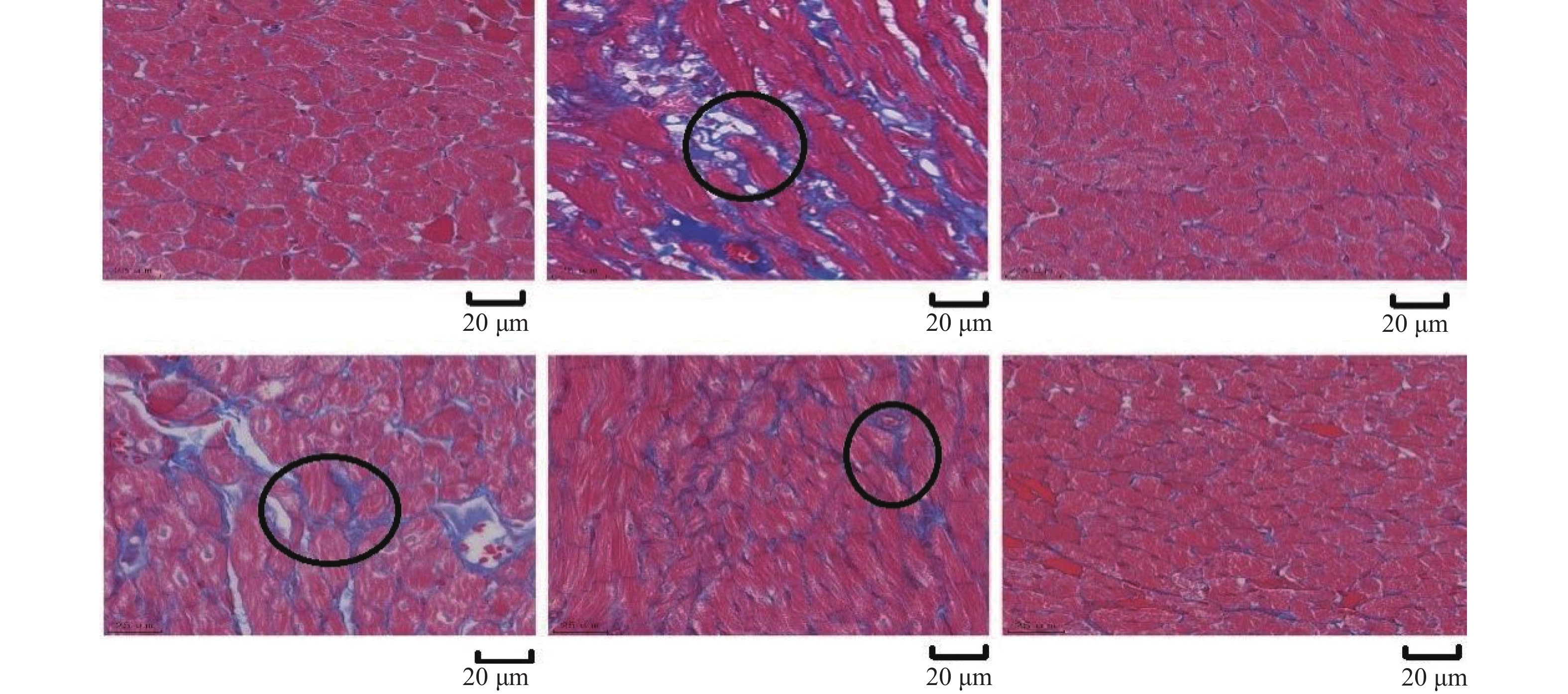
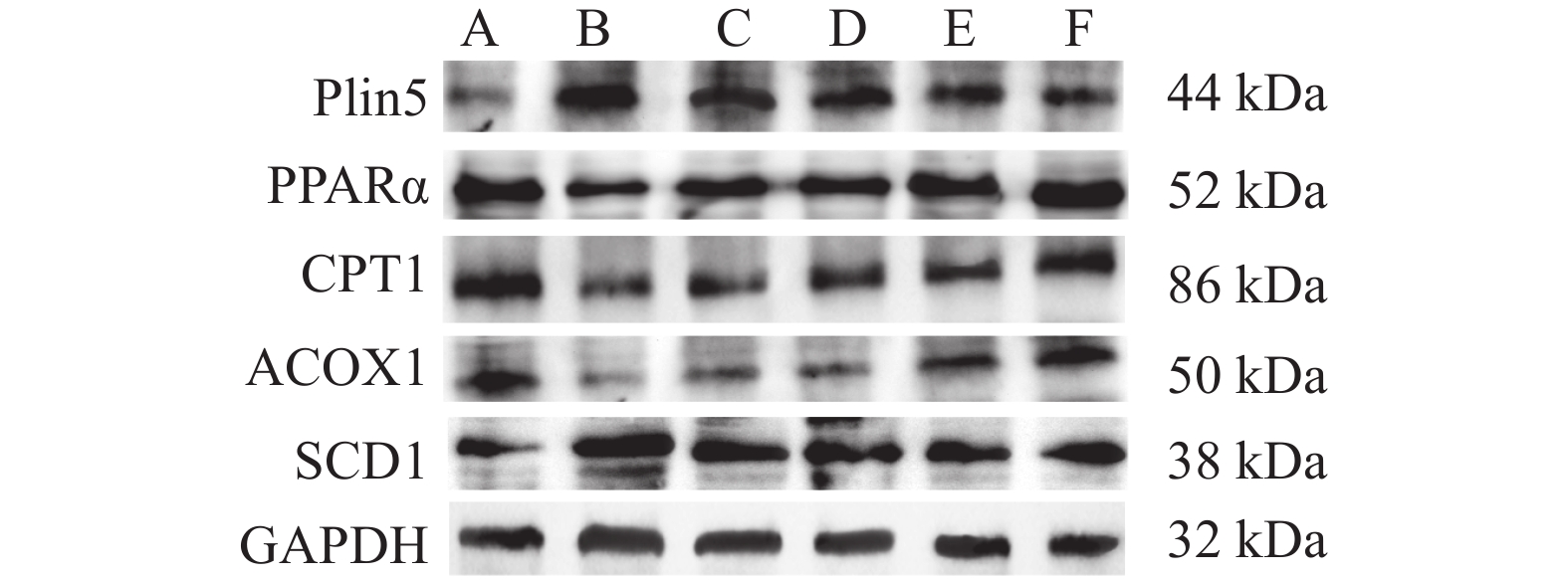
Dendrobium Nobile Polysaccharide Improves Myocardial Injury in Diabetic Rats by Regulating Plin5 Mediated Fatty Acid β Oxidation of Cardiomyocytes
-
摘要:
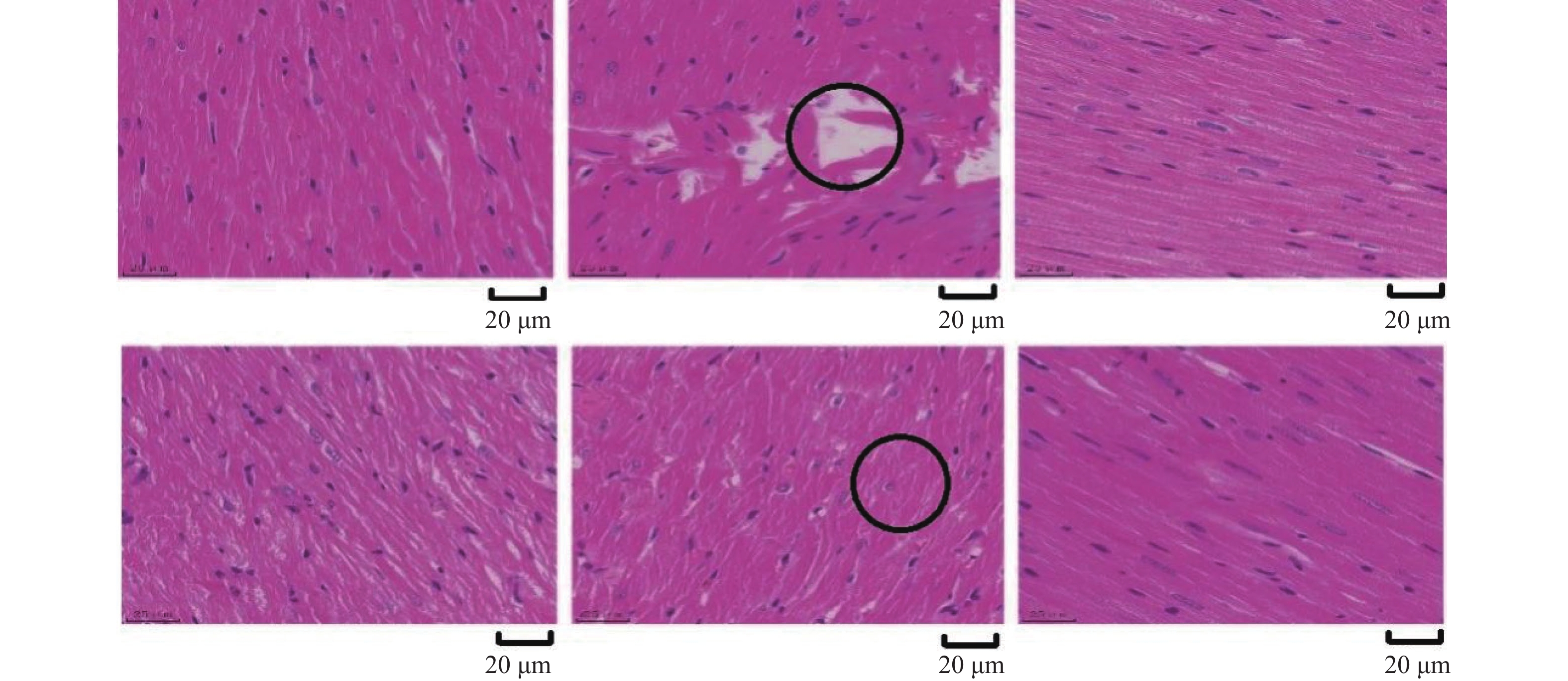
目的 从Plin5调节心肌细胞脂肪酸β氧化信号通路探究金钗石斛多糖干预糖尿病大鼠心肌损伤的作用机制。 方法 清洁级雄性SD大鼠共72只,将其分为正常组(n = 10)以及造模组(n = 62),正常组给予普通饲料喂养,造模组给予高脂高糖饮食2月+一次性STZ注射构建糖尿病心肌损伤动物模型,构建模型成功后,将造模组SD大鼠分为5组,分别为模型组、单硝酸异山梨酯片组、金钗石斛多糖低剂量组、中剂量组和高剂量组(n = 10),药物治疗4周后,处死大鼠,收集心脏,检测心肌组织病理学(HE和Masson染色)、心脂质含量(TG、TC、HDL-C和LDL-C)、心肌炎性因子(IL-1α、IL-1β、IL-6和TNF-α)和抗氧化指标(SOD、GSH、MDA和ROS),收集血液检测心功能、胰岛素和血糖水平,采用Western blot检测心肌组织中Plin5、SCD1、CPT1A、ACOX1和PPARα蛋白表达特点。 结果 组织病理学HE和Masson染色显示正常组大鼠的心肌纤维整齐排列,模型组大鼠出现心肌纤维断裂,与模型组相比,金钗石斛多糖各治疗组可见不同程度减轻;相较于正常组,模型组血糖和胰岛素抵抗指数升高(P < 0.05),胰岛素下降(P < 0.05),相较于模型组,金钗石斛多糖3个组血糖和胰岛素抵抗指数降低(P < 0.05),低剂量组、中剂量和硝酸异山梨酯片组胰岛素水平没有升高(P > 0.05),金钗石斛多糖高剂量组胰岛素含量升高(P < 0.05);相较于正常组,模型组TG、TC和LDL-C升高(P < 0.05),HDL-C降低(P < 0.05),相较于模型组,金钗石斛多糖治疗组以及阳性药物组TG、TC和LDL-C降低(P < 0.05),HDL-C升高(P < 0.05);相较于正常组,模型组AST、CK、CK-MB、LDH、a-HBDH以及CTnl升高(P < 0.05),相较于模型组,金钗石斛多糖3个治疗组和阳性药物组上述6个指标降低(P < 0.05);相较于正常组,模型组心肌IL-1α、IL-1β、IL-6和TNF-α升高(P < 0.05),相较于模型组,金钗石斛多糖3个治疗组和阳性药物组上述炎性因子指标降低(P < 0.05);相较于正常组,模型组SOD和GSH降低(P < 0.05),MDA和ROS升高(P < 0.05);相较于模型组,金钗石斛多糖3个治疗组和阳性药物组SOD及GSH升高(P < 0.05),MDA和ROS降低(P < 0.05);与正常组相比,模型组大鼠心肌组织中Plin5和SCD1升高(P < 0.05),PPARα、CPT1以及ACOX1降低(P < 0.05),与模型组相比,金钗石斛多糖各个治疗组和阳性药物组Plin5和SCD1均降低(P < 0.05),而PPARα、CPT1以及ACOX1升高(P < 0.05)。 结论 金钗石斛多糖能够通过Plin5调节脂肪酸β氧化,进而降低心肌脂质含量、炎症反应和氧化应激,保护糖尿病心肌损伤大鼠心脏,提高心功能。 Abstract:Objective To explore the mechanism of Dendrobium nobium polysaccharide on myocardial injury in diabetic rats by regulating the fatty acid β oxidation signaling pathway of cardiomyocytes from Plin5. Methods A total of 72 male SD rats of a clean grade were divided into a control group (n = 10) and a modeling group (n = 62). The control group was fed regular diet, while the modeling group was given a high-fat, high-sugar diet for two months, along with a single STZ injection to establish a diabetic myocardial injury animal model. After successfully creating the model, the modeling group of SD rats was further divided into five subgroups: the model group, the isosorbide mononitrate tablet group, the low-dose Dendrobium nobium polysaccharide group, the medium-dose group, and the high-dose group (n = 10). After 4 weeks of drug treatment, rats were euthanized, and their hearts were collected to detect the myocardial hemopathology (using HE and Masson staining), lipid levels (TG, TC, HDL-C and LDL-C), inflammatory factors (IL-1α, IL-1β, IL-6 and TNF-α) and antioxidant indexes (SOD, GSH, MDA and ROS). Blood samples were taken to assess cardiac function, insulin and blood sugar levels, and Western blot was used to detect the expression characteristics of Plin5, SCD1, CPT1A, ACOX1 and PPARα proteins. Results Histopathology with HE and Masson staining showed that in the normal group, the myocardial fibers of rats were neatly arranged, whereas in the model group, there was fibrous rupture. Compared to the model group, different degrees of improvement were seen in all treatment groups with Dendrobium polysaccharides. Blood sugar and insulin resistance index increased in the model group compared to the normal group (P < 0.05), with insulin levels decreasing (P < 0.05). Compared to the model group, the three groups treated with Dendrobium polysaccharides showed reduced blood sugar and insulin resistance index (P < 0.05). In the low-dose, mid-dose, and isosorbide dinitrate tablet groups, insulin levels did not increase (P > 0.05), while the high-dose Dendrobium polysaccharides group exhibited an increase in insulin content (P < 0.05). Compared to the normal group, TG, TC and LDL-C in the model group were remarkably increased (P < 0.05), while HDL-C was remarkably decreased (P < 0.05). Compared to the model group, TG, TC and LDL-C in the three Dendrobium polysaccharide groups and the positive drug group were remarkably decreased (P < 0.05), while HDL-C was remarkably increased (P < 0.05). Compared to the normal group, AST, CK, CK-MB, LDH, a-HBDH and CTnl in the model group were remarkably increased (P < 0.05). Compared to the model group, the above 6 indexes in the 3 Dendrobium polysaccharide treatment groups and positive drug group were remarkably decreased (P < 0.05). Compared to the normal group, IL-1α, IL-1β, IL-6 and TNF-α in the the model group were remarkably increased (P < 0.05), and they were remarkably decreased in the three Dendrobium polysaccharide treatment groups and the positive drug group compared to the model group (P < 0.05); compared to normal group, SOD and GSH in model group were remarkably decreased (P < 0.05), MDA and ROS were remarkably increased (P < 0.05), compared to the model group, SOD and GSH in the three treatment groups and the positive drug group were increased (P < 0.05), while MDA and ROS were decreased (P < 0.05). Compared to normal group, Plin5 and SCD1 in model group were increased (P < 0.05), and protein expressions of PPARα, CPT1 and ACOX1 were decreased (P < 0.05). Compared to model group, Plin5 and SCD1 were remarkably increased (P < 0.05). Compared to the model group, Plin5 and SCD1 were remarkably decreased in all treatment groups and positive drug groups (P < 0.05), while PPARα, CPT1 and ACOX1 were remarkably increased (P < 0.05). Conclusion Dendrobium nobium polysaccharide can regulate fatty acid β oxidation through Plin5, thereby reducing myocardial lipid content, inflammation, and oxidative stress, protecting diabetic myocardial injury in rats, and improving heart function. -
根据国际糖尿病联盟的分析,预计到2045年,糖尿病患者的数量将从2030年的5.78亿增加到7.00亿人,这意味着世界各地的公共卫生威胁日益增加[1]。其中2型糖尿病(type 2 diabetes mellitus,T2DM)约占全球所有糖尿病患者的90%, 是一种受遗传和环境风险综合影响的复杂疾病,越来越多的研究表明微生物失调在T2DM 发病机制中的作用[2]。前期研究发现:恒古骨伤愈合剂具有降血糖功效,可改善2型糖尿病db/db小鼠肠道微生态平衡[3]。广谱抗生素引起宿主肠道菌群失调。因此,本实验进一步探索恒古骨伤愈合剂联合广谱抗生素对糖尿病小鼠的作用,为进一步的药物开发提供理论依据。
1. 材料与方法
1.1 实验动物
SPF级10周龄雄性 db/db小鼠和野生型小鼠,购于北京斯贝福公司[SCXK(京)2019-0010],在昆明医科大学[许可证SYXK(滇)2020-0006]饲养:室温(23±2)℃,湿度(52±10)%,明暗交替时间为12 h。小鼠自由采食和饮水。相关操作遵守动物福利要求,试验方案通过伦理委员会批准(KMMU20220903)。
1.2 药物和仪器
恒古骨伤愈合剂(批号:20211211)来自克雷斯制药公司。抗生素(万古霉素,氨苄西林,甲硝唑,新霉素)来自美国Sigma公司。小鼠胰岛素酶联免疫吸附测定试剂盒购自齐一生物科技(上海)公司。血糖检测仪为三诺生物公司制造。
1.3 实验方法
1.3.1 分组与样本采集
适应性饲养2 w后,野生型小鼠为对照组(wt),24只db/db小鼠随机分为模型组(db组)、恒古骨伤愈合剂组(OK组)、广谱抗生素组(ABX组)、恒古骨伤愈合剂与抗生素联合干预组(AO组)(n = 6)。OK组以临床成人常用剂量(按60 kg计算),并按人和动物体表面积换算法折算等效剂量为15.3 g/(kg.2 d),ABX组灌胃等体积广谱抗生素(甲硝唑1 g/L、氨苄西林1 g/L、0.5 g/L 万古霉素和1 g/L新霉素)、对照组、模型组灌胃等体积0.9 %氯化钠溶液,每2 d给药1次。AO组给与恒古骨伤愈合剂与抗生素交替灌胃,共11周。禁食 12 h,测量小鼠体重、空腹血糖,3% 戊巴比妥钠麻醉,采心脏血离心,收集血清检测胰岛素,计算胰岛素抵抗指数(HOMA-IR)。公示为HOMA-IR = 空腹血糖 (mmol/L)× 空腹胰岛素(μU/mL )/ 22.5(校正因子)。取盲结肠内容物0.5 g~1.0 g,分装至无菌 EP 管,过液氮,-80 ℃保存。
1.3.2 肠道菌群16S rDNA测序
由上海百趣公司利用Illumina NovaSeq测序平台测序进行。具体使用Hipure Stool DNA Kits 提取DNA,对16S rDNA V3 + V4区扩增,用AxyPrep DNA Gel Extraction Kit纯化PCR产物,ABI StepOnePlus System定量。原始数据提交SRA数据库,获得ASVs[4],物种注释。并进行菌群多样性分析、差异分析及功能预测。
1.4 统计学处理
采用SPSS 22.0分析数据,计量数据用平均数±标准差表示,组间比较采用单因素方差分析(方差齐) 或秩和检验(方差不齐),多重比较用LSD法或Nemenyi法,P < 0.05为差异有统计学意义。
2. 结果
2.1 广谱抗生素、恒古骨伤愈合剂单独和联合使用改善db/db小鼠胰岛素抵抗
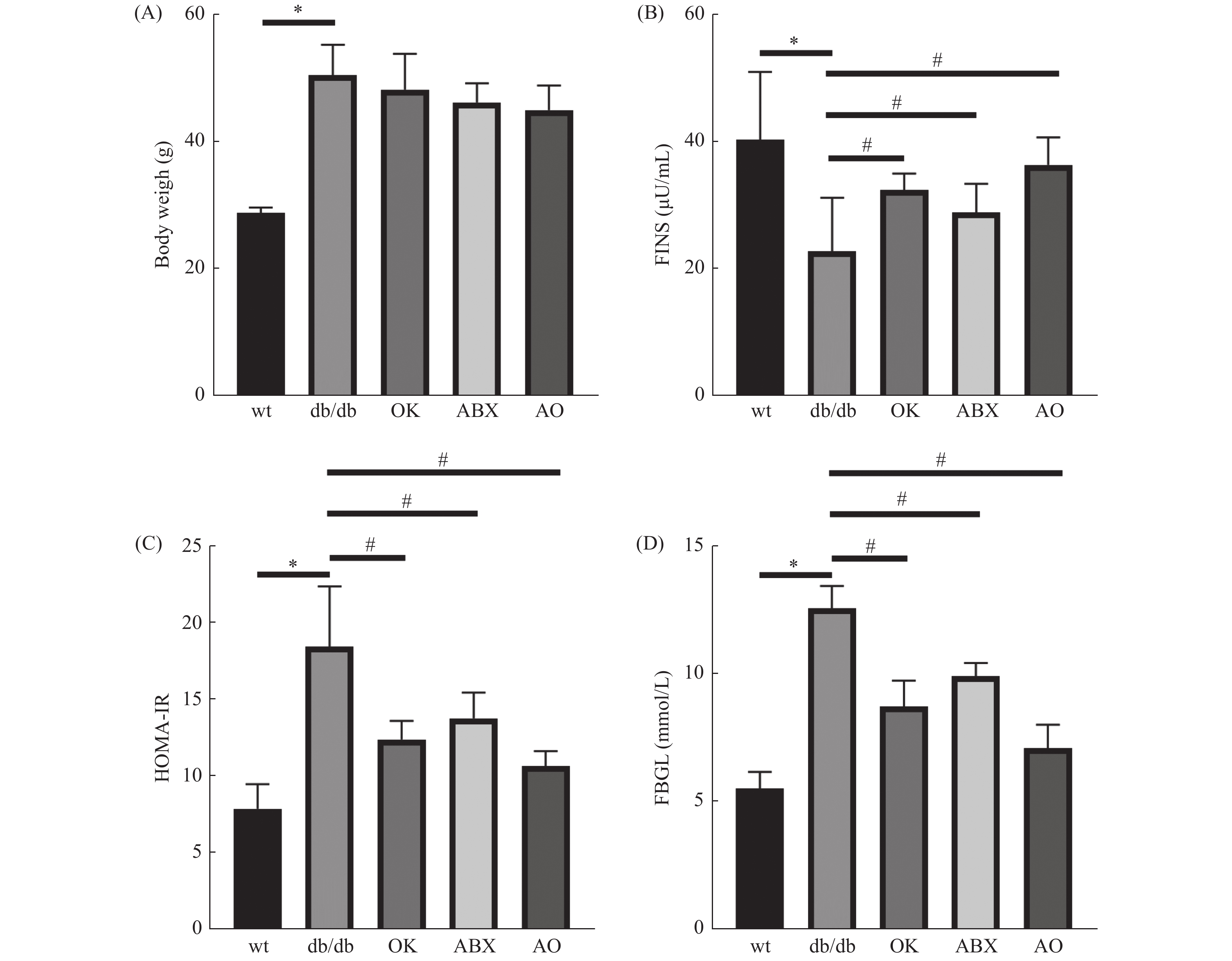
与野生型比较,db/db 小鼠体重(图1A)、空腹血糖(图1B)和胰岛素抵抗指数(图1C)升高,血清胰岛素(图1D)含量降低,差异具有统计学意义(P < 0.05),提示db/db 小鼠出现T2DM的病理症状。与模型组(db/db )比较,广谱抗生素组、恒古骨伤愈合剂组和联合用药组空腹血糖和胰岛素抵抗指数降低,血清胰岛素含量升高,差异具有统计学意义( P < 0.05),但各处理组体重与模型组差异无统计学意义( P > 0.05)( 图1A)。
2.2 肠道菌群测序ASVs和韦恩图
5组小鼠韦恩图呈现小鼠间共有、特有的ASVs,其中对照组
1283 个ASVs;ABX、db/db、OK、AO组的ASVs依次增加(824个、878个、946个、1 330个),ABX组肠道菌群ASVs最少而AO组最多。提示抗生素抑制肠道菌群,恒古骨伤愈合剂改善这种抑制作用。2.3 肠道菌群丰富度和均匀性
与对照组wt小鼠比较,db/db组肠道菌群丰富度和均匀性相关指标chao1、shannon、simpson和pielou-e指数降低(P < 0.05)。与db/db组比较,OK组菌群丰富度和均匀性增加( P < 0.05);ABX组菌群丰富度和均匀性最低,差异具有统计学意义( P < 0.01);AO组菌群丰富度和均匀性介于db/db与ABX组之间,其中chao1和simpson指数与db/db组无统计学差异( P > 0.05)。提示恒古骨伤愈合剂改善抗生素作用下的db/db菌群的丰富度和均匀性。各组样本覆盖度大于99.7%,提示样本中的物种均被检测到( 表1)。
表 1 各组小鼠肠道菌群丰富度和均匀性指数( $ \bar x \pm s $)Table 1. Alpha-diversity index of gut microbiota in the 5 groups ( $ \bar x \pm s $)组别 chao1指数 shannon指数 simpson指数 Pielou-e指数 样本覆盖度 对照组(wt) 557.81 ± 78.59 7.16 ± 0.47 0.98 ± 0.0044 0.79 ± 0.039 1.00 模型组(db/db) 363.52 ± 28.91* 5.87 ± 0.64* 0.92 ± 0.0087 *0.69 ± 0.047* 1.00 广谱抗生素组(ABX) 193.04 ± 32.41## 3.70 ± 0.14## 0.84 ± 0.0091 ##0.49 ± 0.018## 1.00 恒古骨伤愈合剂组(OK) 467.39 ± 51.07# 6.84 ± 0.82# 0.96 ± 0.041# 0.78 ± 0.080# 1.00 联合用药组(AO) 311.28 ± 33.87 4.50 ± 0.50# 0.91 ± 0.013 0.59 ± 0.032# 1.00 F 4.01 15.75 13.41 11.69 — P 0.0132 < 0.0001 < 0.0001 < 0.0001 — 与对照组比较,*P < 0.05;与模型组比较, #P < 0.05, ##P < 0.01。 2.4 门与科水平的肠道菌群丰度
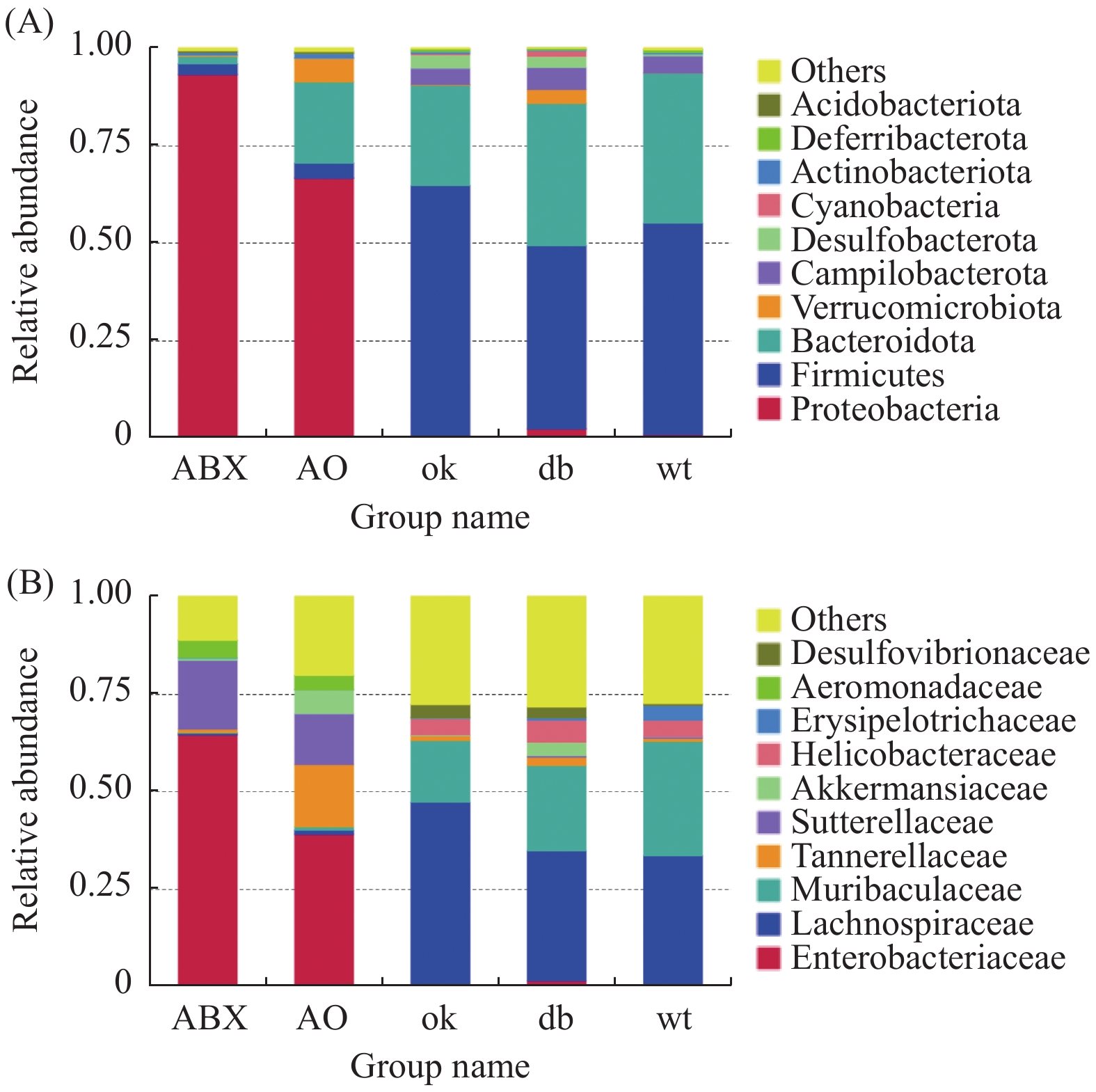
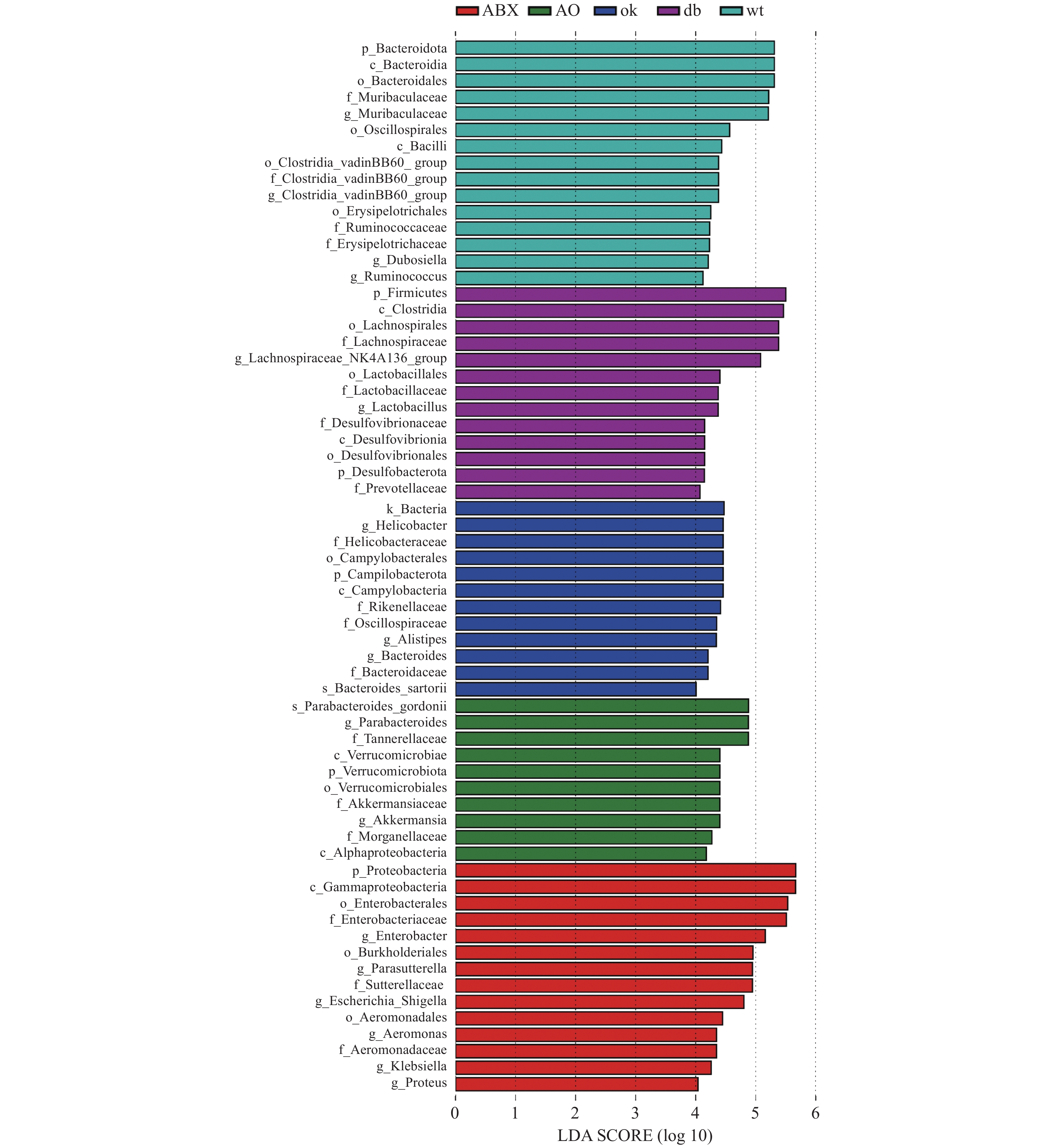
门水平上(图2A),db/db 和OK组厚壁菌门(Firmicutes)(0.471,0.644)和拟杆菌门(Bacteroidota)相对丰度最高(0.366,0.259)。ABX组变形菌门(Proteobacteria)的相对丰度高达0.934,AO组变形菌门相对丰度为0.667,其次为拟杆菌门(0.209)。进一步分析发现各组小鼠肠道菌门构成有明显差异,相对与db/db 组,OK组厚壁菌门(P < 0.05)、ABX( P < 0.01)和AO组( P < 0.01)变形菌门的丰度升高,OK组( P < 0.05)、AO组( P < 0.01)和ABX( P < 0.01)的拟杆菌门相对丰度降低。
科层面(图2B),db/db 小鼠和OK组毛螺菌科(Lachnospiraceae)(0.333、0.472)和Muribaculaceae科(0.219、0.159)群丰度最高。ABX组肠杆菌科(Enterobacteriaceae)和萨特菌科(Sutterellaceae)(0.650,0.178)丰度最高,AO组肠杆菌科、坦纳菌科(Tannerellaceae)和萨特菌科(0.390、0.162、0.130)丰度最高。5组小鼠肠道菌科构成差异明显,与db/db 组比较,OK组毛螺菌科(P < 0.05)、ABX和AO组肠杆菌科、萨特菌科及AO组坦纳菌科的丰度均升高( P < 0.01),而OK组Muribaculaceae丰度降低( P < 0.05)。
2.5 差异菌属筛
LEFse分析组间菌群差异(图3),野生型小鼠肠道菌群的优势物种为拟杆菌门Muribaculaceae属和厚壁菌门瓦丁梭菌BB60群(VadinBB60-group)、杜氏乳杆菌(Dubosiella)、瘤胃球菌属(Ruminococcus)。db/db小鼠的优势菌属为弯曲杆菌门螺旋杆菌属(Helicobacter)和拟杆菌门另枝菌属(Alistipes)和拟杆菌属(Bacteroides)。OK组的优势菌种为厚壁菌门毛螺菌科NK4A136组(Lachnospiraceae NK4A136 group)和乳杆菌属(Lactobacillus),脱硫杆菌门(Desulfobacterota)脱硫杆菌科(Desulfobacteraceae) )和拟杆菌门的普雷沃氏菌科(Prevotellaceae)等。ABX组的优势菌均为变形菌门,包括γ-变形菌纲肠杆菌科(Enterobacteriaceae)、气单胞菌科(Aeromonadaceae)和β-变形菌纲的萨特氏菌科(Sutterellaceae)等。AO组肠道菌群的优势物种包括古氏副拟杆菌(Parabacteroides gordonii),疣微菌门阿克曼菌属(Akkermansia)和变形菌门γ-变形菌纲肠杆菌目的摩根菌科(Morganellaceae)及α-变形菌纲(Alphaproteobacteria)等。 3. 讨论
糖尿病在中医学中属于“消渴病”范畴,由于先天不足、劳倦内伤、饮食失节、情志失调等导致。中医药治疗糖尿病已千余年,在“天人合一”的整体观念指导下,注重对患者全身功能的调节[5]。彝药恒古骨伤愈合剂由陈皮、红花、三七、杜仲、人参、黄芪、洋金花、钻地风、鳖甲等配方制成,功能主治为活血益气、补肝肾,符合中药治疗糖尿病的原则。课题前期发现恒古骨伤愈合剂改善db/db小鼠糖尿病性骨代谢异常和降低空腹血糖[3]。由于胰岛素在糖脂代谢中发挥重要作用,胰岛素抵抗指数(homeostasis model assessment-insulin resistance,HOMA-IR)是判断胰岛素抵抗的有效指标[6],本实验进一步发现恒古骨伤愈合剂改善db/db小鼠胰岛素抵抗。
肠道菌通过微生物-宿主共代谢系统影响药物代谢。通过抗生素处理杀灭微生物是研究肠道菌群变化的替代方法。抗生素可通过种类、剂量、给药途径及时间的不同,影响肠道菌群组成和结构[7]。抗生素诱导的肠道群的改变与代谢性疾病相关,如肥胖、糖尿病等[4]。选择广谱抑菌且肠道内吸收率相对缓慢的抗生素,其中包括万古霉素、氨苄西林、甲硝唑、新霉素的组合,已被证明对肠道菌群有杀伤作用[8]。本实验选择了以上混合抗生素,通过比对抗生素、恒古骨伤愈合剂单独和联合作用于db/db小鼠的效应。结果显示:经11周干预,抗生素、恒古骨伤愈合剂单独和联合作用,均降低2型糖尿病db/db小鼠空腹血糖和胰岛素抵抗,联合用药组效果更优。与本实验结果类似,出生至成年的长期抗生素暴露,通过调节肠道菌群在一定程度上抵抗高脂饮食负荷小鼠的糖代谢失调[9]。万古霉素降低NOD小鼠的糖尿病发病率[10]。广谱抗生素(给药12 d)降低db/db小鼠随机血糖,改变肠道微生物群组成[11]。本实验也表明广谱抗生素降低 db/db小鼠肠道菌群的丰富度与均匀性。广谱抗生素主要通过抑制拟杆菌门等常见的共生专性厌氧菌,并扩大正常情况下丰度较低变形杆菌门等兼性厌氧菌重塑肠道微生态[12-13]。相似地,本实验也发现ABX和AO组拟杆菌门相对丰度降低,变形菌门的丰度升高。而恒古骨伤愈合剂改善抗生素作用下的菌群的多样性。
LEFse分析显示野生型小鼠拟杆菌门Muribaculaceae属和厚壁菌门瓦丁梭菌BB60群(VadinBB60 -group)、杜氏乳杆菌(Dubosiella)、瘤胃球菌属(Ruminococcus)等益生菌富集。Muribaculaceae属在肠道的能量代谢、血糖血脂等发挥作用。Dubosiella的代谢产物为丁酸,可预防、改善肥胖。VadinBB60-group的代谢产物为短链脂肪酸,与丙酸盐比例呈正相关[14]。较高比例的丙酸盐会导致糖异生增加,减低肥胖[15]。提示野生型小鼠的标志菌在调节血糖中发挥作用。在中国人群中,拟杆菌属(Bacteroides)和另枝菌属(Alistipes)在2型糖尿病患者中比在糖代谢正常的人中更为丰富。另外,它们也与胆固醇水平升高相关[16]。糖尿病患者的幽门螺旋杆菌感染率明显更高[17]。以上临床结果与本实验中db/db小鼠螺旋杆菌属、Alistipes和Bacteroides富集相符。大豆不溶性膳食纤维通过增加乳杆菌属(Lactobacillus)和毛螺菌科NK4A136组(Lachnospiraceae _NK4A136_group)与肥胖相关的潜在有益菌的相对丰度,预防高脂肪饮食喂养小鼠肥胖[18]。黄芩和黄连处理增加产生短链脂肪酸的细菌Prevotellaceae UCG-001等的富集,改善2型糖尿病大鼠糖脂代谢[19]。提示本实验恒古骨伤愈合剂通过Lachnospiraceae NK4A136 group、Lactobacillus和Prevotellaceae等的富集,调节糖脂代谢。而广谱抗生素组中变形菌门γ-和β-变形菌纲的相关致病菌富集,提示抗生素虽然降低血糖,但可能导致重要的肠道微生物共生体从各群体中消失,使能够提供重要健康益处的细菌物种减少[20]。此外,本实验发现恒古骨伤愈合剂联合广谱抗生素干预组中,表现为有益菌Parabacteroides、Akkermansia和有害菌Morganellaceae、Alphaproteobacteria并存富集的状态。提示尽管ABS、OK和联合干预下db/db小鼠血糖及胰岛素抵抗均有所改善,但从肠道优势菌群富集的角度,各干预组涉及的具体调控机制可能有所不同。已有研究表明狄氏副拟杆菌胞外多糖粗提物具良好的免疫调节功能,由可减轻肥胖、缓解结肠炎[21]。阿克曼菌与肥胖、糖尿病、肝脏和心血管疾病等的发生发展相关,在调节机体代谢紊乱方面发挥着至关重要的作用[22-23]。恒古骨伤愈合剂改善抗生素导致肠道微生态紊乱,增加改善糖代谢的有益微生物的富集。推测恒古骨伤愈合剂改善血糖、胰岛素抵抗与这些有益微生物的富集有关,值得进一步探讨。
综上,广谱抗生素、恒古骨伤愈合剂单独和联合作用,均降低2型糖尿病db/db小鼠空腹血糖和胰岛素抵抗。且恒古骨伤愈合剂可改善抗生素对db/db肠道微生态损害。
-
表 1 各组大鼠血糖、胰岛素以及胰岛素抵抗指数比较 ($\bar x \pm s $ )
Table 1. Comparison of blood glucose,insulin and insulin resistance index of rats in each group ($\bar x \pm s $ )
组别 血糖(U/L) 胰岛素(IL/L) 胰岛素抵抗指数 正常组 5.52 ± 0.80 18.65 ± 2.42 4.35 ± 0.23 模型组 21.76 ± 4.52* 12.65 ± 4.50* 12.13 ± 0.95* 阳性药组 21.30 ± 4.35 13.25 ± 2.43 13.06 ± 0.84 低剂量组 18.34 ± 3.86#^ 13.48 ± 3.04 11.05 ± 0.78#^ 中剂量组 17.40 ± 4.17#^ 13.78 ± 2.79 10.86 ± 0.63#^ 高剂量组 16.62 ± 3.40#^ 14.32 ± 2.35# 9.56 ± 0.56#^ F 61.1324 8.9705 340.7878 P 0.00△ 0.001△ 0.00△ 与正常组相比,*P < 0.05;与模型组相比,#P < 0.05;与阳性药物组相比,^P < 0.05;△P < 0.05。 表 2 各组大鼠心肌组织中血脂相关指标比较 [($\bar x \pm s $ ),mmol/L]
Table 2. Comparison of blood lipid related indexes in myocardial tissue of rats in each group [($\bar x \pm s $),mmol/L]
组别/指标 心肌TG 心肌TC 心肌HDL-C 心肌LDL-C 正常组 0.44 ± 0.06 2.56 ± 0.28 1.58 ± 0.22 0.72 ± 0.09 模型组 1.28 ± 0.12* 4.45 ± 0.76* 0.91 ± 0.20* 2.08 ± 0.23* 阳性药组 1.07 ± 0.10# 4.10 ± 0.56# 1.04 ± 0.21# 1.85 ± 0.30# 低剂量组 0.84 ± 0.14#^ 3.28 ± 0.32#^ 1.23 ± 0.28#^ 1.40 ± 0.32#^ 中剂量组 0.79 ± 0.14#^ 3.19 ± 0.34#^ 1.30 ± 0.23#^ 1.36 ± 0.29#^ 高剂量组 0.76 ± 0.16#^ 3.13 ± 0.30#^ 1.34 ± 0.19#^ 1.28 ± 0.26#^ F 204.7500 31.2820 28.5811 105.2781 P 0.00△ 0.00△ 0.00△ 0.00△ 注:与正常组相比,*P < 0.05;与模型组相比,#P < 0.05;与阳性药物组相比,^P < 0.05;△P < 0.05。 表 3 各组大鼠心肌酶谱指标比较($\bar x \pm s $)
Table 3. Comparison of myocardial zymogram indexes of rats in each group($\bar x \pm s $)
组别/指标 AST(U/L) CK(U/L) CK-MB(U/L) LDH(U/L) a-HBDH(U/L) CTnl(ng/L) 正常组 68.54 ± 7.74 22.50 ± 3.38 18.46 ± 2.36 80.25 ± 10.24 150.23 ± 7.71 55.32 ± 7.89 模型组 169.67 ± 35.62* 50.35 ± 10.23* 44.32 ± 9.87* 168.35 ± 20.25* 330.65 ± 47.65* 128.68 ± 20.21* 阳性药组 120.24 ± 28.32# 30.26 ± 6.89# 27.40 ± 6.25# 110.54 ± 15.45# 230.34 ± 40.24# 76.34 ± 15.14# 低剂量组 146.52 ± 28.54# 45.37 ± 8.92# 38.65 ± 5.79# 144.32 ± 17.65# 286.54 ± 42.36# 106.34 ± 16.72# 中剂量组 135.54 ± 26.65# 40.35 ± 8.05# 33.48 ± 6.82# 136.48 ± 16.36# 270.35 ± 35.14# 90.24 ± 19.23# 高剂量组 133.54 ± 25.65# 38.28 ± 7.78# 29.79 ± 4.89# 130.36 ± 13.26# 265.25 ± 38.14# 85.32 ± 17.30# F 36.0055 37.8922 36.4293 79.7554 62.0769 61.1717 P 0.00△ 0.00△ 0.00△ 0.00△ 0.00△ 0.00△ 注:与正常组相比,*P < 0.05;与模型组相比,#P < 0.05;与阳性药物组相比,^P < 0.05;△P < 0.05。 表 4 各组大鼠炎性因子比较[($\bar x \pm s $ ),ng/L]
Table 4. Comparison of inflammatory factors of rats in each group[($\bar x \pm s $ ),ng/L]
组别/指标 心肌IL-1a 心肌IL-1b 心肌IL-6 心肌TNF-a 正常组 0.08 ± 0.01 0.14 ± 0.02 0.46 ± 0.08 1.45 ± 0.12 模型组 0.32 ± 0.08* 0.38 ± 0.08* 1.21 ± 0.25* 4.65 ± 0.32* 阳性药组 0.27 ± 0.12# 0.29 ± 0.03# 0.84 ± 0.07# 3.23 ± 0.43# 低剂量组 0.24 ± 0.03#^ 0.25 ± 0.04#^ 0.86 ± 0.15# 3.30 ± 0.35# 中剂量组 0.20 ± 0.04#^ 0.21 ± 0.03#^ 0.74 ± 0.12#^ 2.65 ± 0.30#^ 高剂量组 0.16 ± 0.02#^ 0.17 ± 0.02#^ 0.64 ± 0.10#^ 2.18 ± 0.32#^ F值 23.0144 57.2727 57.1680 255.6314 P值 0.00△ 0.00△ 0.00△ 0.00△ 与正常组相比,*P < 0.05;与模型组相比,#P < 0.05;与阳性药物组相比,^P < 0.05;△P < 0.05。 表 5 各组大鼠心肌组织中氧化应激指标比较[($\bar x \pm s $),ng/L]
Table 5. Comparison of oxidative stress indexes in myocardial tissues of rats in each group [($\bar x \pm s $),ng/L]
组别/指标 心肌MDA(nmol/L) 心肌SOD(U/mL) 心肌ROS(%) 心肌GSH(ug/L) 正常组 5.50 ± 0.52 880.56 ± 28.89 30.56 ± 5.08 654.23 ± 29.37 模型组 10.45 ± 0.76* 290.42 ± 120.32* 88.92 ± 9.02* 230.54 ± 30.50* 阳性药组 7.68 ± 0.57# 470.72 ± 98.35# 54.26 ± 7.08# 326.56 ± 37.92# 低剂量组 7.79 ± 0.62# 465.57 ± 67.56# 56.62 ± 8.84# 330.46 ± 44.45# 中剂量组 6.92 ± 0.56#^ 592.34 ± 80.24#^ 44.09 ± 7.92#^ 396.78 ± 40.42#^ 高剂量组 6.22 ± 0.58#^ 763.68 ± 99.41#^ 38.09 ± 9.03#^ 440.24 ± 38.45#^ F 157.4209 109.8176 164.3076 458.2505 P 0.00△ 0.00△ 0.00△ 0.00△ 注:与正常组相比,*P < 0.05;与模型组相比,#P < 0.05;与阳性药物组相比,^P < 0.05;△P < 0.05。 表 6 大鼠心肌组织中Plin5及其介导的脂肪酸β氧化相关蛋白的相对表达量[($\bar x \pm s $ ),相应蛋白/GAPDH]
Table 6. Relative expression of Plin5 and its related proteins mediated fatty acid Beta oxidation in rat myocardial tissue[($\bar x \pm s $),Corresponding protein /GAPDH]
组别/指标 Plin5 PPARα CPT1 ACOX1 SCD1 正常组 0.26 ± 0.04 1.15 ± 0.08 1.32 ± 0.06 0.62 ± 0.04 0.54 ± 0.07 模型组 1.07 ± 0.12* 0.56 ± 0.05* 0.32 ± 0.07* 0.19 ± 0.03* 1.52 ± 0.10* 阳性药组 0.85 ± 0.08# 0.76 ± 0.06# 0.40 ± 0.06# 0.24 ± 0.04# 1.28 ± 0.08# 低剂量组 0.72 ± 0.08#^ 0.85 ± 8.92#^ 0.56 ± 0.08#^ 0.25 ± 0.03# 1.25 ± 0.09# 中剂量组 0.57 ± 0.06#^ 1.14 ± 8.05#^ 0.58 ± 0.06#^ 0.48 ± 0.05#^ 1.05 ± -0.10#^ 高剂量组 0.45 ± 0.05#^ 1.38 ± 0.78#^ 1.08 ± 0.07#^ 0.58 ± 0.06#^ 1.06 ± 0.12#^ F 70.4866 64.8240 229.6860 121.3902 110.2535 P 0.00△ 0.00△ 0.00△ 0.00△ 0.00△ 与正常组比较,*P < 0.05;与模型组相比,#P < 0.05;与阳性药物组相比,^P < 0.05;△P < 0.05。 -
[1] Ghosh N,Chacko L,Bhattacharya H,et al. Exploring the complex relationship between diabetes and cardiovascular complications: Understanding diabetic cardiomyopathy and promising therapies[J]. Biomedicines,2023,11(4):1126. doi: 10.3390/biomedicines11041126 [2] Bansal S,Burman A,Tripathi A K. Advanced glycation end products: Key mediator and therapeutic target of cardiovascular complications in diabetes[J]. World Journal of Diabetes,2023,14(8):1146. doi: 10.4239/wjd.v14.i8.1146 [3] 郑天圣,佟雪巍,张伊桐,等. 2 型糖尿病心血管并发症发病机制的研究进展[J]. 基础医学与临床,2022,42(5):814. doi: 10.3969/j.issn.1001-6325.2022.05.020 [4] 郭振,樊迪,唐其柱. 活性氧在糖尿病心肌病中的作用机制研究进展[J]. 解放军医学杂志,2020,45(12):1294-1298. doi: 10.11855/j.issn.0577-7402.2020.12.14 [5] 葛淑瑜,杨文娟,孙萍萍,等. 心肌底物能量代谢在糖尿病心肌病中的研究进展[J]. 心电与循环,2021,40(4):450-452. [6] Miner G E,So C M,Edwards W,et al. PLIN5 interacts with FATP4 at membrane contact sites to promote lipid droplet-to-mitochondria fatty acid transport[J]. Developmental Cell,2023,58(14): 1250-1265. e6. [7] Blaibel D,Fernandez C J,Pappachan J M. Acute worsening of microvascular complications of diabetes mellitus during rapid glycemic control: The pathobiology and therapeutic implications[J]. World Journal of Diabetes,2024,15(3):311. doi: 10.4239/wjd.v15.i3.311 [8] Alkholifi F K,Devi S,Yusufoglu H S,et al. The cardioprotective effect of Corosolic Acid in the Diabetic rats: A possible mechanism of the PPAR-γ pathway[J]. Molecules,2023,28(3):929. doi: 10.3390/molecules28030929 [9] ALTamimi J Z,AlFaris N A,Alshammari G M,et al. Esculeoside A decreases diabetic cardiomyopathy in streptozotocin-treated rats by attenuating oxidative stress,inflammation,fibrosis,and apoptosis: Impressive role of Nrf2[J]. Medicina,2023,59(10):1830. doi: 10.3390/medicina59101830 [10] Verma V K,Malik S,Mutneja E,et al. Morin ameliorates myocardial injury in diabetic rats via modulation of inflammatory pathways[J]. Laboratory Animal Research,2024,40(1):1-13. doi: 10.1186/s42826-024-00192-9 [11] Huang Q,Tian H,Tian L,et al. Inhibiting Rev-erbα-mediated ferroptosis alleviates susceptibility to myocardial ischemia-reperfusion injury in type 2 diabetes[J]. Free Radical Biology and Medicine,2023,209:135-150. doi: 10.1016/j.freeradbiomed.2023.09.034 [12] 吴梦龄,范艳,杨榆青,等. 金钗石斛多糖通过下调CYP2E1表达改善非酒精性脂肪肝病大鼠症状[J]. 西部医学,2020,32(4):505-509,514. doi: 10.3969/j.issn.1672-3511.2020.04.010 [13] 张俊青,吴芹,龚其海,等. 金钗石斛生物总碱对脂多糖激活星形胶质细胞产生炎症因子的影响[J]. 中国药理学通报,2011,27(6):824-827. doi: 10.3969/j.issn.1001-1978.2011.06.020 [14] 刘莹莹,武俊紫,李伟,等. 金钗石斛多糖对2型糖尿病大鼠心肌组织凋亡相关蛋白的影响[J]. 中西医结合心脑血管病杂志,2018,16(18):2621-2626. doi: 10.12102/j.issn.1672-1349.2018.18.007 [15] 刘莹莹,武俊紫,李伟,等. 金钗石斛多糖对2型糖尿病大鼠心肌组织中RIP蛋白表达影响[J]. 辽宁中医药大学学报,2017,19(12):44-48. [16] Pang M,Li Y,Gu W,et al. Recent advances in epigenetics of macrovascular complications in diabetes mellitus[J]. Heart,Lung and Circulation,2021,30(2):186-196. [17] Da Dalt L,Cabodevilla A G,Goldberg I J,et al. Cardiac lipid metabolism,mitochondrial function,and heart failure[J]. Cardiovascular Research,2023,119(10):1905-1914. doi: 10.1093/cvr/cvad100 [18] Krizanac M,Mass Sanchez P B,Schröder S K,et al. Lipid-independent regulation of PLIN5 via IL-6 through the JAK/STAT3 axis in Hep3B cells[J]. International Journal of Molecular Sciences,2023,24(8):7219. doi: 10.3390/ijms24087219 [19] Cinato M,Mardani I,Miljanovic A,et al. Cardiac Plin5 interacts with SERCA2 and promotes calcium handling and cardiomyocyte contractility[J]. Life Science Alliance,2023,6(4):e202201690 . doi: 10.26508/lsa.202201690 [20] Du T,Xiang L,Zhang J,et al. Vitamin D improves hepatic steatosis in NAFLD via regulation of fatty acid uptake and β-oxidation[J]. Frontiers in Endocrinology,2023,14:1138078. doi: 10.3389/fendo.2023.1138078 [21] Burgin H,Sharpe A J,Nie S,et al. Loss of mitochondrial fatty acid β‐oxidation protein short‐chain Enoyl‐CoA hydratase disrupts oxidative phosphorylation protein complex stability and function[J]. The FEBS Journal,2023,290(1):225-246. doi: 10.1111/febs.16595 -






 下载:
下载:
 下载:
下载: