Study on Predictive Parameters of Liver Fibrosis Risk in Patients with Metabolic Dysfunction-associated Fatty Liver Disease Complicated with Chronic Hepatitis B
-
摘要:
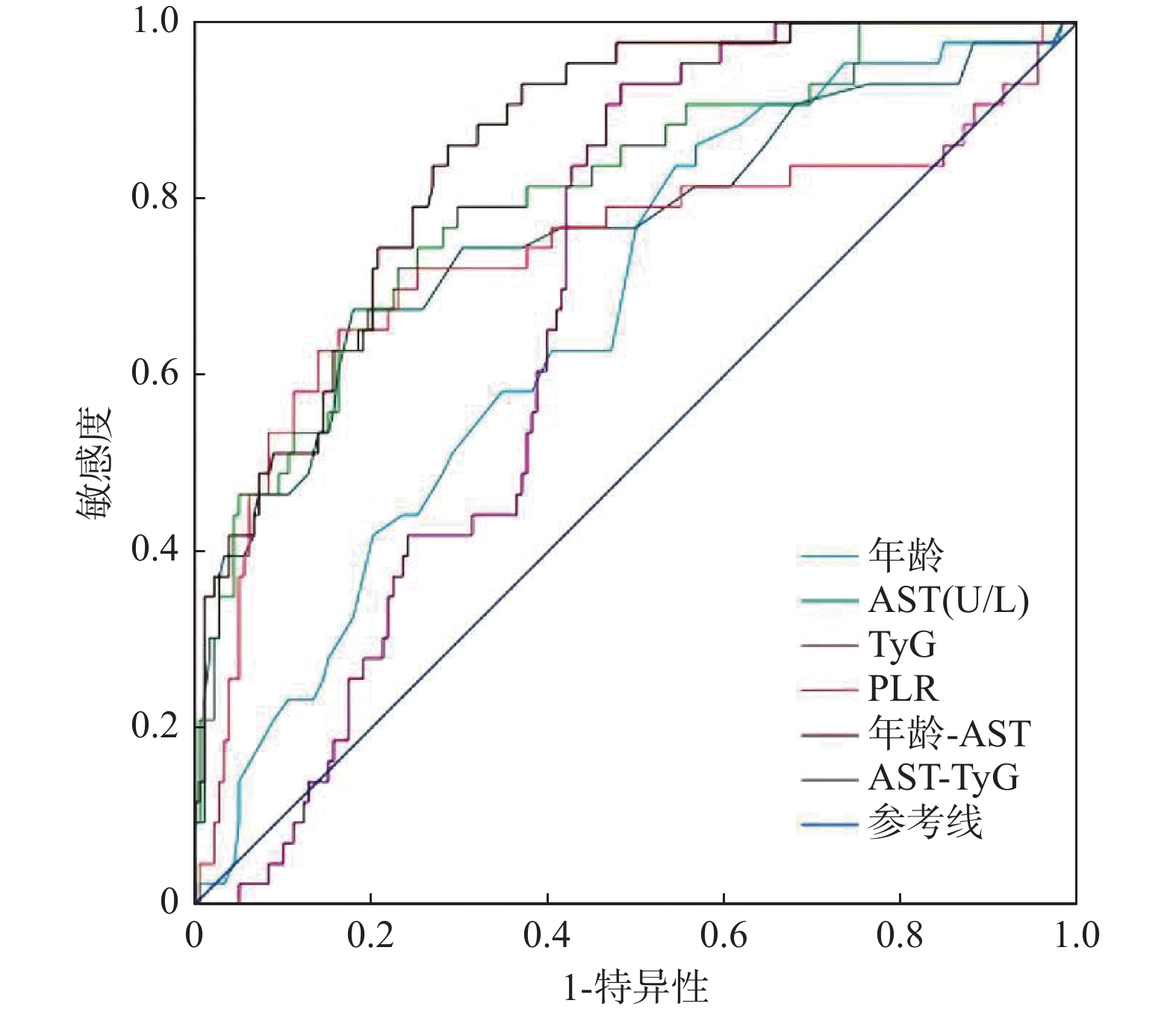
目的 探讨代谢相关脂肪性肝病(MAFLD)合并慢性乙型肝炎(CHB)患者肝纤维化的影响因素,并寻找临床实用方便的预测参数以评估患者肝纤维化的风险。 方法 采用回顾性研究方法,纳入兰州大学第二医院2015年1月至2023年8月期间诊治的MAFLD合并CHB病例221例。依据肝纤维化4因子(FIB-4)指数将患者分为肝纤维化低危组(FIB-4<1.30,n = 84)、肝纤维化中危组(1.30≤FIB-4≤2.67,n = 94)和肝纤维化高危组(FIB-4>2.67,n = 43),比较三组间患者的一般临床资料、实验室检验指标以及复合指标。通过向前逐步回归法筛选变量,进一步实施二分类Logistic回归分析确定肝纤维化独立预测因子,构建预测模型后通过受试者工作特征(ROC)曲线量化效能。 结果 年龄、AST、甘油三酯葡萄糖乘积指数(TyG)是MAFLD合并CHB患者肝纤维化的独立危险因素,血小板/淋巴细胞比值(PLR)是MAFLD合并CHB患者肝纤维化的独立保护因素(均P < 0.05)。ROC曲线分析显示年龄、AST、TyG、PLR、年龄-AST、AST-TyG预测肝纤维化的曲线下面积(AUC)分别为0.668、0.764、0.680、0.738、0.856、0.805,当取截断值时,其敏感度分别为86.0%、67.4%、93.0%、62.8%、86.0%、79.1%,特异度分别为43.3%、82.0%、51.7%、86.0%、71.3%、70.2%。 结论 年龄、AST、TyG、PLR是MAFLD合并CHB患者肝纤维化的影响因素,基于此建立的参数可能可以预测MAFLD合并CHB患者发生肝纤维化的风险,联合预测可以提高预测效能。 -
关键词:
- 代谢相关脂肪性肝病 /
- 慢性乙型肝炎 /
- 肝纤维化 /
- 甘油三酯葡萄糖乘积指数 /
- 血小板/淋巴细胞比值
Abstract:Objective To explore the influencing factors of liver fibrosis in patients with metabolic dysfunction-associated fatty liver disease (MAFLD) complicated with chronic hepatitis B (CHB), and to find clinically convenient predictive parameters for assessing liver fibrosis risk. Methods A retrospective analysis was conducted on 221 cases of MAFLD with CHB diagnosed and treated at the Second Hospital of Lanzhou University between January 2015 and August 2022. Patients were divided into low-risk (FIB-4<1.30, n = 84), medium-risk (1.30≤FIB-4≤2.67, n = 94) and high-risk (FIB-4>2.67, n = 43) liver fibrosis groups based on the Fibrosis-4 (FIB-4) index. General clinical data, laboratory indicators and composite indicators were compared among the three groups. Variables were screened using forward stepwise regression, and binary logistic regression analysis was performed to determine independent predictors of liver fibrosis. A prediction model was constructed and evaluated using receiver operating characteristic (ROC) curve analysis. Results Age, AST and Triglyceride-glucose index(TyG) were independent risk factors for liver fibrosis in patients with MAFLD combined with CHB, while platelet-to-lymphocyte ratio (PLR) was an independent protective factor (all P < 0.05). ROC curve analysis showed that the area under the curve (AUC) for age, AST, TyG, PLR, age-AST and AST-TyG in predicting liver fibrosis were 0.668, 0.764, 0.680, 0.738, 0.856 and 0.805, respectively. At the optimal cut-off values, the sensitivities were 86.0%, 67.4%, 93.0%, 62.8%, 86.0% and 79.1%, and the specificities were 43.3%, 82.0%, 51.7%, 86.0%, 71.3% and 70.2%. Conclusion Age, AST, TyG and PLR are influencing factors of liver fibrosis in MAFLD combined with CHB patients. The parameters established based on these factors may predict the risk of liver fibrosis, and combined prediction can improve predictive efficacy. -
表 1 计算指标
Table 1. Calculation of indicators
表 2 三组患者一般资料水平的比较 [n(%) / ($\bar x \pm s $) / M(P25,P75)]
Table 2. Comparison of general characteristics in three groups of patients [n(%) / ($\bar x \pm s $) / M(P25,P75)]
变量 肝纤维化低危组
(n=84)肝纤维化中危组
(n=94)肝纤维化高危组
(n=43)χ2/F/H P 性别 男 60(27.1) 55(24.9) 28(12.7) 3.245 0.197 女 24(10.9) 39(17.6) 15(6.8) 年龄(岁) 48.08±10.01ab 57.90±9.65 59.39±9.53 29.247 <0.001*** 体重(kg) 77.10±12.41a 72.86±11.48 73.65±10.06 3.157 0.044* 身高(m) 1.70(1.65,1.75) 1.68(1.62,1.73) 1.70(1.62,1.73) 3.836 0.147 BMI(kg/m2) 26.26(24.83,28.07) 25.68(23.75,28.43) 25.65(24.03,28.73) 2.718 0.257 合并吸烟史 49(58.3) 54(57.4) 24(55.8) 0.074 0.964 合并高血压 36(42.9) 45(47.9) 22(51.2) 0.894 0.640 合并冠心病 26(31.0) 29(30.9) 12(27.9) 0.147 0.929 T2DM病程(年) 8.65±2.29 8.55±2.10 8.84±2.17 0.249 0.780 *P < 0.05;***P < 0.001;与肝纤维化中危组比较,aP < 0.05;与肝纤维化高危组比较,bP < 0.05。 表 3 三组患者常规实验室数据的比较 [ ($\bar x \pm s $) / M(P25,P75)]
Table 3. Comparison of routine laboratory data among three groups [ ($\bar x \pm s $) / M(P25,P75)]
变量 肝纤维化低危组
(n=84)肝纤维化中危组
(n=94)肝纤维化高危组
(n=43)F/H P NEU(109/L) 4.34(3.30,5.22)b 3.61(2.82,4.56) 3.32(2.56,4.18) 10.692 0.005** LYM(109/L) 2.03±0.69ab 1.77±0.61c 1.49±0.60 10.650 <0.001*** PLT(109/L) 226(192,262)ab 166(139,195)c 108(78,132) 119.100 <0.001*** FPG(mmol/L) 8.61(6.61,11.29)ab 10.60(7.23,14.62) 11.05(10.17,12.57) 20.931 <0.001*** UA(μmol/L) 340(271,394) 322(255,371) 287(225,370) 4.910 0.086 TBIL(μmol/L) 14.30(11.30,17.28)b 14.75(11.90,18.78)c 19.00(114.50,28.60) 17.869 <0.001*** DBIL(μmol/L) 4.15(3.00,5.50)b 4.25(2.58,5.70)c 5.80(3.50,8.50) 9.097 0.011* IBIL(μmol/L) 9.10(7.13,13.58)b 10.80(7.30,14.83)c 13.20(10.00,17.00) 13.777 0.001** ALT(U/L) 32(22,48) 26(19,38)c 43(24,87) 13.951 0.002** AST(U/L) 24(19,31)b 25(20,30)c 42(26,74) 29.780 <0.001*** GGT(U/L) 38(27,49)b 32(23,59)c 79(32,257) 18.380 <0.001*** ALP(U/L) 82(66,98)b 80(63,106)c 97(75,137) 11.147 0.004** ALB(g/L) 45.45(41.90,47.48)b 43.95(41.35,46.53) 42.00(39.70,45.30) 11.840 0.003** TC(mmol/L) 4.49(3.81,5.35)ab 3.99(3.33,4.88) 3.96(3.51,4.44) 8.579 0.014** TG(mmol/L) 1.90(1.25,2.87) 1.86(1.42,2.45) 2.14(1.85,2.48) 4.612 0.100 HDL-C(mmol/L) 1.07(0.91,1.25) 1.03(0.81,1.22) 1.11(0.95,1.27) 4.017 0.134 LDL-C(mmol/L) 3.04(2.49,3.60)ab 2.50(1.96,3.24) 2.43(1.93,3.00) 13.415 0.001** *P < 0.05;**P < 0.01;***P < 0.001;与肝纤维化中危组比较,aP < 0.05;与肝纤维化高危组比较,bP < 0.05;与肝纤维化高危组比较,cP < 0.05。 表 4 三组患者复合指标的比较 [ ($\bar x \pm s $) / M(P25,P75)]
Table 4. Comparison of composite indicators among three groups [ ($\bar x \pm s $) / M(P25,P75)]
变量 肝纤维化低危组
(n=84)肝纤维化中危组
(n=94)肝纤维化高危组
(n=43)F/H P TyG 7.93±0.81b 8.10±0.74c 8.37±0.41 5.218 0.006** TyG-BMI 205.40(189.67,234.63) 207.67(190.40,227.32) 214.88(205.04,238.82) 4.435 0.109 PLR 105.28(90.10,149.05)ab 97.98(77.71,125.05)c 67.55(51.11,95.35) 30.163 <0.001*** NLR 2.03(1.51,2.77) 2.09(1.54,3.02) 2.20(1.38,3.72) 0.927 0.629 TG/ HDL-C 1.75(1.09,2.88) 1.76(1.28,3.14) 1.84(1.60,2.61) 1.216 0.544 TC/ HDL-C 4.36(3.53,5.00)b 4.10(3.16,5.00) 3.61(3.19,4.07) 7.815 0.020** LDL-C/ HDL-C 2.87±0.91b 2.69±0.99c 2.24±0.75 6.679 0.002** **P < 0.01;***P < 0.001;与肝纤维化中危组比较,aP < 0.05;与肝纤维化高危组比较,bP < 0.05;与肝纤维化高危组比较,cP < 0.05。 表 5 肝纤维化影响因素的多因素Logistic回归分析
Table 5. Multifactorial Logistic regression analysis of factors influencing liver fibrosis
自变量 β SE Wald P OR(95%CI) VIF 常数项 −1.421 0.170 69.892 <0.001*** 0.242 − 年龄 0.111 0.025 19.840 <0.001*** 1.117 (1.064~1.173) 1.013 AST 0.063 0.012 28.210 <0.001*** 1.065 (1.041~1.090) 1.028 TyG 0.739 0.318 5.411 0.020* 2.094 (1.123~3.903) 1.020 PLR −0.016 0.006 6.769 0.009** 0.984 (0.972~0.996) 1.037 通过线性回归计算方差膨胀因子(variance inflation factor,VIF)评估自变量间的多重共线性,上述变量的VIF均小于10,表明不存在严重共线性问题(通常以VIF≥10为阈值);*P < 0.05;**P < 0.01;***P < 0.001。 表 6 年龄、AST、TyG、PLR对肝纤维化预测价值的ROC曲线分析
Table 6. ROC curve analysis of age,AST,TyG and PLR in predicting liver fibrosis
变量 AUC P 截断值 敏感度(%) 特异度(%) 95%CI 年龄 0.668 0.001** 50.5 86.0 43.3 0.585~0.752 AST 0.764 <0.001*** 34.5 67.4 82.0 0.675~0.854 TyG 0.680 <0.001*** 7.96 93.0 51.7 0.610~0.750 PLR 0.738 <0.001*** 75.78 62.8 86.0 0.637~0.838 年龄-AST 0.856 <0.001*** 0.15 86.0 71.3 0.801~0.911 AST-TyG 0.805 <0.001*** 0.14 79.1 70.2 0.730~0.880 **P < 0.01;***P < 0.001。 -
[1] 罗小露, 谢宝刚, 邱芳, 等. 慢性乙型肝炎患者抗病毒治疗不同阶段HBV RNA的检测及意义[J]. 中国感染与化疗杂志, 2022, 22(3): 289-294. [2] Davis T. Diabetes and metabolic dysfunction-associated fatty liver disease[J]. Metabolism: Clinical and Experimental, 2021, 123: 154868. doi: 10.1016/j.metabol.2021.154868 [3] Campos-Murgu í a A, Ruiz-Marg á in A, Gonz á lez-Regueiro J A, et al. Clinical assessment and management of liver fibrosis in non-alcoholic fatty liver disease[J]. World Journal of Gastroenterology, 2020, 26(39): 5919-5943. doi: 10.3748/wjg.v26.i39.5919 [4] 中华医学会肝病学分会脂肪肝和酒精性肝病学组, 中国医师协会脂肪性肝病专家委员会. 非酒精性脂肪性肝病防治指南(2018年更新版)[J]. 临床肝胆病杂志, 2018, 34(5): 947-957. doi: 10.3969/j.issn.1001-5256.2018.05.007 [5] 范建高, 徐小元, 南月敏, 等. 代谢相关(非酒精性)脂肪性肝病防治指南(2024年版)[J]. 实用肝脏病杂志, 2024, 27(4): 494-510. [6] 尤红, 王福生, 李太生, 等. 慢性乙型肝炎防治指南(2022年版)[J]. 实用肝脏病杂志, 2023, 26(3): 457-478. [7] Yan X, Jiahui X, Man L, et al. Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: Triglyceride glucose index-related parameters[J]. Frontiers in Endocrinology, 2022, 13: 951689. doi: 10.3389/fendo.2022.951689 [8] 黄媞, 戴霞, 张莉莉, 等. 高血压合并2型糖尿病患者颈动脉内-中膜增厚的影响因素和甘油三酯-葡萄糖指数评估颈动脉内-中膜增厚的价值研究[J]. 内科, 2023, 18(1): 14-17+32. [9] Ramdas Nayak V K, Satheesh P, Shenoy M T, et al. Triglyceride Glucose (TyG) Index: A surrogate biomarker of insulin resistance[J]. The Journal of the Pakistan Medical Association, 2022, 72(5): 986-988. doi: 10.47391/JPMA.22-63 [10] 乔晶, 刘乙君, 王彦. 甘油三酯-葡萄糖指数与胰岛素抵抗相关代谢性疾病的关系[J]. 国际内分泌代谢杂志, 2022, 42(3): 223-226. doi: 10.3760/cma.j.cn121383-20210206-02013 [11] 努尔吉马·阿合尼牙孜, 刘一佳, 梁灿灿, 等. 甘油三酯葡萄糖指数与非酒精性脂肪性肝病脂肪变程度及肝纤维化的相关性研究[J]. 海南医学院学报, 2023, 29(23): 1794-1800. [12] Eslam M, Sanyal J A, George J, et al. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease[J]. Gastroenterology, 2020, 158(7): 1999-2014. e1. [13] Helda T, Fatemeh N, Alireza O. Triglyceride glucose (TyG) index and the progression of liver fibrosis: A cross-sectional study[J]. Clinical Nutrition ESPEN, 2021, 44: 483-487. doi: 10.1016/j.clnesp.2021.04.025 [14] 赵玲玲, 王贝贝, 姚勇利, 等. T2DM合并非酒精性脂肪性肝病伴进展性肝纤维化患者血清抵抗素和TyG指数变化及其临床意义探讨[J]. 实用肝脏病杂志, 2023, 26(3): 356-359. doi: 10.3969/j.issn.1672-5069.2023.03.014 [15] 赵晨, 姜美玲, 邱懿雯. 肝纤维化血清学和影像学诊断的研究进展[J]. 国际消化病杂志, 2024, 44(1): 13-16+35. doi: 10.3969/j.issn.1673-534X.2024.01.004 [16] Martina C, Dominik S, Carsten D, et al. Macrophages and platelets in liver fibrosis and hepatocellular carcinoma[J]. Frontiers in Immunology, 2023, 14: 1277808. doi: 10.3389/fimmu.2023.1277808 [17] 赵芯, 李晖, 邓秀秀, 等. 抗血小板药物与肝纤维化[J]. 实用医学杂志, 2024, 40(8): 1083-1087. doi: 10.3969/j.issn.1006-5725.2024.08.011 [18] Chauhan A, Adams D H, Watson S P, et al. Platelets: No longer bystanders in liver disease[J]. Hepatology, 2016, 64(5): 1774-1784. doi: 10.1002/hep.28526 [19] 薛芮, 范建高. 代谢相关脂肪性肝病新定义的国际专家共识简介[J]. 临床肝胆病杂志, 2020, 36(6): 1224-1227. doi: 10.3969/j.issn.1001-5256.2020.06.007 [20] Ayman A, Mostafa E, Imam W. Platelets-to-lymphocyte ratio is a good predictor of liver fibrosis and insulin resistance in hepatitis C virus-related liver disease[J]. European Journal of Gastroenterology & Hepatology, 2018, 30(2): 207-211. [21] 牛兴杰, 刘志慧, 崔凤梅, 等. 相关炎症指标预测慢性乙型肝炎患者肝纤维化程度的价值[J]. 中华医院感染学杂志, 2020, 30(5): 703-708. [22] Cornberg M, Wong V W, Locarnini S, et al. The role of quantitative hepatitis B surface antigen revisited[J]. J Hepatol, 2017, 66(2): 398-411. doi: 10.1016/j.jhep.2016.08.009 [23] Paccoud O, Surgers L, Lacombe K. Hepatitis B virus infection: Natural history, clinical manifestations and therapeutic approach[J]. Rev Med Interne, 2019, 40(9): 590-598. doi: 10.1016/j.revmed.2019.03.333 [24] 谢放, 孟庆华, 侯维, 等. HBeAg阳性慢性乙型肝炎合并非酒精性脂肪肝患者的临床与病理学特征[J]. 中华实验和临床感染病杂志(电子版), 2018, 12(3): 256-261. [25] Chen Y, Fan C, Chen Y, et al. Effect of hepatic steatosis on the progression of chronic hepatitis B: A prospective cohort and in vitro study[J]. Oncotarget, 2017, 8(35): 58601-58610. doi: 10.18632/oncotarget.17380 [26] 刘伟鸿, 刘晖, 丁惠国, 等. 慢性乙型肝炎合并代谢相关性脂肪性肝病的临床特征及预后影响因素分析[J]. 临床肝胆病杂志, 2022, 38(10): 2230-2235. doi: 10.3969/j.issn.1001-5256.2022.10.007 [27] 阳韬, 李军. 慢性乙型肝炎合并非酒精性脂肪性肝病: 当前的认识与争议[J]. 临床肝胆病杂志, 2023, 39(8): 1797-1804. [28] Xuan Y, Wu D, Zhang Q, et al. Elevated ALT/AST ratio as a marker for NAFLD risk and severity: Insights from a cross-sectional analysis in the United States[J]. Frontiers in Endocrinology, 2024, 15: 1457598. doi: 10.3389/fendo.2024.1457598 -





 下载:
下载: